Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
The CHOICES Study: Facility Level Comparative Cost, Resource Utilization, and Outcomes Analysis of Myomectomy Compared to Transcervical Fibroid Ablation
Authors Brooks EA , Singer AM, Delvadia DR , Forstein D , Beaudoin TJ, Bauserman RL, Yuen MW, Little CA, Zambelli-Weiner A
Received 19 March 2020
Accepted for publication 28 May 2020
Published 12 June 2020 Volume 2020:12 Pages 299—306
DOI https://doi.org/10.2147/CEOR.S253891
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Elizabeth A Brooks,1 Allison M Singer,2 Dipak R Delvadia,3 David Forstein,4,5 Teresa J Beaudoin,6 Robert L Bauserman,1 Matt W Yuen,1 Carter A Little,1 April Zambelli-Weiner1
1TTi Health Research & Economics, Westminster, MD, USA; 2Benaroya Research Institute at Virginia Mason Medical Center, Seattle, WA, USA; 3Drexel University College of Medicine, Philadelphia, PA, USA; 4Greenville Health System, Greenville, SC, USA; 5Touro College of Osteopathic Medicine, New York, NY, USA; 6Mercy Clinical Minimally Invasive Gynecology, St. Louis, MO, USA
Correspondence: Elizabeth A Brooks
TTi Health Research & Economics, 1231 Tech Court, Suite 201, Westminster, MD 21157, USA
Tel/ Fax +1 800-580-2990
Email [email protected]
Purpose: The CHOICES study compared short-term resource utilization, facility costs, and perioperative patient outcomes between transcervical fibroid ablation (TFA) with the Sonata® system and myomectomy through a case-matched comparative trial design. This is the first facility-level comparative study conducted for TFA.
Patients and Methods: The study enrolled 88 patients from 4 centers equally divided among the two cohorts. The TFA arm consisted of 44 women who had enrolled in the SONATA Pivotal IDE trial, whereas the myomectomy arm included 44 patients who were identified through retrospective case-matching to the enrolled SONATA patients at the same 4 centers.
Results: TFA had a significantly lower mean operating room duration (90 minutes) and length of stay (5.2 hours) than myomectomy (143 minutes and 45.8 hours, respectively). The average total mean facility costs for TFA procedure ($7,563) were significantly lower than those associated with myomectomy ($11,425; p=0.002). TFA mean facility costs were also compared with other stratifications of myomectomy (inpatient or outpatient and surgical route). TFA facility costs were significantly lower than that associated with inpatient, abdominal, or laparoscopic myomectomy (all p< 0.001).
Conclusion: TFA using the Sonata system has a significantly shorter operating room time and length of stay than myomectomy for the treatment of symptomatic uterine fibroids. All procedure, anesthesia, laboratory, pathology, and pharmacy costs were significantly higher for myomectomy as compared to TFA. TFA was also associated with significantly lower facility procedure-related costs compared to myomectomy, including inpatient, abdominal, or laparoscopic myomectomy.
Keywords: uterine fibroids, Sonata, transcervical ablation, uterine preserving, cost, radiofrequency ablation
Introduction
Uterine fibroids, also known as leiomyomata uteri, are common benign uterine tumors typically found in women of reproductive age. While the true prevalence is unknown and likely underestimated, it has been reported that the prevalence of fibroids in premenopausal women is 70–80% or greater, depending on ethnicity.1,2 The symptoms of fibroids include heavy menstrual bleeding, subfertility, urinary frequency, pelvic pressure and pain, and dyspareunia.1
Current treatment of uterine fibroids is economically burdensome, with costs estimated at $4.1 billion-$9.4 billion in annual US health care costs and an additional $1.55 billion-$17.2 billion lost in annual work hours, in part due to the invasive nature of existing interventions.3,4 Myomectomy is the most common uterine-preserving surgical procedure to treat uterine fibroids.5
Uterine fibroids may also be treated with a less invasive approach such as transcervical fibroid ablation (TFA) with the Sonata system. The transcervical route eliminates the need for incisions and the complications related to open and laparoscopic surgery, preserves the uterus and uterine myometrium, and minimizes the disruption to a woman’s life. The Sonata system combines a single-use radiofrequency ablation handpiece with a reusable intrauterine ultrasound probe to form a single integrated device, eliminating the need to coordinate multiple devices. The procedure has been associated with a significant reduction in symptoms, short recovery time, and length of stay (LOS).6,7 The clinical safety and effectiveness of this procedure has been previously reported.7–9
The purpose of the CHOICES study was to compare short-term resource utilization, facility costs, and perioperative patient outcomes between TFA and myomectomy through a case-matched comparative study design. This is the first facility-level comparative study conducted for TFA with the Sonata system.
Patients and Methods
A comparative case-matched, 30-day outcomes and facility cost analysis was conducted. Costs and procedure-related complications data were collected prospectively for the TFA arm within a multi-center longitudinal clinical trial called SONATA and retrospectively for the myomectomy arm through case-matched patient data collection. As such, original SONATA trial centers were invited to participate if they fulfilled the following criteria: 1) their number of patients enrolled for the SONATA study was >5, 2) the center was interested in participating and had an adequate volume of myomectomy cases for inclusion in the comparator arm, and 3) is located within the continental United States.
Patient Selection
The TFA arm was derived from the SONATA clinical trial, a multicenter, prospective, longitudinal, single-arm clinical trial that verified the safety and effectiveness of the TFA procedure in the treatment of symptomatic uterine fibroids.7 SONATA enrolled and treated patients with TFA between April 2015 and October 2016. Key patient eligibility criteria included women who were premenopausal and between 25 and 50 years of age, had experienced heavy menstrual bleeding associated with fibroids for at least the previous three months, had 1–10 fibroids of International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and/or type 2–5 with diameter between 1 and 5 cm, and had at least one fibroid that indented or abutted the endometrial cavity. Patients for the myomectomy arm were identified by case-matching to TFA arm participants at the same centers. Each site in the SONATA clinical trial gained local IRB approval or ethics committee approval, and the patients used in the SONATA trial provided appropriate consent for the data collected and reported in this study. The data in the myomectomy arm were collected retrospectively, and proper IRB approval or waiver was given by each site that provided data to the study. This study was in accordance with the Declaration of Helsinki. In addition, written informed consent was provided by the patients. See the Appendix for a list of institutions participating in this study. Data used in both the TFA arm and the myomectomy arm were collected from the same sites. There were no additional sites utilized for this study.
Comparator Arm Case-Matching Criteria
The criteria for identifying retrospectively matched myomectomy patients were based on 1) the dates during which SONATA patients were enrolled at that center, 2) the patient’s Body Mass Index (BMI), and 3) the patient’s age. Centers who agreed to participate were provided with data on the range of dates for TFA procedures at the center as well as the associated range of age and BMI from those cases to use in identifying matches. These ranges were based on the means and standard deviations (SDs) for patients recruited at those centers for TFA in the SONATA trial. The centers then reviewed their electronic medical records (EMR) to identify myomectomy patients meeting the criteria. If centers encountered difficulty in locating several matches equivalent to the number of TFA patients that participated at that center, the ranges for matching criteria were broadened until adequate matches were identified.
Data Collection
The primary outcomes of interest were operating room (OR) duration (defined as time patient entered treatment room to time patient exited treatment room), LOS (defined as time of admission to time of discharge or time of eligibility for discharge as reported by the investigators), and the facility costs associated with the index procedure (TFA and myomectomy), including the index procedure hospitalization and any 30-day readmissions. Facility costs were derived from de-identified institution billing forms (UB-04) and health care provider billing forms (HCFA-1500) contributed by the centers for the procedures. A reimbursement specialist identified total charges from these forms and further divided them into the categories of procedure, anesthesia, supply (including the Sonata system), lab, pathology, and pharmacy charges based on each charge’s description. For example, the procedure category included costs for operating room and recovery room time, among others, while supply costs included items such as sterile and non-sterile supplies. Fixed costs are defined by the NIH as those costs which do not vary with the quality of production. The categories defined above are the procedural fixed cost associated with each procedure. Once charges were identified for each center and study arm, cost-to-charge ratios (CCRs) were applied to each center to generate costs, with separate CCRs for procedures done in an inpatient or outpatient setting (Table 1). Specifically, all charges were converted into facility costs using each facility’s own CCR obtained from the American Hospital Directory.10
 |
Table 1 Cost-to-Charge Ratios by Center |
The secondary outcome of interest was the occurrence of complications during the index procedure or hospital stay, and any 30-day readmissions following the procedure. TFA patients’ perioperative complications and readmissions were reported in the SONATA trial and included in this analysis. Data on perioperative complications and readmissions for patients enrolled in the myomectomy arm were obtained from each facility’s EMR.
Analysis
All analyses were conducted in Stata (version 13; StataCorp LLC, College Station, Texas). Patient characteristics were reported for each study arm and compared with chi-square and Fisher’s exact tests for categorical variables or paired t-tests for continuous variables. Means for OR duration, LOS, total costs, cost stratifications, and cost subcategories were reported (using 2016 dollars) and compared using paired t-tests for unequal variances. Stratifications included site of service (inpatient or outpatient) and procedure route (abdominal, laparoscopic, hysteroscopic). Cost subcategories included procedure, anesthesia, supply, laboratory (blood or serum collection and analysis), pathology (cell or tissue collection and analysis), and pharmacy costs. Median and interquartile range values for the same outcomes can be found in the Appendix. Finally, Fisher’s exact test was used to compare the incidence of perioperative complications between the study arms. In all analyses, p-values <0.05 indicate statistical significance.
Results
Seven of the original 22 SONATA trial centers that fulfilled the inclusion criteria participated in this comparative case-matched study. Of the 7 centers that met the inclusion criteria, 1 center declined to participate and 2 centers were unable to provide cost data. As a result, 4 of the original SONATA trial centers provided 44 matched myomectomy patient records with usable cost, resource use, and perioperative outcomes data for this study. Thus, there were 44 patients in each of the 2 study arms (Table 2). TFA patients were compared with myomectomy patients on the demographic characteristics of age, BMI, race, and ethnicity. Because age was a matching characteristic, we expected the groups to be similar in this regard. However, some study centers had difficulty identifying myomectomy patients within the original age range requested, and in these instances the centers could extend the age range for matching patients to younger ages. Myomectomy patients were significantly younger than those in the TFA group, with a mean age of 37.5 ± 6.5 years as compared to 44.3 ± 3.6 (p<0.001). Mean BMI was significantly lower for myomectomy patients (27 ± 6 kg/m2) than TFA patients (30 ± 7 kg/m2; p=0.010). The study arms did not significantly differ in race (p=0.250) or in ethnicity (p=0.494).
 |
Table 2 Characteristics of Study Participants |
OR Duration and LOS
Average OR duration and LOS were compared between the study arms (Table 3). Average OR duration (in minutes) was significantly lower for TFA (90 ± 38) than for myomectomy (143 ± 79; p<0.001). Similarly, LOS was significantly lower for TFA (5.2 ± 1.4 hours) than for myomectomy (45.8 ± 53.7 hours; p<0.001).
 |
Table 3 Average OR Duration and Length of Stay |
Procedure Costs
Figure 1 shows the total mean facility costs of the index procedure inclusive of hospital stay for each arm. The total mean facility costs were significantly lower for TFA ($7,563 ± $2,369) than for myomectomy ($11,425 ± $7,608; p=0.002). Each arm was further stratified by site of service and costs were compared (Table 4). Since TFA is an outpatient procedure, there is no cost data for inpatient. As shown, the total mean costs of TFA ($7,563 ± $2,369) were significantly lower than inpatient myomectomy procedures ($19,811 ± $6,330; p<0.001). The cost of TFA did not significantly differ from outpatient myomectomy procedures, inclusive of hysteroscopic procedures ($7,087 ± $3,420; p=0.517).
 |
Table 4 Procedure Cost by Site of Service |
 |
Figure 1 Mean facility costs. Note: p = 0.002. Abbreviations: SE, standard error; TFA, transcervical fibroid ablation. |
An additional analysis was carried out to compare costs by procedure route (Table 5). As shown, the total mean facility costs of TFA ($7,563 ± $2,369) were significantly lower than both abdominal myomectomy ($18,373 ± $5,548; p<0.001) and laparoscopic myomectomy ($10,352 ± $7,161; p<0.001). As there were only 7 cases of hysteroscopic myomectomy in the myomectomy arm, a statistical comparison could not be made; however, the mean cost of this procedure, which were all outpatient, trended lower in comparison to TFA.
 |
Table 5 Procedure Cost by Route |
Finally, total costs for the TFA and myomectomy arms were broken down by specific subcategories of facility costs, including procedure, anesthesia, supply, laboratory, pathology, and pharmacy costs. As shown in Table 6, all non-supply-related costs associated with TFA were significantly lower than those associated with myomectomy. As expected, supply costs of TFA (which included the Sonata treatment device) were significantly higher than the supply costs of myomectomy, which are mainly surgical tools. However, despite this difference in supply costs, the total mean facility costs of TFA remained significantly lower than myomectomy. This may be explained by the reduced procedure time and LOS associated with TFA due to the efficient transcervical route of delivery.
 |
Table 6 Procedure and Hospitalization Cost |
Complications
The 30-day complications requiring readmission and associated costs were sought for all patients, but no such readmissions were reported for either study arm. As such, total costs reflect the index procedure and hospitalization only. Patient outcome comparisons, in the form of complications, also reflect the index procedure hospitalization only. Any complications serious enough to require readmission within 30 days would have been captured in the study data. The occurrence of complications between TFA and myomectomy was compared. There were 3 complications reported in this study, all being blood loss requiring transfusion in the myomectomy arm patients (p=0.078; Table 7).
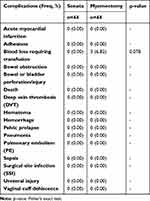 |
Table 7 Complications During Index Procedure or Index Procedure Hospitalization Compared to Sonata |
Discussion
CHOICES is the first comparative study evaluating myomectomy and TFA with the Sonata system. Results for procedure costs, resource utilization, and perioperative patient outcomes favored TFA over myomectomy. Specifically, TFA had a significantly lower OR duration and LOS. Furthermore, TFA had a significantly lower total mean facility cost than myomectomy (all combined), inpatient myomectomy, abdominal myomectomy, and laparoscopic myomectomy. The subcategories of all hospital costs other than the procedure supplies were significantly lower for TFA than myomectomy. Many of these cost differences may be associated with the significantly longer OR duration and LOS for myomectomy as compared to TFA. Neither arm reported any 30-day readmissions, and the only perioperative complications noted occurred in the myomectomy arm.
To identify possible cost drivers, various stratification analyses were conducted. Stratifications included site of service (inpatient or outpatient) and procedure route (abdominal, laparoscopic, hysteroscopic). Cost subcategories included procedure, anesthesia, supply, laboratory, pathology, and pharmacy costs. Stratification analyses by site of service and procedure route yielded predictable results. When comparing outpatient myomectomy procedures with TFA, costs did not significantly differ, as one would expect, because TFA is an outpatient treatment. In addition, cost of hysteroscopic myomectomy trended lower than TFA. Hysteroscopic myomectomy is a transcervical procedure with a limited range of treatable fibroid types (ie, only submucosal fibroids) and a different cost profile from either laparoscopic or abdominal myomectomies.11 TFA can treat all non-pedunculated fibroids (including submucosal, transmural, intramural, and selected subserosal fibroids) that are not amenable to hysteroscopic myomectomy (which is limited to intracavitary and small indenting fibroids). Therefore, the treatment of fibroids with laparoscopic or abdominal myomectomies vs TFA is the more relevant comparison given the range of treatable fibroid types. Despite this lower cost trend for hysteroscopic myomectomy, the total mean facility cost of TFA was significantly lower than all combined myomectomy procedures.
Analysis of hospital cost subcategories also yielded expected results. All non-supply-related costs were significantly lower for TFA than myomectomy. Predictably, the mean cost of TFA supplies, which is mainly driven by the Sonata device cost, was higher than cost of supplies (ie, surgical tools) for myomectomy.
Facility costs reported elsewhere in the literature vary substantially for surgical myomectomy, depending on the source of the cost data, which costs were considered, and if procedure route data were collected. Mauskopf et al examined uterine fibroid treatment costs in the United States and found a range of $8,058 to $18,199 for myomectomies (converted from 2004 dollars to 2016 dollars).12 The highest mean cost by route reported in our study ($18,373 for abdominal myomectomy) was closely aligned with the highest estimates found in Mauskopf’s review. However, our lower mean cost by procedure route ($10,352 for laparoscopic myomectomy) was slightly higher than the lower estimate provided in the literature. In Mauskopf et al, 2005, some of the studies included hysteroscopic myomectomy data, which as we report is considerably lower than the other routes (mean $4,493).12 This could explain why average laparoscopic myomectomy cost in our study was slightly higher than the low estimate, but still within the reported ranges.
Blood loss-related complications reported for the myomectomy patients in this study were also frequently reported in the literature. An article by Sheyn et al reported perioperative blood transfusion as the most common complication for laparoscopic myomectomy within 30 days of the index procedure.13 Several other complications reported by the same article, namely, intraoperative cystotomy, superficial surgical site infection, and wound dehiscence as well as perioperative transfusion, which are possibly due to the more invasive nature of myomectomies, would be eliminated with TFA given the incisionless transcervical approach.
The same study centers were used for the myomectomy comparison arm as those who participated in the original SONATA trial. Myomectomy procedures that occurred at the same time frame as the SONATA trial procedures were selected for data collection. Furthermore, patients were matched based on age and BMI (although in practice, myomectomy patients were younger as a group). This supports our conclusions by controlling for potential biases such as prior procedural experience, internal procedures and protocols, and billing practices of the study centers. The pool of myomectomies included in the study represented a mix of inpatient and outpatient, and abdominal, laparoscopic, and hysteroscopic procedures. Therefore, the cost comparisons reported here are relevant to health care facilities as they utilize a wide mix of treatment routes and patient setting in performing the procedure.
The two groups had statistically significant differences in both age and BMI. The age difference between the TFA and myomectomy patients may reflect the original SONATA trial inclusion criteria.7 The BMI difference is complicated by missing data from one center, but represents a real, but clinically insignificant difference.
As this was a comparative case-matched study, the sample size was restricted by the number of patients enrolled in the original, prospective SONATA clinical trial centers. This limited our ability to match the patients between the two study arms using multiple variables while also maintaining a meaningful sample size. Because of this, we were unable to use patient fibroid characteristics as a matching criteria. The small sample sizes in each arm also may have limited detection of adverse events and hospital readmissions. Therefore, the low number of adverse events and lack of complications seen in the study was not clinically meaningful, and may not be generalizable to a larger population.
While information on fibroid characteristics would have strengthened the comparison between the two patient arms, the lack of appropriate data made a comparison unfeasible. Available details on the myomectomy procedures were limited to the definitions of the CPT codes used to retrospectively identify these patients (Appendix, Table 5). For example, code 58146 is defined as
Myomectomy, excision of fibroid tumor(s) of uterus, 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 grams, abdominal approach.14
Under this definition the number of fibroids, their location, and the total mass of the fibroids are uncertain. As participants in a clinical trial design, the TFA patients had data on some specifics, such as the number of fibroids treated. However, it was not possible to categorize the TFA patient fibroids in such a way that a direct comparison between the two arms would be appropriate. Despite this limitation, the TFA and myomectomy patients were similar enough in fibroid characteristics that the study sites determined both groups could be treated using procedures that preserved the uterus. Considering this study does not evaluate effectiveness or clinical outcomes, strict case matching based on clinical details, while ideal, was not absolutely necessary for this health economic study.
Although each site provided an equal number of patients in both arms, we were unable to control for the confounding that occurs as a result of collecting from four different sites. Each site may have had different protocols for collecting LOS, OR time, or adverse events for the retrospectively identified myomectomy procedures, so the site contributing the most number of patients may have disproportionally influenced the data. However, given the magnitude of the measured differences in LOS, specifically, it is unlikely that differences in protocols changed the results in a meaningful way.
A notable difference among the study arms pertains to the procedure settings. TFA is an entirely outpatient procedure, and therefore, the comparison to inpatient myomectomy may not be most appropriate. Despite remaining a fully outpatient procedure, TFA has been shown to successfully treat the same types of fibroids traditionally treated by abdominal or laparoscopic myomectomy.7 This makes a comparison between the two procedures appropriate, regardless of patient care setting.
These results provide the first comparative analysis of costs and outcomes for TFA as compared to myomectomy. The data demonstrate that TFA offers facilities the potential for reduced health care resource utilization and related costs in comparison to myomectomy procedures.
Conclusion
TFA using the Sonata system has a significantly shorter operating room time and length of stay than myomectomy for the treatment of symptomatic uterine fibroids. All procedure, anesthesia, laboratory, pathology, and pharmacy costs were significantly higher for myomectomy as compared to TFA. TFA was also associated with significantly lower facility procedure-related total costs compared to most stratifications of myomectomy, including inpatient, abdominal, or laparoscopic myomectomy.
Acknowledgments
Gynesonics provided funding for the research presented and the development of the manuscript. Gynesonics provided support during the review of the manuscript, but was not involved in the interpretation of the analyses. Gynesonics also provided funding to TTi Health Research & Economics, for other research presented in other articles published in this journal.
Disclosure
EAB, RLB, MWY, CAL, and AZ-W are employees of TTi Health Research & Economics. DRD is now affiliated with Virtua Ob/Gyn, Voorhees, NJ, USA. The authors report no other conflicts of interest in this work.
References
1. Williams VSL, Jones G, Mauskopf J, Spalding J, Duchane J. Uterine fibroids: a review of health-related quality of life assessment. J Womens Health. 2006;15(7):818–829. doi:10.1089/jwh.2006.15.818
2. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi:10.1067/mob.2003.99
3. Côté I, Jacobs P, Cumming D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol. 2002;100(4):683–687. doi:10.1016/S0029-7844(02)02094-X
4. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):
5. Barrett M, Weiss A, Stocks C, Steiner C, Myers E Procedures to Treat Benign Uterine Fibroids in Hospital Inpatient and Hospital-Based Ambulatory Surgery Settings, 2013 #200; 2016. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp.
6. Brölmann H, Bongers M, Garza-Leal JG, et al. The FAST-EU trial: 12-month clinical outcomes of women after intrauterine sonography-guided transcervical radiofrequency ablation of uterine fibroids. Gynecol Surg. 2016;13:27–35. doi:10.1007/s10397-015-0915-3
7. Chudnoff S, Guido R, Roy K, Levine D, Mihalov L, Garza-Leal J. Ultrasound-guided transcervical ablation of uterine leiomyomas. Obstet Gynecol. 2019;133(1):13–22. doi:10.1097/AOG.0000000000003032
8. Garza-Leal JG, Toub D, León IH, et al. Transcervical, intrauterine ultrasound-guided radiofrequency ablation of uterine fibroids with the VizAblate System: safety, tolerability, and ablation results in a closed abdomen setting. Gynecol Surg. 2011;8(3):327–334. doi:10.1007/s10397-010-0655-3
9. Bongers M, Brölmann H, Gupta J, Garza-Leal JG, Toub D. Transcervical, intrauterine ultrasound-guided radiofrequency ablation of uterine fibroids with the VizAblate® System: three- and six-month endpoint results from the FAST-EU study. Gynecol Surg. 2015;12(1):61–70. doi:10.1007/s10397-014-0873-1
10. American Hospital Directory. Available from: https://www.ahd.com/.
11. Rakotomahenina H, Rajaonarison J, Wong L, Brun J. Myomectomy: technique and current indications. Minerva Ginecol. 2017;69(4):357–369. doi:10.23736/S0026-4784.17.04073-4
12. Mauskopf J, Flynn M, Thieda P, Spalding J, Duchane J. The economic impact of uterine fibroids in the United States: a summary of published estimates. J Womens Health. 2005;14(8):692–703. doi:10.1089/jwh.2005.14.692
13. Sheyn D, Bretschnieder CE, Mahajan ST, El-Nashar S, Billow M, Ninivaggio CS. Comparison of 30-day complication rates between laparoscopic myomectomy and total laparoscopic hysterectomy for the treatment of uterine leiomyoma in women older than age 40. J Minim Invasive Gynecol. 2019;26(6):1076–1082. doi:10.1016/j.jmig.2018.10.018.
14. TCI Super Coder. New myomectomy codes require complete documentation to ensure proper payment; 2003. Available from: https://www.supercoder.com/coding-newsletters/my-ob-gyn-coding-alert/new-myomectomy-codes-require-complete-documentation-to-ensure-proper-payment-article.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
