Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
The Chinese Version of Oxford Depression Questionnaire: A Validation Study in Patients with Mood Disorders
Authors Zhu Y, Wu L, Ye S, Fu Y, Huang H, Lai J , Shi C, Hu S
Received 6 November 2022
Accepted for publication 27 January 2023
Published 7 March 2023 Volume 2023:19 Pages 547—556
DOI https://doi.org/10.2147/NDT.S396356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yiyi Zhu,1 Lingling Wu,2– 5 Shuling Ye,6– 9 Yaoyang Fu,2– 5 Huimin Huang,1 Jianbo Lai,2– 5 Chuan Shi,6– 9 Shaohua Hu1– 5,10
1School of Mental Health, Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Department of Psychiatry, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3The Key Laboratory of Mental Disorder’s Management in Zhejiang Province, Hangzhou, People’s Republic of China; 4Brain Research Institute of Zhejiang University, Hangzhou, People’s Republic of China; 5Zhejiang Engineering Center for Mathematical Mental Health, Hangzhou, People’s Republic of China; 6Peking University Sixth Hospital, Beijing, People’s Republic of China; 7Peking University Institute of Mental Health, Beijing, People’s Republic of China; 8NHC Key Laboratory of Mental Health (Peking University), Beijing, People’s Republic of China; 9National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, People’s Republic of China; 10Department of Neurobiology, NHC and CAMS Key Laboratory of Medical Neurobiology, School of Brain Science and Brian Medicine, and MOE Frontier Science Center for Brain Science and Brain-Machine Integration, Zhejiang University School of Medicine, Hangzhou, 310058, People’s Republic of China
Correspondence: Shaohua Hu, School of Mental Health, Wenzhou Medical University, Wenzhou, People’s Republic of China, Email [email protected] Chuan Shi, Peking University Sixth Hospital, Beijing, 100191, People’s Republic of China, Email [email protected]
Background: Emotional blunting is prevalent in patients with mood disorders and adversely affects the overall treatment outcome. The Oxford Depression Questionnaire is a validated psychometric instrument for assessing emotional blunting. We aimed to evaluate the reliability and validity of the Chinese version of the ODQ (ODQ) in Chinese patients with mood disorders.
Methods: 136 mood disorders patients and 95 healthy control participants were recruited at the First Affiliated Hospital of Zhejiang University, School of Medicine. Patients were assessed using the ODQ, Beck Depression Inventory-II (BDI-II), and Montgomery-Asberg Depression Rating Scale (MADRS). Internal consistency reliability and test-retest reliability were analyzed. Confirmatory factor analysis and correlation analysis were used to evaluate construct and convergent validity.
Results: A total of 136 patients with mood disorders and 95 healthy controls participated in this study. Cronbach α values were 0.928 (ODQ-20) and 0.945 (ODQ-26). Test-retest reliability coefficients were 0.798 (ODQ-20) and 0.836 (ODQ-26) (p< 0.05); intraclass correlation coefficient values were 0.777 (ODQ-20) and 0.781 (ODQ-26) (p< 0.01). The score of ODQ was positively correlated with BDI-II and MADRS (r=0.326~0.719, 0.235~0.537, p< 0.01). The differences in the ODQ scores between the patient and control groups were statistically significant.
Conclusion: The reliability, structural validity, and criterion validity of the ODQ applied to patients with mood disorders meet the psychometric requirements, and the scale can be used to assess emotional blunting in Chinese patients with mood disorders.
Keywords: emotional blunting, Chinese, mood disorder, psychometrics, validation
Introduction
Mood disorders, a very common type of psychiatric disorder, refer to a superordinate grouping of bipolar disorder (BD) and major depressive disorder (MDD). The total prevalence of mood disorders is approximately 10% in the Chinese population.1 Patients with mood disorders present with a variety of clinical symptoms, some of which lack specificity or are atypical. Recently, many researchers have reported the presence of emotional blunting in patients with mental illness. Emotional blunting refers to decreased emotional sensitivity. It may be a common symptom of many psychiatric disorders, including MDD, BD, schizophrenia, and post-traumatic stress disorder (PTSD).2–4 However, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), published in 2013, does not define emotional blunting or outline its diagnostic criteria. At present, most physicians define emotional blunting as a deficit of all emotions in daily life, including both positive and negative emotions. However, there is no agreement on how to determine the presence of emotional blunting in patients.
There is growing clinical interest in the symptom cluster of emotional deficits in patients with psychiatric disorders. Emotional blunting has been shown as the main reason for treatment discontinuation in patients with unipolar and bipolar depression.3 In a large international cohort study, 66.1% of the respondents receiving antidepressant medication reported feeling emotionally numb.5 Similarly, a large online survey found that most patients experienced emotional blunting while taking commonly used antidepressants.6 One study found that 80% of the patients with major depressive episodes reported emotional blunting due to sexual dysfunction caused by selective serotonin reuptake inhibitors. This suggests that emotional blunting plays an important role in the diagnosis and treatment of psychiatric disorders. Reportedly, patients with emotional blunting have more severe residual depressive symptoms and delayed remission of depressive symptoms.7,8 Moreover, emotional blunting alienates patients from their families, interferes with their parenting abilities, impairs social skills, and negatively affects relationships at home and work. Furthermore, patients with emotional blunting are unaware of the danger around them and remain irresponsive when in danger.9 Ignoring emotional blunting may lead to poorer treatment outcomes and, consequently, lower adherence to treatment. Therefore, identifying emotional blunting is particularly important in the treatment of psychiatric disorders.
Some instruments currently used to assess emotional blunting include the Laukes Emotional Intensity Scale (LEIS) and the Bell–Shipman Apathy/Emotional blunting Questionnaire (BSAQ).10,11 However, both scales have limitations in practical application and neither has been scientifically validated. Moreover, it was indicated that the BSAQ was under development, but the subsequent relevant results were not published. Thus, to compensate for the shortcomings of the existing instruments, there is a need to introduce and validate a new assessing instrument for emotional blunting. The Oxford Depression Questionnaire, developed by Price et al at the University of Oxford, is a patient-centered, self-reported questionnaire which can qualitatively understand the patient’s experiences in depth.
Materials and Methods
Participants
Participants with mood disorders registered as outpatients or inpatients with the First Affiliated Hospital of Zhejiang University, School of Medicine, and healthy control participants, recruited through the internet, registered with the physical examination center of the hospital between November 2021 and April 2022, and were enrolled in the study. A total of 231 participants participated in this study, which comprised 136 mood disorders patients and 95 healthy control participants. The inclusion criteria were as follows: 1) All participants were Han Chinese ethnicity with Han parents; 2) currently diagnosed with MDD or BD according to the Mini International Neuropsychiatric Interview for DSM-5;12,13 3) aged 13–65 years; and 4) able to complete the self-assessment questionnaires. The objectives and methods of this study were clearly explained to the participants, and all participants signed an institutional review board-approved informed consent form before being enrolled in the study. The exclusion criteria were: 1) currently diagnosed with neurological diseases or other major psychiatric disorders, such as schizophrenia, cognitive impairment, and substance abuse; and/or 2) diagnosed with severe somatic illnesses, such as diabetes and cardiovascular diseases.
Healthy control participants were screened for depression using the Patient Health Questionnaire-9 (PHQ-9). It was ensured that the control participants were healthy and free of physical and mental illnesses, and that they had never received any psychiatric treatment, including antidepressant medication, antipsychotics, and psychotherapy.
In order to determine face validity and content validity, 10 experts who hold PhDs and senior titles in the psychiatric field were interviewed. Experts anonymously completed two survey questions on the ODQ after reviewing it. In total, experts answered six survey questions about face validity. The face validity survey question was scored using a five-point Likert scale (score range 1–5 with 1 = not well designed and 5 = well designed). To quantify the CVI,14 experts evaluated each item of the scale for relevance and cultural appropriateness on a 4-point rating scale: (1) not relevant; (2) slightly relevant; (3) relevant; and (4) completely relevant. To quantify the CVR,15 experts assessed the necessity of each item on a 3-point rating scale: (1) not essential; (2) useful but not essential; (3) essential.
Procedure
All psychiatrists who evaluated the participants of this study had completed a prior standard training, including standardized instructions on the inclusion and exclusion criteria, procedures for completing the scales, precautions, and interpretation of guidelines and terminology. The participants filled out a demographic questionnaire, the ODQ, Beck Depression Inventory-II (BDI-II), and Montgomery–Asberg Depression Rating Scale (MADRS) during the survey. The participants’ responses were checked after completion, and any missing responses were added based on the patients’ experiences to ensure the accuracy and completeness of the scale.
33 of the original 136 participants in the clinical group were assessed ODQ twice, approximately 2–4 weeks apart, to determine the test-retest reliability of the ODQ.
All manipulations were approved by the Institutional Research Ethics Committee of the First Affiliated Hospital of Zhejiang University, School of Medicine.
Measures
ODQ: The ODQ 26-item questionnaire is divided into three sections: i) patient’s experience in the past week; ii) patient’s experience before the illness worsened, and iii) patient’s emotional feelings about receiving antidepressant treatment (antidepressant- as-cause, [AC] domain), and it contains four dimensions of “emotional blunting”: not caring (NC), emotional detachment from others (ED), reduction in positive emotions (RP), and a general reduction in emotions (GR).16,17 Patients not taking antidepressants are required to only rate the first two sections for a total score of 20–100, while those taking antidepressants are required to complete all items for a total score of 26–130. Patients not taking antidepressants were required to answer only questions 0–20; thus, the two versions of the scale (Included-AC and Non-AC versions) are hereafter referred to as ODQ-26 and ODQ-20 (indicating 26-item and 20-item versions of ODQ). Each item on the scale describes the patient’s emotional experience on a 5-point Likert scale. Higher scores indicate more severe emotional blunting. We expected higher scores on all domains for patients compared to the control group. The ODQ used in this study was authorized by Price et al. The scale was translated into Simplified Chinese from the Clinical Outcomes at Oxford University Innovation Limited.
BDI-II:18 The BDI-II is a widely used scale to screen the severity of depressive symptoms. It is a 21-item, self-reported scale about how the patient has been feeling in the past two weeks.
MADRS:19 The MADRS is a structured, validated, 10-item rating scale designed to measure depressive symptoms quantitatively that was administered by an assessor.
PHQ-9:20,21 A score of 4 or less indicated an absence of depression. The sensitivity and specificity of PHQ-9 were 0.86 and 0.85, respectively.
Data Analyses
Statistical analyses were performed using the IBM SPSS Statistics 22.0 program and AMOS 21.0 program. The Shapiro–Wilk method was used to test the normality of the continuous variables in the participants’ demographic data. Normally distributed variables were presented as means ± SD; other variables were expressed as median (25% quantile, 75% quantile). Cronbach’s α was used to assess the internal consistency reliability. The scale reliability (Cronbach α) values were calculated separately for the two scales based on their designs. Test-retest reliability was estimated using Pearson’s correlation coefficient and intraclass correlation coefficient (ICC).22 Face validity was presented as the percentage of the number of the experts who scored ≥ 4.23 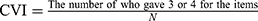 .14,24
.14,24 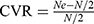 , Ne is the number of experts who rate the item as “essential”, and N is the total number of experts.15 Construct validity was estimated through confirmatory factor analysis. We calculated the following goodness-of-fit indices: X2/df, Comparative Fit Index (CFI), Tucker-Lewis index (TLI), Incremental fit indexes(IFI), Root Mean Square Error of Approximation (RMSEA). Spearman correlation coefficient was calculated to analyze the correlation between ODQ and the other scales (BDI-II, MADRS). A p-value of <0.05 was considered to be statistically significant.
, Ne is the number of experts who rate the item as “essential”, and N is the total number of experts.15 Construct validity was estimated through confirmatory factor analysis. We calculated the following goodness-of-fit indices: X2/df, Comparative Fit Index (CFI), Tucker-Lewis index (TLI), Incremental fit indexes(IFI), Root Mean Square Error of Approximation (RMSEA). Spearman correlation coefficient was calculated to analyze the correlation between ODQ and the other scales (BDI-II, MADRS). A p-value of <0.05 was considered to be statistically significant.
Results
Demographic Characteristics
In total, 136 participants completed the assessment; their demographic characteristics are presented in Table 1. A total of 102 participants completed ODQ-26 and 34 completed the ODQ-20. The health controls only completed ODQ-20. The participants comprised 39 (28.68%) men and 97 (71.32%) women, with a mean age of 21 (17, 26) years. A total of 95 participants were enrolled in the control group, including 34 men and 61 women, with a mean age of 23 (20, 26) years. All healthy control participants’ PHQ-9 scores were lower than 4, with a mean score of 1.84 (0, 3). No statistically significant differences in age were found between the two groups. Using convenience sampling, 33 participants were selected from the patient group for scale retesting 2–4 weeks after the initial assessment, including 5 men and 28 women, with a mean age of 20 (17, 25) years. All 231 questionnaires distributed were completed and returned, with an effective response rate of 100%.
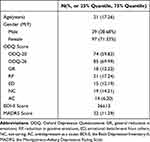 |
Table 1 Demographic and Clinical Characteristics of the Study Participants with Mood Disorders (Bipolar Disorder/Major Depressive Disorder) |
Reliability of the ODQ
Internal Consistency Reliability
The Cronbach’s α coefficients for the total score of the two versions of the ODQ were 0.928 (ODQ-20) and 0.945 (ODQ-26), indicating good internal consistency reliability. The confidence interval for Cronbach’s α uses 95% confidence.
Test-Retest Reliability
Among the 136 participants included in this study, 33 completed the retest of the ODQ 2–4 weeks after the initial assessment; the ICC Cronbach α values of the ODQ were 0.888 (ODQ-20) and 0.911 (ODQ-26); Pearson’s correlation coefficients and ICC coefficients for each dimension are presented in Table 2. The ICC coefficients in this study were all higher than 0.75, except for ED (0.638) and NC (0.627), and the results indicate that the ODQ has good test-retest reliability.
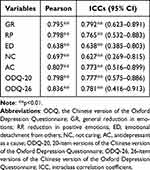 |
Table 2 Test-Retest Reliability of ODQ |
Validity of the ODQ
Face Validity
In total, the experts answered 6 survey questions. The scores of face validity survey questions were all above 4 points, which showed good face validity. The results are reported in Table 3. Data presented as the percentage of the number of the experts who scored ≥ 4.
 |
Table 3 Face Validity Survey Questions |
Content Validity
Results of the content validity are shown in Table 4. The whole scale of CVI and CVR was 0.85, 0.7, respectively, which indicating adequate content validity.
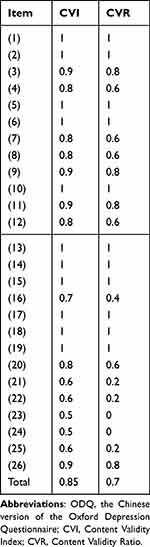 |
Table 4 Content Validity of the ODQ |
Construct Validity
Using confirmatory factor analysis, the scale’s goodness-of-fit indexes were as follows: X2/df=1.899, CFI=0.845, TLI=0.826, IFI=0.848, RMSEA=0.094.
Convergent Validity
Following the normality test, the Shapiro–Wilk test p-values were < 0.05. Spearman correlation was used to evaluate the associations between the ODQ and BDI-II and MADRS. The ED and GR dimensions showed weak positive correlations with the BDI-II scores, whereas the PR and NC dimensions showed strong positive correlations. The specific results of Spearman correlation analysis of the ODQ dimension scores and the total score with the other scales are summarized in Table 5.
 |
Table 5 The Correlations Between the ODQ and Other Scales |
Discriminant Validity
Mann–Whitney U-test was used to evaluate the correlations of the ODQ scores between the patient and control groups; the results are presented in Table 6 and show that for all dimensions of the ODQ and the total score, the scores were significantly higher in the patient group than in the control group.
 |
Table 6 The Correlations Between Mood Disorder Patients and Healthy Controls |
Discussion
The purpose of this study was to examine the psychometric properties of the ODQ for Chinese patients with mood disorders. The results of this study suggest that the ODQ has good reliability and validity, which provides evidence to support its use as a rapid assessing tool for emotional blunting in Chinese patients with mood disorders. Additionally, most of the patients completed the ODQ within two minutes. It can effectively help clinicians assess emotional blunting and provide targeted interventions for patients with mood disorders.
Psychiatric clinicians often ignore the symptoms of emotional blunting. Currently, there are few studies on emotional blunting in mood disorders, and there is a lack of corresponding psychological assessment tools. Thus, there is an urgent need for more scientific tools to reflect the heterogeneity and complexity of mood disorders. The ODQ was developed to assess emotional blunting in depression,25 and it is currently the most scientific and comprehensive tool among all existing scales for assessing emotional blunting.
In terms of reliability, the Cronbach α values of the ODQ in this study were 0.928 (ODQ-20) and 0.945 (ODQ-26). After an interval of 2–4 weeks, the test-reliability values of the ODQ were 0.888 (ODQ-20) and 0.911 (ODQ-26). Price et al used the ODQ to study emotional blunting in depressed patients;16 in their study, Cronbach’s α value for the total score was 0.93. In a recent study in China, Cronbach’s α statistic for the total score on ODQ was 0.91.25 The results of the present study are consistent with these previous studies, showing that the ODQ has excellent internal consistency and stability across time.
This study suggests that the ODQ has favorable validity. Face Validity assesses whether the tools are appropriate for screening symptoms of emotional blunting in patients with mood disorders. The ODQ’ face validity obtains endorsement by experts. All experts confirmed the ODQ measures what is supposed to be measured. The correlation coefficient between ODQ-26 and BDI-II was 0.719, and that between ODQ-26 and MADRS was 0.537. The scores of ODQ-26 and those of each dimension were correlated with BDI-II and MADRS (p<0.05); the correlation coefficients of BDI-II and MADRS scores with the ED and NC dimensions of ODQ were slightly lower. The PR and NC dimensions were closely associated with depressive symptoms. This result is consistent with the data presented by Price et al and Christensen et al16,26 The correlation coefficients of scores among ODQ dimensions are less than the total score correlation, suggesting both consistency and variability between the scales. This suggests that the ODQ does measure emotional blunting dimensions that are not measured by other classical scales. This may be due to the following reasons. On the one hand, as the ODQ, BDI-II, and MADRS assessed decreased positive emotional response in patients with mood disorders, their total scores showed good convergence; on the other hand, there were differences in the content, number of items, and scoring methods between the three tools, which may have weakened the correlations between the scales. For example, BDI-II does not assess the intensity and frequency of sadness, anger, anxiety while ODQ asks participants whether they feel unpleasant, numbed. MADRS has an item to estimate “anhedonia”, namely, having difficulty experiencing pleasure during normally pleasurable activities, whereas the ODQ is an assessment of the patient’s “overall emotional blunting”, including positive and negative. MADRS has an item to estimate “inability to feel”, whereas the ODQ has more detailed measure, such as “Loss of sympathy”,’detachment’. The discriminant validity results showed that the ODQ can assess patients with emotional blunting from healthy individuals.
Confirmatory factor analysis was performed to validate the factor structure. The results showed that the partial model goodness-of-fit indexes of ODQ were not ideal, which might be due to the portion between sample size and number of items.
Recently, Jing Chen et al25 published another validation of the ODQ. However, both studies have many differences. The data comes from different hospitals. And we recruited the participants with mood disorder who do not take antidepressant. We propose that the emotional blunting may not the cause of antidepressant but the mood disorders participants with various residual symptoms. We invited 10 experts to do the face and content validity which would increase the credibility of ODQ. Our study used different scales to do the convergent validity which enrich the relevant research about ODQ. Most of importantly, our study recruited the mood disorders participants which broaden the spectrum of ODQ’ clinical use in the future.
There are some limitations in this study. First, all participants were recruited from a single center. The sample size should be expanded in the future. Second, the patients in this study had prolonged illness and severe symptoms, which may limit the application of the scale to patients with less severe symptoms. Third, the sensitivity, specificity, and cut-off values to the variability of the scale scores remain to be explored in depth. If so, the ODQ would be the gold standard for screening emotional blunting instead of assessing.
Conclusion
Taken together, the ODQ can be used to assess for emotional blunting in Chinese patients with mood disorders, which can help clinicians to prescribe the right medication to alleviate patients’ degree of disability and burden associated with mood disorders. The ODQ is valid and reliable to be used for rapid assessment of emotional blunting in clinical settings.
Abbreviations
ODQ, the Chinese version of the Oxford Depression Questionnaire; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ODQ-20, 20-item versions of the Chinese version of the Oxford Depression Questionnaire; ODQ-26, 26-item versions of the Chinese version of the Oxford Depression Questionnaire; BDI-II, the Beck Depression Inventory-II; MADRS, the Montgomery-Asberg Depression Rating Scale; GR, general reduction in emotions; RP, reduction in positive emotions; ED, emotional detachment from others; NC, not caring; AC, antidepressant as a cause; BD, bipolar disorder; MDD, major depressive disorder; PTSD, post-traumatic stress disorder; LEIS, Laukes Emotional Intensity Scale; BSAQ, Bell–Shipman Apathy/Emotional blunting Questionnaire; ICC, intraclass correlation coefficient; Comparative Fit Index (CFI), Tucker-Lewis index (TLI), Incremental fit indexes(IFI), Root Mean Square Error of Approximation (RMSEA).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author Shaohua HU upon reasonable request.
Ethics Approval and Informed Consent
This study protocol was reviewed and approved by the Institutional Research Ethic Committee of First Affiliated Hospital of Zhejiang University, School of Medicine, approval number [IIT20220240B-R1]. All participants wrote informed consent. We followed the guidelines outlined in the Declaration of Helsinki.
Acknowledgments
We thank our research group that helped and supported us for this study at the Department of psychiatry, First Affiliated Hospital, Zhejiang University School of Medicine, China.
Funding
This study was supported by the Zhejiang Provincial Key Research and Development Program (No. 2021C03107) and the Leading Talent of Scientific and Technological Innovation - “Ten Thousand Talents Program” of Zhejiang Province (No. 2021R52016), and the Innovation Team for Precision Diagnosis and Treatment of Major Brain Diseases (No. 2020R01001). The funders had no role in the preparation of data or the manuscript.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Yueqin H, Wang Y, Hong W, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/s2215-0366(18)30511-x
2. Price J, Cole V, Goodwin M. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211–217. doi:10.1192/bjp.bp.108.051110
3. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116–120. doi:10.1016/j.jad.2018.09.027
4. Litz BT, Litz BT, Gray MJ. Emotional numbing in posttraumatic stress disorder: current and future research directions. Aust N Z J Psychiatry. 2002;36(2):198–204. doi:10.1046/j.1440-1614.2002.01002.x
5. Read J, Williams J. Adverse effects of antidepressants reported by a large international cohort: emotional blunting, suicidality, and withdrawal effects. Curr Drug Saf. 2018;13(3):176–186. doi:10.2174/1574886313666180605095130
6. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67–73. doi:10.1016/j.psychres.2014.01.042
7. Delaveau P, Sanchez TA, Steffen R, et al. Default mode and task-positive networks connectivity during the N-Back task in remitted depressed patients with or without emotional residual symptoms. Hum Brain Mapp. 2017;38(7):3491–3501. doi:10.1002/hbm.23603
8. Xiao L, Feng L, Zhu XQ, et al. Comparison of residual depressive symptoms and functional impairment between fully and partially remitted patients with major depressive disorder: a multicenter study. Psychiatry Res. 2018;261:547–553. doi:10.1016/j.psychres.2018.01.020
9. Grigoriu JR, Grigoriu M, Gee A, Diggle J, Butler H. The positive and negative experiences of 342 antidepressant users. Community Ment Health J. 2020;56(4):744–752. doi:10.1007/s10597-019-00535-0
10. Opbroek A, Delgado PL, Laukes C, McGahuey C, Katsanis J, Moreno FAMR. Emotional blunting associated with SSRI-induced sexual dysfunction. Do SSRIs inhibit emotional responses? Int J Neuropsychopharmacol. 2002;5(2):147–151. doi:10.1017/S1461145702002870
11. Bell S, Shipman M, Haifley T. Fluoxetine treatment and testosterone levels. Ann Clin Psychiatry. 2006;18(1):19–22. doi:10.1080/10401230500464612
12. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33;quiz 34–57.
13. Association AP. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2013.
14. Polit DF, Beck CT. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res Nurs Health. 2006;29(5):489–497. doi:10.1002/nur.20147
15. Lawshe CH. A Quantitative Approach to Content Validity. Pers Psychol. 1975;28(4):563–575. doi:10.1111/j.1744-6570.1975.tb01393.x
16. Price J, Cole V, Doll H, Goodwin GM. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66–74. doi:10.1016/j.jad.2012.01.030
17. Goodwin GM, Price J, Bodinat C, Laredo J. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31–35. doi:10.1016/j.jad.2017.05.048
18. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi:10.1207/s15327752jpa6703_13
19. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi:10.1192/bjp.134.4.382
20. Costantini L, Pasquarella C, Odone A, et al. Screening for depression in primary care with patient health questionnaire-9 (PHQ-9): a systematic review. J Affect Disord. 2021;279:473–483. doi:10.1016/j.jad.2020.09.131
21. Inagaki M, Ohtsuki T, Yonemoto N, et al. Validity of the patient health questionnaire (PHQ)-9 and PHQ-2 in general internal medicine primary care at a Japanese rural hospital: a cross-sectional study. Gen Hosp Psychiatry. 2013;35(6):592–597. doi:10.1016/j.genhosppsych.2013.08.001
22. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6(4):284–290. doi:10.1037/1040-3590.6.4.284
23. McBride KE, Steffens D, Lambert T, Glozier N, Roberts R, Solomon MJ. Acceptability and face validity of two mental health screening tools for use in the routine surgical setting. BMC Psychol. 2021;9(1):171. doi:10.1186/s40359-021-00672-w
24. Khosravi S, Rafiei F, Norozy M, Khanmohamadi Hezave A, Ebrahimabadi M. Cross-cultural adaptation of the Persian version of test of the adherence to inhalers (TAI). Patient Prefer Adherence. 2019;13:1693–1699. doi:10.2147/PPA.S222096
25. Jing C, Wei C, Hongyan Z, et al. Reliability and validity of the Chinese version of the oxford depression questionnaire (ODQ-Chinese). J Affect Disord. 2022;313:278–282. doi:10.1016/j.jad.2022.06.044
26. Cronquist CM, Andrea F, Ioana F, Henrik L, Cuomo Alessandro MGG, Goodwin GM. Validation of the oxford depression questionnaire: sensitivity to change, minimal clinically important difference, and response threshold for the assessment of emotional blunting. J Affect Disord. 2021;294:924–931. doi:10.1016/j.jad.2021.07.099
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
