Back to Journals » Journal of Asthma and Allergy » Volume 16
The Comparative Bioavailability of Fluticasone and Azelastine Delivered as a Single Fixed Dose Combination (MP-AzeFlu) in Comparison to Two Different Formulations of Azelastine and Fluticasone Propionate Following Intranasal Administration in Healthy Chinese Volunteers
Authors Qi W, Liu Y, Xue W, Li K, Gorski JC, Liu M, Yan T, Nguyen DT, Ramalingam RK
Received 6 April 2023
Accepted for publication 14 June 2023
Published 30 June 2023 Volume 2023:16 Pages 667—677
DOI https://doi.org/10.2147/JAA.S416095
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Luis Garcia-Marcos
Wenyuan Qi,1,2 Yue Liu,1,2 Wei Xue,1,2 Kexin Li,1,2 J Christopher Gorski,3 Mark Liu,3 Tieliang Yan,3 Duc Tung Nguyen,4 Rajesh Kumar Ramalingam5
1Clinical Trial Center, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Assessment of Clinical Drugs Risk and Individual Application Key Laboratory, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 3Global Clinical Pharmacology, Mylan Pharmacetical Inc. (Now Viatris), Morgantown, WV, USA; 4Global Clinical Sciences, MEDA Pharma GmbH & Co. KG (A Viatris Company), Bad-Homburg, Germany; 5Global Medical Affairs, Mylan Pharmaceuticals Private Limited (Now Viatris), Bengaluru, India
Correspondence: Kexin Li, Clinical Trial Centre, Beijing Hospital, No. 1 Dahua Road, Dongdan Dongcheng District, Beijing, People’s Republic of China, Email [email protected]
Objective: Two studies (Study I and Study II) were conducted in healthy Chinese volunteers to confirm that there was no pharmacokinetic drug interaction between AZE and FLU in MP-AzeFlu. The secondary objective was to evaluate the pharmacokinetic parameters of MP-AzeFlu compared with the commercially available mono-components.
Methods: Both studies were a randomized, open-label, three-period, six-sequence, single-dose cross-over trial (William’s design) conducted at Beijing Hospital (Beijing, China) in September and October of 2019 in 30 healthy adult male and female volunteers. The natural log transformed parameters: AUC0-tlast, AUC0-∞ and Cmax were analyzed.
Results: The comparison of PK parameters between MP-AzeFlu and Aze (commercially available) showed that the LS mean ratios (90% CI) values for, AUC0–tlast, AUC 0–∞ and Cmax were 100.29% (94.31– 106.66%), 100.76% (94.60– 107.32%) and 93.14% (81.47– 106.48%). The comparison of PK parameters between MP-AzeFlu and Flu (commercially available) for the bioavailability evaluation showed that the LS mean ratios (90% CI) values for, AUC0–tlast, AUC 0–∞ and Cmax were 83.48% (69.81– 99.82%), 100.19% (87.34– 114.94%) and 81.91% (68.50– 97.95%).
Conclusion: The study results confirm that neither the FLU or the AZE component in the combination product (MP-AzeFlu), nor the existing qualitative and quantitative differences in the formulation between the currently marketed AZE and FLU mono-product, display significant potential to impact the systemic exposure of AZE or FLU in Chinese subjects.
Keywords: MP-AzeFlu, azelastine, fluticasone, allergic rhinitis, pharmacokinetics
Introduction
MP-AzeFlu Nasal Spray consists of a fixed-dose combination of azelastine hydrochloride (AZE) and fluticasone propionate (FLU) that is used for the relief of symptoms of moderate to severe seasonal (SAR) and perennial allergic rhinitis (PAR).1,2 Due to different primary mechanisms of action of the individual agents, MP-AzeFlu nasal spray provides greater efficacy than therapy with each agent (azelastine hydrochloride and fluticasone propionate) alone. The fixed drug combination has been approved in 65 countries worldwide and it has been marketed in the US since September 2012 and in Europe since March 2013 for use by adults and adolescents aged ≥12 years and in the US since February 2015 for children aged ≥6 years. The recent ARIA guidelines recommend the use of an INCS and intranasal antihistamine combination for the treatment of AR.3 MP-AzeFlu could be a useful option for those who need an intranasal steroid and antihistamine to control their allergic rhinitis.4,5
Azelastine hydrochloride (AZE) is a selective, non-sedating H1-antagonist with antihistaminic, anti-inflammatory, and mast cell stabilizing property.6 Azelastine hydrochloride nasal spray, 137 µg/spray, is indicated for the treatment of the symptoms of seasonal AR such as rhinorrhea, sneezing, and nasal pruritus in adults and children 5 years of age and older. Azelastine predominantly inhibits early phase reaction via antagonizing histamine activity at H1-receptor level. After intranasal administration, the systemic bioavailability of azelastine HCl is approximately 40%. Maximum plasma concentrations (Cmax) are achieved in 2–3 hours. Azelastine is metabolized by N-demethylation to N-desmethylazelastine mainly via CYP3A4, CYP2D6, and CYP1A2 in human liver microsomes.7 Azelastine binds to the H1-receptors on effector cells and prevents them from releasing histamine and other mediators, with ensuing early and late allergic responses.8
Fluticasone propionate nasal spray, 50 µg, is indicated for the relief of symptoms of seasonal and perennial allergic and non-allergic rhinitis in adults and children 4 years of age and older. Fluticasone propionate is a synthetic, trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone propionate delivered by the intranasal route has an absolute bioavailability averaging less than 1% and a terminal elimination half-life of approximately 7.8 hours.9 Fluticasone propionate is principally metabolized to an inactive metabolite by the cytochrome P450 (CYP) isoenzyme CYP3A4.10 Fluticasone mainly suppresses late phase inflammatory reactions through inhibition of cytokine production via its trans repressive activity. Anti‐inflammatory genes are activated when glucocorticoids (GCs) diffuse across the cell membrane to bind to GC‐responsive elements (GREs) on the promoter region of target genes.11
In previous clinical trials, the combination demonstrated superior efficacy in the treatment of SAR compared with placebo, azelastine HCl, and fluticasone propionate in adults and adolescents in a total of approximately 4000 patients. Other studies have also shown the safety and efficacy of MP-AzeFlu in controlling chronic AR including PAR and vasomotor rhinitis.12 In a pharmacokinetic study in Caucasians, no interaction between AZE and FLU was observed in MP-AzeFlu.13 However, no such pharmacokinetic study was performed in a Chinese population. Thus, two studies (Study I and Study II) were conducted in healthy Chinese volunteers to confirm the lack of a pharmacokinetic drug interaction between AZE and FLU in MP-AzeFlu. A secondary objective was to evaluate the pharmacokinetic parameters of MP-AzeFlu compared with the commercially available mono-products. Both AZE and FLU are approved in China and are marketed as monotherapies.
Study I had the primary objective to assess the effect of fluticasone propionate (FLU) on the relative bioavailability of azelastine hydrochloride (AZE) when administered as fixed AZE-FLU combination product (MP-AzeFlu) compared to similar AZE formulation without containing FLU (ie, AZE alone in the MP-AzeFlu vehicle; REF1). The secondary objective was to compare the relative bioavailability of AZE when administered either as fixed AZE-FLU combination product (TEST) or as marketed AZE product (COMP1) for comparing the effects of FLU on other pharmacokinetic parameters of AZE (AUC0-∞, CL/f, tmax, t½); and to assess incidence of adverse events for the TEST, REF1, and COMP1 treatments. Study II had the primary objective to assess the effect of azelastine hydrochloride (AZE) on the relative bioavailability of fluticasone propionate (FLU) when administered as fixed AZE-FLU combination product (TEST) compared to a similar formulation without AZE (ie, FLU alone in the MP-AzeFlu vehicle; REF2). The secondary objective was to compare the relative bioavailability of FLU when administered either as fixed AZE-FLU combination product (TEST) or as marketed FLU product (COMP2) for comparing the effects of AZE on other pharmacokinetic parameters of FLU (AUC0-∞, CL/f, tmax, t½); and to assess incidence of adverse events for the TEST, REF2, and COMP2 treatments.
Methods
Study Design
Study I and Study II were a randomized, open-label, three-period, six-sequence, single-dose cross-over trial (William’s design) conducted at Beijing Hospital (Beijing, China) in September and October of 2019 in 30 healthy adult male and female volunteers (Table 1). Figure 1 shows the design of the two studies (Study I and Study II). Volunteers were determined to be in good health on the basis of medical histories, physical examinations, vital signs, electrocardiograms, and laboratory evaluations. Exclusion criteria included any clinically relevant abnormality identified at the physical examination, laboratory screening, the use of any medication within 14 days prior to drug administration, blood donation before the start of the study and a documented history of drug allergy.
 |
Table 1 Summary of Mean ± SD Demographic Data for Subjects in Azelastine Study |
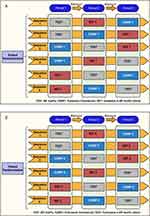 |
Figure 1 Study design of (A) Study I, (B) Study II. Created with BioRender.com. |
Subjects were housed from the day prior to dosing and until at least 12 h post dosing. Subjects received dinner the evening prior to dosing. Overnight fasting of at least 10 h was required for dosage administration. Each subject received a standardized meal approximately 4 hours after drug administration. For each subject, 150 mL of ambient temperature water was given to the subjects (both Study I and II) 2 h post-dose. Between 4 and 24 h p.a. decaffeinated coffee (sugar was allowed but no caloric-free artificial sweetener) or water was allowed (total volume 0–24 h p.a.: ≤3 L). Subjects were monitored for any adverse events (AE) from the signing of the informed consent until the end-of-study visit (within 10–21 days after last dose of study medication).
Study I
Each subject in Study I self-administered four sprays (two per nostril) intranasally for a single azelastine dose of 548 μg (4 × 137 μg/spray) from either MP-AzeFlu Nasal Spray (azelastine HCl 137 μg and fluticasone propionate 50 μg/spray), Azelastine alone in MP-AzeFlu Vehicle Nasal Spray (137 μg/spray), or Aze (commercially available) Nasal Spray (137 μg/spray).
Study II
Each subject in Study II self-administered four sprays (two per nostril) intranasally for a single fluticasone dose of 200 μg (4 × 50 μg/spray) from either MP-AzeFlu Nasal Spray (azelastine HCl 137 μg and fluticasone propionate 50 μg/spray), Fluticasone alone in MP-AzeFlu Vehicle Nasal Spray (50 μg/spray) or Flu (commercially available) Nasal Spray (50 μg/spray).
Ethics
The protocol and informed consent documents were approved by Ethics Committee of Beijing Hospital (Study I: IRB approval letter no. 2018BJYYEC-189-02, Study II: IRB approval letter no. 2018BJYYEC-190-02). This study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization (ICH) guideline on Good Clinical Practice (GCP) (ICH E6 (R2)), General Data Protection Regulation, local regulations as applicable and the Beijing Hospital Standard Operating Procedures. After providing written informed consent, participants were enrolled in the study.
Study Population
Study I and Study II had the same inclusion and exclusion criterion. Male and female (non-pregnant, non-lactating) volunteers aged between 18 and 45 years, with a Body Mass Index (BMI) between 19.0 and 28.0 kg/m2 who were judged to be healthy based on a pre-study physical examination and clinical laboratory tests were eligible for the study. Key exclusion criterion included (1) allergic reaction or sensitivity to azelastine hydrochloride, fluticasone propionate or one of the excipients (eg, benzalkonium chloride, phenyl-ethyl alcohol, microcrystalline cellulose), (2) evidence of clinically relevant acute or chronic diseases which may pose a safety risk for the subject and (3) pregnant or lactating women.
Drug Concentration Measurements
Sample Collection
Study I
Blood samples (1 × 6 mL) were collected from each subjects within 30 min pre-dosing (0 h) and in intervals of 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 48, 72, 96, and 120 h post-dosing. The blood samples were stored in K2EDTA (anticoagulant) tubes.
Study II
Blood samples (1 × 6 mL) were collected in K2EDTA tubes from each subject within 30 minutes prior to dose administration (0 hour) and post-dose at: 8, 15, 30, 45 minutes, 1, 1.25, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 36 hours. Approximately 306 mL of blood was collected from each subject for pharmacokinetic samples over the course of the study.
Sample Processing
Tubes were inverted 5–10 times after collection and immediately placed in an ice bath or a sample cooling rack. The samples were then centrifuged under refrigeration (4° ± 3°C) at approximately 1900g ± 400g for 10 minutes. The plasma was then returned to an ice bath and transferred into labeled polypropylene tubes. Each plasma sample was divided into two aliquots prior to freezing. Plasma samples were frozen in an upright position in a freezer at −70±10°C until shipment for analysis.
Analytical methods
Quantification of Azelastine
Human plasma (0.10 mL) samples were assayed for azelastine at Covance Pharmaceutical R&D (Shanghai) Co., Ltd (China) from the period of November 8, 2019 – November 20, 2019. The method quantitated azelastine over a range of 2.00 to 1000 pg/mL. The lower limit of quantitation for azelastine was 2.00 pg/mL. Human plasma containing the analytes of interest, azelastine and its internal standards, D4-Azelastine, was processed by liquid–liquid extraction. Analytes were chromatographed using High-performance Liquid Chromatography (HPLC) column prior to introduction to a mass spectrometer (Applied Biosciences Sciex API-5500 LC/MS/MS) operating in the positive ion selective reaction mode with Turbo-Ionspray. Quantitation was performed using linear least squares regression analysis generated from calibration standards prepared immediately prior to each run.
Quantification of Fluticasone
Human plasma (0.50 mL) samples were assayed for fluticasone at Covance Pharmaceutical R&D (Shanghai) Co., Ltd (China) from the period of November 25, 2019 – December 7, 2019. The method quantitated fluticasone over a range of 0.25 to 50 pg/mL. The lower limit of quantitation for fluticasone was 0.25 pg/mL. Human plasma containing the analytes of interest, fluticasone and its internal standard, D5-fluticasone propionate, was processed by solid-phase extraction. Analytes were chromatographed using High-performance Liquid Chromatography (HPLC) column prior to introduction to a mass spectrometer (Applied Biosciences Sciex API-6500 LC/MS/MS) operating in the positive ion selective reaction mode with Turbo-V™. Quantitation was performed using linear least squares regression analysis generated from calibration standards prepared immediately prior to each run.
Statistical Analysis
Sample Size Determination
A total of 24 subjects were required to achieve 95% power and 90% Confidence interval completely between 50% and 200% assuming a true treatment ratio of the AUC0-tlast and Cmax TEST/REF not higher than 125% and a CV not higher than 50%. Thirty (30) subjects were randomized to account for up to 20% potential dropouts. The sample size for this study was chosen to be comparable to that used for a similar and already performed study in Caucasians.13 Subjects who failed to complete the study (dropouts) were not replaced. The sample size determination was the same for both the studies.
Pharmacokinetic and Statistical Analysis
Single-dose pharmacokinetic parameters for azelastine and fluticasone were calculated using non-compartmental techniques. Statistical analyses were performed on the pharmacokinetic parameters using the Mixed Models Procedure (PROC MIXED) of SAS Software (SAS Institute, Cary, NC).
The natural log transformed parameters: AUC0-tlast, AUC0-∞ and Cmax were analyzed. The tests were performed to analyze for statistically significant differences in the pharmacokinetic parameters and to determine the test to reference ratios of the pharmacokinetic parameters using Least Squares Means. Ninety (90%) percent confidence intervals were constructed. The maximum blood concentration (Cmax) and the corresponding time of the maximum concentration (tmax) were identified by visual inspection of the data. The elimination rate constant (λz) was determined as the slope of the linear regression or the terminal log-linear portion of the concentration-time curve. A terminal half-life (t½) was calculated as 0.693/λz. The area under the concentration time curve (AUC from zero to the final detectable azelastine concentration) was determined by a combination of linear and logarithmic trapezoidal methods with extrapolation to infinity (AUC∞). Data from the PP population set, which comprised all randomized subjects without major protocol deviations with potential relevance for the PK analyses, was utilized in the pharmacokinetic and statistical analysis of azelastine and fluticasone.
Results
Study I- Azelastine
Study Population
A total of 30 subjects out of 117 subjects were enrolled and dosed in the azelastine study. Twenty-six (26) subjects completed the study as scheduled. Twenty two (22) subjects were included in the PP population and data from these subjects was utilized in the pharmacokinetic and statistical analysis of azelastine. The mean demographic values for all subjects participating in the azelastine study are presented in Table 1.
Pharmacokinetic (PK) Properties
The mean AZE plasma concentration in the subjects after dosing is shown in Figure 2.
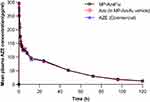 |
Figure 2 Mean azelastine plasma concentrations time profile of PP population. |
Geometric LS mean and arithmetic mean (%CV) of azelastine pharmacokinetic parameters of 22 subjects following a single intranasal dose of 548 μg (4 × 137 μg/spray; 2 sprays per nostril) azelastine under fasting conditions are presented in Table 2
 |
Table 2 Statistical Summary of the Comparative Bioavailability Data for Azelastine (PP Population) |
A comparison of the relative bioavailability of MP-AzeFlu Nasal Spray (TEST), Azelastine alone in MP-AzeFlu Vehicle Nasal Spray (REF1), and AZE Nasal Spray (COMP1) is presented in Table 3.
 |
Table 3 Summary of the Comparison of Azelastine Bioavailability Following Intranasal Administration of Azelastine 548 μg (4 × 137 μg/Spray) Using Three Different Nasal Sprays (PP Population) |
The comparison of primary PK parameters between MP-AzeFlu and Aze in MP-AzeFlu vehicle showed that the LS mean ratios (90% CI) values for, AUC0–tlast, AUC 0–∞ and Cmax were 101.09% (95.55–106.95%), 101.59% (96.24–107.25%) and 99.44% (86.94–113.74%). The comparison of PK parameters between MP-AzeFlu and Aze (commercially available) showed that the LS mean ratios (90% CI) values for, AUC0–tlast, AUC 0–∞ and Cmax were 100.29% (94.31–106.66%), 100.76% (94.60–107.32%) and 93.14% (81.47–106.48%). All of the calculated 90% CIs were within the predefined equivalence margin of 50.00% to 200.00% and even within 80.00%–125.00%.
Safety
Fifteen (15) of 30 (50%) subjects experienced 22 adverse events over the course of the study. Out of 22 AEs, 13 adverse events were considered not to be TEAEs (treatment emergent adverse events) as they were reported more than 6 days after the last investigational product administration. All TEAEs were unlikely to be related to the administration of the study drugs. There were no deaths and no SAEs related to the study medication reported.
Study II- Fluticasone
Study Population
Disposition
A total of 30 subjects out of 125 subjects were enrolled and dosed in Group II. All the subjects completed the study as scheduled. The mean demographic parameters for the fluticasone study are presented in Table 4.
 |
Table 4 Summary of Mean ± SD Demographic Data for All Dosed Subjects in the Fluticasone Study |
Demographics and Baseline Characteristics
The mean (SD) parameters of the volunteers were: age 31.3 (5.3) years, height 166.6 (8.4) cm, weight 63.6 (9.8) kg and BMI 22.8 (2.4) kg/m2 (Table 4).
Population Analysis Sets
All 30 subjects were included in the population for safety analysis (SAF); however, 23 subjects were included in the PP population.
Pharmacokinetic (PK) Properties
The mean FLU plasma concentration in the subjects after dosing is shown in Figure 3.
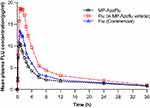 |
Figure 3 Mean Fluticasone concentration in blood plasma of PP population. |
Geometric LS mean and arithmetic mean (%CV) fluticasone pharmacokinetic parameters in twenty-three (23) healthy adult subjects following a single intranasal dose of 200 μg (4 × 50 μg/spray; 2 sprays per nostril) fluticasone propionate under fasting conditions are presented in Table 5. A comparison of the relative bioavailability of MP-AzeFlu Nasal Spray (TEST), fluticasone alone in MP-AzeFlu Vehicle Nasal Spray (REF2), and FLU Nasal Spray (COMP2) is presented in Table 6. The comparison of primary PK parameters between MP-AzeFlu and Flu in MP-AzeFlu vehicle for the bioavailability evaluation showed that the LS mean ratios (90% CI) values for, AUC0–tlast, AUC 0–∞ and Cmax were 61.38% (54.27–69.42%), 72.96% (61.15–87.05%) and 57.47% (50.69–65.16%). The comparison of PK parameters between MP-AzeFlu and Flu (commercially available) for the bioavailability evaluation showed that the LS mean ratios (90% CI) values for, AUC0–tlast, AUC 0–∞ and Cmax were 83.48% (69.81–99.82%), 100.19% (87.34–114.94%) and 81.91% (68.50–97.95%). All of the calculated 90% CIs were within the predefined equivalence margin of 50.00% to 200.00%.
 |
Table 5 Statistical Summary of the Comparative Bioavailability Data for Fluticasone (PP Population) |
 |
Table 6 Summary of the Comparison of Fluticasone Bioavailability Following Intranasal Administration of Fluticasone 200 μg (4 × 50 μg/Spray) Using Three Different Nasal Sprays (PP Population) |
Safety
Most adverse events were mild in intensity, only 2 adverse events (anemia × 2) were moderate. Six adverse events (oropharyngeal pain, nasal obstruction, skin infection, rash, furuncle, upper respiratory tract infection) experienced by four (4) subjects were considered to be TEAEs as they occurred within six days of investigational product administration. All other adverse events were considered to be unlikely or unrelated/not related to study medication. There were no deaths and no SAEs related to the study medication reported.
Discussion
The present study focused on one of the two possible drug–drug interaction scenarios, ie, on the assessment whether the active FLU/AZE component or any excipient of the MP-AzeFlu Nasal Spray may have the potential to perpetrate the systemic exposure and/or the overall disposition/PK of the FLU/AZE component in the formulation. The range 50–200% was chosen as it indicates moderate interactions based on FDA guidance for drug interaction studies.14 Both primary PK parameters for the assessment of a potential PK-based interaction (ie, AUC0-tlast and Cmax) between products confirm that all investigational products employed in the present study display similar in vivo performance in terms of AZE or FLU bioavailability and disposition. The 90%-confidence intervals of the point estimates for TEST/REF and TEST/COMP ratios were completely included in the range 50–200% and even in the range 80–125% for AZE bioavailability. The same results were also obtained for AUC0-∞ which was investigated as secondary PK parameter in this study.
The study results confirm that neither the FLU or the AZE component in the combination product (TEST) nor the existing qualitative and quantitative differences in the formulation between the currently marketed AZE and FLU mono-product and the MP-AzeFlu Nasal Spray display significant potential to impact the systemic exposure of AZE or FLU in Chinese subjects confirming previous results in Caucasians.13 The overall bioavailability and systemic exposure of AZE following MP-AzeFlu administration is the same as compared to the already marketed product confirming the same systemic safety of MP-AzeFlu with regard to AZE component. A single oral dose of AZE (4 mg) has a half-life of 25h15 compared to 35.7 h for AZE in MP-AzeFlu nasal spray. In another study, 200 µg of oral fluticasone had a tmax of 2 h.16 The FLU present in MP-AzeFlu has a tmax 0.75 h. In studies with oral fluticasone, the systemic bioavailability is reported to be <1%.17 Fluticasone propionate delivered by the intranasal route has an absolute bioavailability averaging less than 2%. Overall, MP-AzeFlu Nasal Spray (azelastine HCl 137 μg/spray and fluticasone propionate 50 μg/spray) was well tolerated as single intranasal doses administered as 2 sprays per nostril under fasting conditions.
A direct metabolic interaction between the two drugs can be excluded with the utmost probability even if many microsomal CYPs (eg, CYP2A6, 2B6, 2C, 3A) have been detected in human nasal mucosa18 because both compounds are partly metabolized via different CYP isoenzymes. The lack of metabolic interaction between AZE and FLU has been confirmed in two PK studies in Caucasians.13 The PK parameters of MP-AzeFlu were not higher than of the commercial mono products in Chinese subjects. Consequently, systemically the combination should be as safe as the mono products. Previous study in Caucasians showed that the FLU concentration was very low for all investigational products suggesting no clinically meaningful pharmacodynamic differences in terms of systemic safety13 which was similar to the present study.
Conclusions
The study results confirm that neither the FLU or the AZE component in the combination product (MP-AzeFlu) nor the existing qualitative and quantitative differences in the formulation between the currently marketed AZE and FLU mono-product and the MP-AzeFlu Nasal Spray display significant potential to impact the systemic exposure of AZE or FLU in Chinese subjects.
MP-AzeFlu Nasal Spray was shown to exert similar systemic exposure compared to the marketed nasal spray formulations AZE and FLU as evidenced by the AUC0-tlast and AUC0-∞ values. All study medications were well tolerated as single intranasal doses administered 2 sprays per nostril under fasting conditions.
Data Sharing Statement
Raw data were generated at Viatris. Derived data supporting the findings of this study are available from the corresponding author K.L. on request.
Acknowledgments
The authors acknowledge Arghya Bhattacharya, Ph.D., and Aswin Kumar A, MBBS, for medical writing support (Viatris).
Funding
The present study was funded by MEDA Pharma GmbH & Co. KG (a Mylan Company) now Viatris. The study sponsor was not involved in the study design; the collection, analysis, and interpretation of data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosure
JCG, ML, TY, DTN and RKR are employees of Viatris. All other authors have no declaration for this manuscript.
References
1. Price D, Shah S, Bhatia S, et al. A new therapy (MP29-02) is effective for the long-term treatment of chronic rhinitis. J Invest Allergol Clin Immunol. 2013;23(7):495–503.
2. Li W, Wang Y, Pei Y, Xia Y. Pharmacokinetics and bioequivalence evaluation of two montelukast sodium chewable tablets in healthy Chinese volunteers under fasted and fed conditions. Drug Des Devel Ther. 2021;15:1091–1099. doi:10.2147/DDDT.S298355
3. Bousquet J, Schünemann HJ, Togias A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80.e73. doi:10.1016/j.jaci.2019.06.049
4. Bousquet J, Meltzer EO, Couroux P, et al. Onset of action of the fixed combination intranasal azelastine-fluticasone propionate in an allergen exposure chamber. J Allergy Clin Immunol Pract. 2018;6(5):1726–1732 e1726. doi:10.1016/j.jaip.2018.01.031
5. Bousquet J, Schunemann HJ, Fonseca J, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 2015;70(11):1372–1392. doi:10.1111/all.12686
6. Williams PB, Crandall E, Sheppard JD. Azelastine hydrochloride, a dual-acting anti-inflammatory ophthalmic solution, for treatment of allergic conjunctivitis. Clin Ophthalmol. 2010;4:993–1001. doi:10.2147/OPTH.S13479
7. Ciprandi G, Pronzato C, Passalacqua G, et al. Topical azelastine reduces eosinophil activation and intercellular adhesion molecule-1 expression on nasal epithelial cells: an antiallergic activity. J Allergy Clin Immunol. 1996;98(6):1088–1096. doi:10.1016/S0091-6749(96)80196-5
8. Berger WE, Mustakov TB, Kralimarkova TZ, Christoff G, Popov TA. Treatment with azelastine hydrochloride and fluticasone propionate in a single delivery device of young children and adolescents with allergic rhinitis.
9. Mollmann H, Wagner M, Meibohm B, et al. Pharmacokinetic and pharmacodynamic evaluation of fluticasone propionate after inhaled administration. Eur J Clin Pharmacol. 1998;53(6):459–467. doi:10.1007/s002280050407
10. McKeage K, Keam SJ. Salmeterol/fluticasone propionate: a review of its use in asthma. Drugs. 2009;69(13):1799–1828. doi:10.2165/11202210-000000000-00000
11. Vicens-Artes S, Roca-Ferrer J, Tubita V, et al. Superior effect of MP-AzeFlu compared to monotherapy with fluticasone propionate or azelastine on GILZ, MKP-1 and TTP anti-inflammatory gene expression in healthy and inflamed upper airway mucosa. Clin Exp Allergy. 2022;52(6):788–791. doi:10.1111/cea.14099
12. Berger WE, Shah S, Lieberman P, et al. Long-term, randomized safety study of MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) in subjects with chronic rhinitis. J Allergy Clin Immunol Pract. 2014;2(2):179–185. doi:10.1016/j.jaip.2013.09.019
13. Derendorf H, Munzel U, Petzold U, et al. Bioavailability and disposition of azelastine and fluticasone propionate when delivered by MP29-02, a novel aqueous nasal spray. Br J Clin Pharmacol. 2012;74(1):125–133. doi:10.1111/j.1365-2125.2012.04222.x
14. Food and Drug Administration. Guidance for Industry Drug Interaction Studies —Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. Food and Drug Administration; 2012.
15. McTavish D, Sorkin EM. Azelastine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1989;38(5):778–800. doi:10.2165/00003495-198938050-00005
16. Thorsson L, Dahlstrom K, Edsbacker S, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43(2):155–161. doi:10.1046/j.1365-2125.1997.d01-1425.x
17. Hochhaus G. New developments in corticosteroids. Proc Am Thorac Soc. 2004;1(3):269–274. doi:10.1513/pats.200402-007MS
18. Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43(1):149–173. doi:10.1146/annurev.pharmtox.43.100901.140251
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
