Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
The Challenges in Investigating the Pathogenesis of Sensitive Skin by Noninvasive Measurements: A Systematic Review
Authors Yan S, Zhao J, Han Y, Wang R, Bai K, Ge J, Pan Y , Zhao H
Received 10 October 2022
Accepted for publication 14 January 2023
Published 26 January 2023 Volume 2023:16 Pages 237—251
DOI https://doi.org/10.2147/CCID.S392925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Shiyu Yan,1,2 Jinfeng Zhao,1,2 Yuqing Han,1,2 Rui Wang,1,2 Kexuan Bai,1,2 Junxin Ge,1,2 Yao Pan,1,2 Hua Zhao1,2
1Department of Cosmetics, College of Chemistry and Materials Engineering, Beijing Technology and Business University, Beijing, 100048, People’s Republic of China; 2Beijing Key Laboratory of Plant Research and Development, Beijing, 100048, People’s Republic of China
Correspondence: Yao Pan, Department of Cosmetics, College of Chemistry and Materials Engineering, Beijing Technology and Business University, 11 Fu Cheng Road, Hai Dian District, Beijing, 10048, People’s Republic of China, Tel +86-10-68984937, Email [email protected]
Abstract: Sensitive skin (SS) is a common cutaneous condition that seriously affects people’s quality of life, but studies of sensitive skin pathogenesis are unclear, the exploration are ongoing, and the biophysical properties of sensitive skin disagree with the study results. In this paper, we summarize the noninvasive biophysical and imaging instrumental methods used for sensitive skin and provide support for the classification of sensitive skin subtypes to prescribe precise treatment. PubMed and Web of Science databases were searched according to PRISMA guidelines for articles from January 1971 to May 2022 that used noninvasive biophysical or imaging methods to monitor adult subjects with sensitive skin. The quality of the included articles was determined based on 22 items of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. A total of 55 studies were included, representing 8 biophysical and 5 imaging methods and their applications in treatment efficacy evaluation studies. The biophysical parameter and cutaneous morphological property changes in sensitive skin subjects were observed. The quality of the studies was relatively low, and there was high variability in results between studies. Several parameters have shown tremendous potential in exploring the pathogenesis with different sensitive skin subtypes: type I may be detected with higher transepidermal water loss and lower stratum corneum hydration values, as well as with thinner epidermis with a shallower and more irregular honeycomb structure; Type II and III are more prone to higher blood flow, lower current perception threshold than normal skin. This systematic review identifies key reasons for the lack of uniform trends in noninvasive measurements and recommends the use of effective selection instruments or relevant parameters to explore the pathogenesis of sensitive skin, and to differentiate the subtypes of sensitive skin for achieving the precise treatment.
Keywords: sensitive skin, lactic acid sting test, transepidermal water loss, stratum corneum hydration, reflectance confocal microscopy, VISIA
Keywords: sensitive skin, lactic acid sting test, transdermal water loss, stratum corneum hydration, reflectance confocal microscopy, VISIA
Introduction
Modern advances in daily life have resulted in increasing attention to skin health, particularly as the incidence of skin abnormalities, including sensitive skin (SS), has gradually increased in recent years.1,2 The International Forum for the Study of Itch (IFSI) defines sensitive skin as an unpleasant sensation (tingling, burning, pain, itching, etc.) on skin that may be normal or erythematous in appearance. Sensitive skin generally affects the entire body but is especially common on the face.3 According to the epidemiological survey, a worldwide increase shows in the prevalence of sensitive skin.4,5 Some studies have shown that the incidence of sensitive skin decreases with age,6,7 and in most studies it indicates that women’s skin sensitivity is more common than men’s.8,9 Moreover, sensitive skin is often strongly associated with other skin conditions.10 The prevalence of a comorbid dermatological disease was 2 to 4 times higher among subjects with sensitive or very sensitive skin in India,7 while 1/3 of people with very sensitive skin and 1/5 with sensitive skin in Europe suffered from skin disorders including rosacea, seborrheic dermatitis, eczema, psoriasis and acne.4 Compared to those with atopic diathesis (impaired epidermal barrier function and eczema susceptibility), people with sudden skin reddening caused by vascular instability (excessive vascular activity) are more likely to be SS.11 Therefore, the clinical, biophysical and histological characterization of sensitive skin has become an important research topic in the cosmetics and pharmaceutical industries as well as in biomedical research.
Individuals with sensitive skin may have one or more of the following skin physiological changes: increased neurosensory input, enhanced immune responses, and/or reduced barrier function.12 The resulting objective signs, such as skin dryness, facial erythema, or fine scales, are sometimes observed by physicians.13 The pathophysiology of sensitive skin has long been suboptimal; the most common symptom is impaired barrier function, possibly resulting in changes in the nervous system and/or epidermal structure.14 Noninvasive measurement methods have the advantage in exploring the pathophysiology of sensitive skin. Furthermore, the changing trends of these physiological parameters play an important role in the evaluation of the treatment of sensitive skin.15 Based on sensitive skin pathogenesis, impaired barrier function has been divided into three different types. Type I is defined as the low barrier function group; type II is defined as the inflammatory group with normal barrier function; and type III is defined as the pseudohealthy group, with normal barrier function and no inflammatory changes.16 These classification categories might be beneficial in prescribing precision medicine for the SS population.
Many studies have used noninvasive instruments to provide an overview of the cutaneous physiological properties of sensitive skin, but the changes in the biophysical parameters are quite different from those suggested by various study conclusions.17 Lower TEWL (Transepidermal Water Loss) values might be a typical feature of sensitive skin, but this is not always the case in the literature. The diversity of the biophysical properties of sensitive skin makes it difficult to determine the pathogenesis as well as standardize the treatment evaluation system. An up-to-date comprehensive review of these noninvasive instrumental methods is lacking. Thus, the goals of this systematic review were (1) to elucidate the biophysical properties of sensitive skin and screen effective available noninvasive imaging and biophysical instruments, including limitations and precautions; (2) to provide support for the classification of sensitive skin subtypes by the pathogenesis so as to achieve precise treatment; and (3) to provide information to aid in the construction of a biophysical parameter system for evaluating the effectiveness of sensitive skin treatment methods.
Methods
Following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),18 a systematic review search was performed in 2 electronic databases: PubMed and Web of Science. This systematic review was conducted to identify studies on the use of noninvasive imaging and biophysical instruments to explore the characteristics of sensitive skin.
Databases and Research Rules
A literature search was performed for all studies published from January 1971 to May 2022 using the PubMed and Web of Science databases. The following search terms for sensitive skin were used: “sensitive skin”, “sensory skin”, and “sensitivity skin”. The results of the two search databases were screened independently by two reviewers (S. Y and J. Z) based on titles, abstracts, and full articles. The discrepancies about inclusion between the reviewers were decided by discussion.
Study Selection Criteria
Through a relevant search of the titles and abstracts, the following study inclusion criteria were applied: (1) The research object was humans. (2) Sensitive skin detected by the relevant assessment method in the study (questionnaires and chemical probes). (3) Application of noninvasive instruments. (4) Original research with an available full article. Then, the full articles were excluded if: (1) They used therapeutic techniques or were histopathology, in vitro or animal studies. (2) They did not use noninvasive instruments. (3) The objective parameter data were not described in the original study. (4) They were duplicates of article retrieved from the other database.
Article Quality
The research characteristics extracted in this systematic review included the number of participants, age, ethnicity, chemical probe, testing site, parameter, and outcome. Details are presented in Supplementary Table 1 and Supplementary Table 2. The quality of the articles was based on the 22 items of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement19 and was used to judge clinical case–control, cohort and cross-sectional studies.
Results
The specific screening process is shown in Figure 1. In total, 4763 articles were identified, and after removing patents/reviews and conference abstracts, 2374 articles remained. Then, after removing duplicates, 1142 articles remained for screening. After a rigorous evaluation of the abstracts according to the inclusion criteria, 99 articles were eligible for a detailed full-text evaluation. Finally, through evaluation and screening of the full text based on the exclusion criteria, 55 articles were included in this systematic review: 19 articles about treatment efficacy evaluations on sensitive skin and 36 articles concerning other sensitive skin research topics. Another 8 articles classified sensitive skin by imaging techniques: reflectance confocal microscopy (n=3), confocal laser scanning microscopy (n=1), confocal Raman microspectroscopy (n=1), dynamic optical coherence tomography (n=1), and the VISIA® system (n=2). The articles consisted of case–control studies (n = 15), cohort studies (n = 23), and cross-sectional studies (n=18). Overall, 29 articles were classified as Category B (score 60–80%), and the remaining 26 articles were classified as Category C (score <60%). A summary of the biophysical and imaging methods for assessing sensitive skin covered by the included articles is shown in Table 1.
 |
Table 1 Summary of Biophysical and Imaging Methods for Assessing Sensitive Skin |
 |
Figure 1 PRISMA flow diagram. Notes: Adapted from: Page MJ, Moher D, Bossuyt PM et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.18 Creative Commons CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/legalcode). |
Biophysical Methods
Transepidermal Water Loss
Transepidermal water loss (TEWL) is a measurement of the integrity of barrier function as assessed by skin surface water loss.20,21 Twenty-three studies provided data on the TEWL value with a wide range of 5.8–50.1 g/m2/h to assess skin quality.17,22–43 Only two studies reported that SS subjects diagnosed using questionnaires showed lower TEWL values at different sites on the face and forearms;31,32 the majority of the remaining studies, however, reported the opposite results, where SS subjects showed a higher TEWL value than the controls in ten studies.17,22–30 Among them, it was found that Chinese females subjected to semisubjective tests (LAST/CAT) showed higher TEWL values on the cheek. Additionally, ten studies showed no difference in the values on the cheek,33,35 face,27,28,37,38,40 forearm,34,36,37 body34,35 or hand.39
Stratum Corneum Hydration
When maintained within a certain range, the stratum corneum hydration (SCH) value forms the basis of maintaining skin health.44 The SCH value was assessed in nineteen studies via skin capacitance (CAP).22,23,25–33,36,38–43,45 In cross-sectional and cohort studies, a wide range of skin capacitance values was reported (16.7–80.3 AU).23,25,26,28,29,32,33,36,40–43 Among the case–control studies, four articles clearly reported lower SCH values in subjects with SS or stingers at different facial sites (forehead, cheek, nasolabial fold, and chin).22,27,30,45 Of these, two studies used questionnaires,22,30 one used LAST,45 and one used both questionnaires and LAST.27 These studies commonly employed LAST on the faces of female volunteers. Two studies showed no difference between the SCH values of patients and controls on the face38 and hand.39 One study with 66 subjects showed that SS subjects had higher SCH values on the forehead, chin, and left and right cheekbones and cheeks.31
Sebum Content
Sebum content plays a major role in skin barrier function, and abnormal lipid composition can lead to abnormal skin barrier function.31,46 A total of eleven studies included sebum content with a large-scale range of 0.0–239.8 AU.22,23,25,28,29,31,32,38,41,45,47 Three studies clearly reported lower sebum content values in subjects with sensitive skin using a questionnaire.22,31,45 Comparing SS and non-SS (NSS) subjects, Caucasian women remarkably demonstrated a higher frequency of low sebum content on the face than Chinese women (66.67% vs 33.33%). In addition, two studies reported that the sebum content of subjects with SS and LAST positivity was higher.28,47 However, five studies reported that the sebum content was not different between subjects with SS and NSS32 or between stingers and nonstingers.23,25,29,38
pH
The pH of the skin can be directly detected by a pH meter;48 the resulting value reflects the expression of biological activities of the body in the epidermis,49 with higher skin pH values indicating a lower barrier function to water permeability.50 Nine studies involved the measurement of pH.22,23,25,28,29,31,41,45,51 Two studies enrolled Caucasians, one which showed a higher pH value on the cheek,51 while the other showed a lower pH value on the forehead, chin, cheeks and right forearm in subjects with SS.45 However, in six studies, the pH measurements were not different between subjects with sensitive and normal skin31 or between stingers and nonstingers.23,25,28,29,51 Overall, 75.00% of the studies showed no difference in pH value between subjects with SS or stingers and healthy individuals.
L* and a* Values
The L*a*b* chromaticity system, specified by the International Commission on Illumination (CIE), has been widely used in recent years to reflect the changes in the depth of skin color.52 Three studies reported the L* value,22,28,53 and seven studies included the a* value.22,28,34,36,39,43,53 Three studies showed lower L* values and higher a* values in SS and LAST-positive (LASTP) patients on the forearm53 and face.22,28 However, three studies showed no differences in the a* value between patients with SS34,39 and lactic acid stingers and controls.36
Erythema Index
The erythema index (EI) can directly reflect the hemoglobin content in the papillary dermis; most subjects with sensitive skin are more prone to erythema.45,54 The EI value was measured in six studies.23,25,28,31,35,47 One study clearly demonstrated that in both SS and LASTP subjects, the EI was higher on the forehead, cheek and chin.28 Another study showed higher EI values among subjects with SS on the forehead, nasolabial folds, nose and chin.47 Only one study indicated that the EI value was lower on the forehead, chin, and left and right cheekbones and cheeks.31 However, three studies indicated that there was no difference in the EI value on the cheek between the SS and NSS groups35 or between the stinger and no-stinger groups.23,25
Blood Flow
A colorimeter can detect the size of erythematous patches on the skin surface to determine the blood flow. Skin blood flow can also be measured with laser Doppler.22,55 Six studies assessed the monitoring of blood flow ranging at 6.2–25.1.36,39,42,53,56,57 Two studies found that blood flow was higher in LASTP subjects on the forearm;53,56 however, another three studies showed no difference in blood flow on the hand,39 nasolabial fold57 or forearm.36 Interestingly, using LAST on the forearm, female LASTP subjects showed high blood flow,53,56 but no difference was observed between male LASTP and LAST-negative (LASTN) subjects.36 Through a comparison of the above studies, females subjected to LAST were typically shown to demonstrate higher blood flow on the forearm.
Current Perception Threshold
The current perception threshold (CPT) can be used to assess the integrity of sensory nerve function and quantitatively detect skin sensitivity and pruritus thresholds.46 Five studies were interested in quantifying the CPT in sensitive skin.24,36,57–59 Three studies found that the CPT value was lower in LASTP and lactic acid itch responders than in LASTN and lactic acid non-itch responders at 5 Hz and 250 Hz.24,57,59 Only one study showed that the pretest 250 Hz and 2 kHz CPT values of male subjects were not different between the LASTP and LASTN groups, while a low 5 Hz CPT was observed among male SS subjects.36
Imaging Techniques
Reflectance Confocal Microscopy
Reflectance confocal microscopy (RCM) is used to observe the skin structure based on the different refractive indices of the skin tissue.60 Three studies focused on using the structure of epidermal lesions determined by RCM to assess sensitive skin.37,61,62 Notably, SS and NSS subjects differed in honeycomb structure depth or structure. In one study, the honeycomb structure was shallower in SS than in NSS subjects,61 while in the other two studies, patients with lactic acid stingers had irregular honeycomb structures on the face.37,62
Confocal Laser Scanning Microscope
Confocal laser scanning microscopy (CLSM) converts an optical signal to an electrical signal and transmits it to a computer to produce a clear image of the entire skin.63 In a study using CLSM to explore the epidermal thickness between SS and NSS subjects, the blood vessels were shown to be distorted into earthworm-like shapes, and the epidermis in SS subjects was thinner than in NSS subjects (P = 0.001).64
Confocal Raman Microspectroscopy
Confocal Raman microspectroscopy (CRM) is widely used in dermatology and cosmetology to analyze the concentration of skin components (lipids, natural moisturizing factor molecules, water) and the depth of penetration of treatment/medical formulations in the human stratum corneum (SC).65 Richters et al attempted to uncover differences between NSS and SS with CRM. The authors found no difference between SS and NSS in terms of stratum corneum thickness, water, and natural moisturizing factor (NMF) content, but ceramides/fatty acids on the cheek in SS subjects showed a lower trend than that of NSS subjects.32
Dynamic Optical Coherence Tomography
Dynamic optical coherence tomography (D-OCT) can be used to scan the skin blood flow signal value and obtain information on the microvasculature.66 One study used D-OCT to monitor vessel depth and found that compared with those of the LASTN group, the vascular vessels were closer to the epidermis in LASTP individuals.67 Additionally, they had more frequent mesh and branching vessels and even a higher blood vessel density than the normal population. Importantly, the vascular depth was closely negatively correlated with face flushing and the sum of the sting scores, and the vascular shapes were positively correlated with face flushing and the sum of the burning scores.
Visia
VISIA Red images were developed to document and measure facial skin erythema. Generally, the higher the degree of erythema is, the worse the skin condition.68 Two studies used VISIA to monitor erythema in sensitive skin.23,69 One study used images of erythema taken by VISIA observation to analyze skin structure, revealing erythema in 99% of sensitive skin patients.69 Another study found wider ranges of red spots in LASTP patients.23
Applications
A summary of treatment efficacy evaluations on sensitive skin is shown in Table 2.
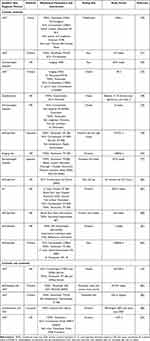 |
Table 2 Summary for Treatment Efficacy Evaluations on Sensitive Skin |
A total of 19 articles described the application of sensitive skin products. Among the methods used to screen sensitive skin, LAST was used in 5 articles.70–74 Three out of the five articles applied lactic acid to the nasolabial folds,70,71,73 one applied sodium lauryl sulfate to the forearm,74 and another applied lactic acid to the cheek.72 In addition to the classical LAST, dermatologists’ diagnostics and questionnaires were also used. Four studies asked dermatologists to differentiate sensitive skin groups,75–77 while five studies involved screening using self-assessments.78–82 Regarding testing sites, 73.68% (14/19) chose to test the products on the face,70–73,75–80,82–85 and 26.32% (5/19) only tested them on the forearm,55,74,81,86,87 while two studies tested the face and forearm together,79,80 and one study tested the face and leg.83
For both products and raw materials, their efficacies were mainly assessed in terms of their ability to moisturize, produce anti-allergy and anti-inflammatory reactions, reduce erythema, and enhance or repair barrier function. The selection of biophysical parameters was also slightly different depending on the product, but according to the frequency of use, the following data were obtained: 89.47% were assessed with TEWL (n=17),55,70–74,76–82,84–87 63.16% with SCH (n=12),70–74,76,78,79,82–84,87 31.58% with the EI (n=6),70,72,74,76,81,82 15.79% with the a* value (n=3),72,82,87 10.53% with sebum content (n=2),70,77 10.53% with skin temperature (n=2),79,85 10.53% with pH (n=2),76,79 10.53% with blood flow (n=2),80,87 10.53% with VISIA (n=2),72,75 5.26% with skin elasticity (n=1),76 and 5.26% with the L* value (n=1).72
In studies on the treatment of sensitive skin, compared with baseline or placebo, cosmetic products produced a significant reduction in the TEWL value (vitamin B3 cream, ST11 care, ceramide, M89 care, barrier cream, anti-sensitive skin cream, oral flaxseed oil).55,70–72,74–76,80,82,84–87 Some articles showed a significant increase in SCH values after treatment (moisturizer, functional products).70,71,75,76,78,82–84,87 All articles involving EI values showed decreases after treatment (topical agents, herbal cream).70,72–74,76,82 One randomized controlled trial in sensitive skin subjects using moisturizer showed no significant changes in the TEWL value after treatment compared to placebo without active ingredients.78 In addition, two articles involved multiple products, and lower TEWL values, higher SCH values, and lower EI values were observed relative to the placebo.72,82 Two articles that included multiproduct comparisons noticed no significant changes in the TEWL values with respect to the baseline.74,81
Risk of Bias
A screening of the above results revealed the following sources of bias: (1) Most studies had small sample sizes (n≤50, 50.91%; 50<n≤100, 25.46%; 100<n≤200, 16.36%; n>200, 7.27%). (2) Some studies did not describe the inclusion criteria for volunteers. (3) The evaluation criteria were not uniform; for example, in studies using LAST, different lactic acid concentrations, different application volumes, and even different stimulation sites were employed. (4) The sensitive skin questionnaire was not disclosed, and the questionnaires used were not identical.
Discussion
Noninvasive methods have great potential for studying cutaneous condition. This systematic review includes many studies describing noninvasive biophysical or imaging measurements for sensitive skin. This is particularly useful for research purposes and related treatments and, importantly, the challenges in noninvasive measurements may also help to elucidate the pathogenesis of this cutaneous condition.
For physiological parameters, different grouping methods (questionnaires and chemical probes), measurement sites, and biophysical instruments were used. There were large differences in the indicator measurement trends, but the results did not always give a meaningful degree of discrimination. Otherwise, the quality of the included studies was relatively low, and the results were highly variable between studies, indicating that methods for assessing sensitive skin are not suitably standardized or effective.
The results showed that the biophysical parameters had quite large value ranges, with TEWL values of 5.8–50.1 g/m2/h, skin capacitance values of 16.7–80.3 AU, sebum content values of 0.0–239.8 AU and blood flow of 6.2–25.1. Many factors affect the biophysical parameter results for SS. In epidemiological investigations, the incidence of sensitive skin tended to decrease with age.6,88 Ding et al found that age was negatively correlated with pH value.25 Sex has also been suggested to play a role in this condition;89,90 female LASTP subjects showed higher blood flow on the forearm than LASTN subjects,53,56 but the males showed no difference.36 Different ethnicities had different responses to the same stimulation;91–93 Asians appeared to have greater skin reactivity to sudden changes than European-Americans and African-Americans.94 The testing site could also lead to great differences. Ye et al found that the TEWL value, SCH value, sebum content, and pH value of subjects with sensitive skin varied across different facial areas.41 Moreover, according to the search results, among the semisubjective methods, the most frequently used was the LAST. However, the studies that reported on this test reported differences in terms of concentration, application site, and action time. These findings clearly demonstrate that there is no unified method for assessing SS, and overall, differences in the subject inclusion criteria, selection method and measurement method may potentially lead to different results.
Biophysical instruments are strongly influenced by intrinsic and extrinsic factors, such as age, sex, ethnicity, and testing site. Therefore, data collection is difficult to standardize. This also verifies that the physiological parameters listed in the table do not reflect a unified trend. Furthermore, biophysical instruments are commonly based on the use of probes, which only cover a small area of the skin without representation of the entire face. Imaging techniques such as VISIA can address this deficiency, as the condition of the entire face can be visualized without direct skin contact. However, the image analysis techniques have not been standardized or validated, and different researchers may use different software and description parameters. Therefore, more explorations are needed to further assess these imaging methods.
Despite the above limitations, some instruments show promising value in basic research and treatment efficacy evaluation. Since the biophysical parameters in the existing research do not show obvious regularity, we suggest using specific instruments for different skin conditions. To detect the degree of skin redness, VISIA, chromameters, mexameters, and Laser Doppler flowmeters can be used to measure areas of erythema. For subjects with tingling or itching sensations, CPT can be used to reflect skin nerve sensitivity, and the severity of barrier damage can be measured by RCM, CRS, CLSM, D-OCT, evaporimetry, corneometry and sebumetry. Moreover, we recommend operating the instruments strictly in accordance with a standard operating procedure (SOP) and conducting the study in a controlled environment. Importantly, the instruments should be calibrated regularly, and test sites should be accurately located. In addition, studies with larger sample sizes are required to improve the reliability of the data.
As a complex skin condition, based on the three different types distinguished by the physiological parameters of sensitive skin,15 we inferred that different sensitive skin types could probably be judged by specific sets of noninvasive biophysical parameters. For type I, sensitive skin may be detected with higher TEWL and lower SCH values, lower sebum content and a thinner epidermis with a shallower and more irregular honeycomb structure. Because types II and III are more sensitive to chemical stimulation than normal skin, the type II sensitive skin population may have a lower L* value, higher a* value, higher blood flow, lower CPT, larger erythema area, and more activity in secondary somatosensory regions. Subjects with type III SS may have higher blood flow, lower CPT.
In conclusion, sensitive skin is a subjective feeling, which influences the quality of life of the patients. The study of the pathogenesis is helpful to suit the remedy to the case and achieve precise treatment. Although the factors should be taken into account when using noninvasive imaging and biophysical instruments, such as age, gender, race, etc., it is undeniable that these measurement tools reveal the biophysical and cutaneous morphological property of sensitive skin better than visual inspection. This systematic review gives an overview of the available noninvasive imaging and biophysical instruments for sensitive skin, as well as the factors that influence these results. However, adequate and effective principles for the use of these cutaneous noninvasive tools are needed in the future studies, and it is expected to have better instruments and detection methods to explore the pathogenesis of sensitive skin. Additionally, this review provides technical support for the selection of noninvasive instruments and biophysical parameters for evaluating the efficacy of cosmetics or drugs that are claimed to improve sensitive skin, potentially promoting their research and development.
Data Sharing Statement
All data generated or analyzed during this study are included in Supplementary Tables 1 and 2. Further enquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
An ethics statement is not applicable because this study is based exclusively on published literature.
Consent for Publication
The details of any images etc, can be published, and that the person(s) providing consent have been shown the article contents to be published.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) [grant number 81903361].
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Kim YR, Cheon HI, Misery L, Taieb C, Lee YW. Sensitive skin in Korean population: an epidemiological approach. Skin Res Technol. 2018;24(2):229–234. doi:10.1111/srt.12418
2. Falcone D, Richters R, Uzunbajakava NE, van Erp P, van de Kerkhof P. Risk factors associated with sensitive skin and potential role of lifestyle habits: a cross-sectional study. Clin Exp Dermatol. 2017;42(6):656–658. doi:10.1111/ced.13133
3. Misery L, Ständer S, Szepietowski JC, et al. Definition of Sensitive Skin: an Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm-Venereol. 2017;97(1):4–6. doi:10.2340/00015555-2397
4. Misery L, Boussetta S, Nocera T, Perez-Cullell N, Taieb C. Sensitive skin in Europe. J Eur Acad Dermatol. 2009;23(4):376–381. doi:10.1111/j.1468-3083.2008.03037.x
5. Misery L, Loser K, Stander S. Sensitive skin. J Eur Acad Dermatol. 2016;30(Suppl 1):2–8. doi:10.1111/jdv.13532
6. Guinot C, Malvy D, Mauger E, et al. Self-reported skin sensitivity in a general adult population in France: data of the SU.VI.MAX cohort. J Eur Acad Dermatol. 2006;20(4):380–390. doi:10.1111/j.1468-3083.2006.01455.x
7. Brenaut E, Misery L, Taieb C. Sensitive Skin in the Indian Population: an Epidemiological Approach. Front Med-Lausanne. 2019;6:29. doi:10.3389/fmed.2019.00029
8. Xu F, Yan S, Wu M, et al. Self-declared sensitive skin in China: a community-based study in three top metropolises. J Eur Acad Dermatol. 2013;27(3):370–375. doi:10.1111/j.1468-3083.2012.04648.x
9. Farage MA. Does sensitive skin differ between men and women? Cutan Ocul Toxicol. 2010;29(3):153–163. doi:10.3109/15569521003774990
10. Escalas-Taberner J, Gonzalez-Guerra E, Guerra-Tapia A. Sensitive skin: a complex syndrome. Actas Dermo-Sifilogr. 2011;102(8):563–571. Spanish. doi:10.1016/j.ad.2011.04.011
11. Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Phys. 2015;28(2):75–83. doi:10.1159/000363149
12. Arun CI, Aparna P. Sensitive skin: an overview. Indian J Dermatol Venereol Leprol. 2013;79(1):548.
13. Pons-Guiraud A. Sensitive skin: a complex and multifactorial syndrome. J Cosmet Dermatol-Us. 2004;3(3):6598.
14. Do LHD, Azizi N, Maibach H. Sensitive Skin Syndrome: an Update. Am J Clin Dermatol. 2020;21(3):401–409. doi:10.1007/s40257-019-00499-7
15. Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmetic Sci. 2013;35(1):2–8. doi:10.1111/j.1468-2494.2012.00754.x
16. Yokota T, Matsumoto M, Sakamaki T, et al. Classification of Sensitive Skin and Development of a Treatment System Appropriate for Each Group. IFSCC Mag. 2003;6(4):303–307.
17. Pinto P, Rosado C, Parreirao C, Rodrigues LM. Is there any barrier impairment in sensitive skin?: a quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Res Technol. 2011;17(2):181–185. doi:10.1111/j.1600-0846.2010.00478.x
18. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
19. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi:10.1016/j.ijsu.2014.07.013
20. Imhof RE, De Jesus ME, Xiao P, Ciortea LI, Berg EP. Closed-chamber transepidermal water loss measurement: microclimate, calibration and performance. Int J Cosmetic Sci. 2009;31(2):97–118. doi:10.1111/j.1468-2494.2008.00476.x
21. Warren R, Bauer A, Greif C, et al. Transepidermal water loss dynamics of human vulvar and thigh skin. Skin Pharmacol Phys. 2005;18(3):139–143. doi:10.1159/000084911
22. Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38(6):311–315. doi:10.1111/j.1600-0536.1998.tb05764.x
23. Zheng Y, Liang H, Li Z, Tang M, Song L. Skin microbiome in sensitive skin: the decrease of Staphylococcus epidermidis seems to be related to female lactic acid sting test sensitive skin. J Dermatol Sci. 2020;97(3):225–228. doi:10.1016/j.jdermsci.2019.12.004
24. Li S, Wang X, Gao Y, et al. CPT, the main test method of skin neuronal sensitivity. Cutan Ocul Toxicol. 2015;34(3):208–211. doi:10.3109/15569527.2014.950379
25. Ding DM, Tu Y, Man MQ, et al. Association between lactic acid sting test scores, self‐assessed sensitive skin scores and biophysical properties in Chinese females. Int J Cosmetic Sci. 2019;41(4):398–404. doi:10.1111/ics.12550
26. Yu LL, Wang XM, Zou Y, et al. Correlation between the capsaicin test and objective skin measurements in evaluating sensitive skin in Chinese females. J Dermatol Sci. 2012;68(2):108–109. doi:10.1016/j.jdermsci.2012.08.006
27. Qiao Z, Huang S, Leng F, et al. Analysis of the Bacterial Flora of Sensitive Facial Skin Among Women in Guangzhou. Clin Cosmet Inv Derm. 2021;14:655–664. doi:10.2147/CCID.S307668
28. Pan Y, Ma X, Song Y, Zhao J, Yan S. Questionnaire and Lactic Acid Sting Test Play Different Role on the Assessment of Sensitive Skin: a Cross-sectional Study. Clin Cosmet Inv Derm. 2021;14:1215–1225. doi:10.2147/CCID.S325166
29. An S, Lee E, Kim S, et al. Comparison and correlation between stinging responses to lactic acid and bioengineering parameters. Contact Dermatitis. 2007;57(3):158–162. doi:10.1111/j.1600-0536.2007.01182.x
30. Georgieva F. THE SKIN BARRIER IN SENSITIVE SKIN SYNDROME. J IMAB. 2021;27(4):4120–4124. doi:10.5272/jimab.2021274.4120
31. Fan L, Jia Y, Cui L, Li X, He C. Analysis of sensitive skin barrier function: basic indicators and sebum composition. Int J Cosmetic Sci. 2018;40(2):117–126. doi:10.1111/ics.12442
32. Richters RJ, Falcone D, Uzunbajakava NE, et al. Sensitive Skin: assessment of the Skin Barrier Using Confocal Raman Microspectroscopy. Skin Pharmacol Phys. 2017;30(1):1–12. doi:10.1159/000452152
33. Raj N, Voegeli R, Rawlings AV, et al. A fundamental investigation into aspects of the physiology and biochemistry of the stratum corneum in subjects with sensitive skin. Int J Cosmetic Sci. 2016;39(1):2–10. doi:10.1111/ics.12334
34. Falcone D, Uzunbajakava N, Richters R, van de Kerkhof P, van Erp P. Histamine Iontophoresis as in vivo Model to Study Human Skin Inflammation with Minimal Barrier Impairment: pilot Study Results of Application of the Model to a Sensitive Skin Panel. Skin Pharmacol Phys. 2017;30(5):246–259. doi:10.1159/000477416
35. Cho HJ, Chung BY, Lee HB, Kim HO, Park CW, Lee CH. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J Dermatol. 2012;39(3):295–300. doi:10.1111/j.1346-8138.2011.01406.x
36. Kim SJ, Lim SU, Won YH, et al. The perception threshold measurement can be a useful tool for evaluation of sensitive skin. Int J Cosmetic Sci. 2008;30(5):333–337. doi:10.1111/j.1468-2494.2008.00434.x
37. Ahn HJ, Kim HJ, Ham H, et al. Visualizing the in-vivo application of zinc in sensitive skin using reflectance confocal microscopy. Sci Rep-Uk. 2021;11(1):7738. doi:10.1038/s41598-021-87346-0
38. Bai Y, Wang YJ, Zheng HJ, Tan F, Yuan C. CorrelationBetweenFacialSkinMicrobiota and SkinBarriersinaChineseFemalePopulationwithSensitiveSkin. Infect Drug Resist. 2021;14:219–226. doi:10.2147/IDR.S287844
39. Diogo L, Papoila AL. Is it possible to characterize objectively sensitive skin? Skin Res Technol. 2010;16(1):30–37. doi:10.1111/j.1600-0846.2009.00404.x
40. Boyer G, Belilovsky CD, Brédif S, Baudouin C, Misery L, Bellemère G. Clinical and Instrumental Exploration of Sensitive Skin in a Pediatric Population. Cosmetics-Basel. 2021;8(2):43. doi:10.3390/cosmetics8020043
41. Ye C, Chen J, Yang S, et al. Skin sensitivity evaluation: what could impact the assessment results? J Cosmet Dermatol-Us. 2020;19(5):1231–1238. doi:10.1111/jocd.13128
42. Agner T, Serup J. Individual and instrumental variations in irritant patch-test reactions--clinical evaluation and quantification by bioengineering methods. Clin Exp Dermatol. 1990;15(1):29–33. doi:10.1111/j.1365-2230.1990.tb02014.x
43. Pierard GE, Arrese JE, Rodriguez C, Daskaleros PA. Effects of softened and unsoftened fabrics on sensitive skin. Contact Dermatitis. 1994;30(5):286–291. doi:10.1111/j.1600-0536.1994.tb00600.x
44. Barel AO, Clarys P. In vitro calibration of the capacitance method (Corneometer CM 825) and conductance method (Skicon-200) for the evaluation of the hydration state of the skin. Skin Res Technol. 1997;3(2):107–113. doi:10.1111/j.1600-0846.1997.tb00171.x
45. Roussaki-Schulze AV, Zafiriou E, Nikoulis D, Klimi E, Rallis E, Zintzaras E. Objective biophysical findings in patients with sensitive skin. Drugs Exp Clin Res. 2005;31:Suppl:17–24.
46. Takekuma K, Ando F, Niino N, Shimokata H. Age and gender differences in skin sensory threshold assessed by current perception in community-dwelling Japanese. J Epidemiol. 2000;10(1 Suppl):S33–8. doi:10.2188/jea.10.1sup_33
47. Manav V, Karaali MG, Erdem O, Koku AA. Association between biophysical properties and anxiety in patients with sensitive skin. Skin Res Technol. 2022;28(4):556–563. doi:10.1111/srt.13156
48. Runeman B, Faergemann J, Larko O. Experimental Candida albicans lesions in healthy humans: dependence on skin pH. Acta Derm-Venereol. 2000;80(6):421–424. doi:10.1080/000155500300012819
49. Thune P, Nilsen T, Hanstad IK, Gustavsen T, Dahl HL. The water barrier function of the skin in relation to the water content of stratum corneum, pH and skin lipids. The effect of alkaline soap and syndet on dry skin in elderly, non-atopic patients. Acta Derm-Venereol. 1988;68(4):277–283.
50. Selander C, Zargari A, Mollby R, Rasool O, Scheynius A. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy. 2006;61(8):1002–1008. doi:10.1111/j.1398-9995.2006.01108.x
51. Issachar N, Gall Y, Borrel MT, Poelman MC. pH measurements during lactic acid stinging test in normal and sensitive skin. Contact Dermatitis. 1997;36(3):152–155. doi:10.1111/j.1600-0536.1997.tb00399.x
52. Berardesca E, Cespa M, Farinelli N, Rabbiosi G, Maibach H. In vivo transcutaneous penetration of nicotinates and sensitive skin. Contact Dermatitis. 1991;25(1):35–38. doi:10.1111/j.1600-0536.1991.tb01770.x
53. Chen SY, Yin J, Wang XM, Liu YQ, Gao YR, Liu XP. A new discussion of the cutaneous vascular reactivity in sensitive skin: a sub‐group of SS? Skin Res Technol. 2018;24(3):432–439. doi:10.1111/srt.12446
54. Robinson MK, Perkins MA. Evaluation of a quantitative clinical method for assessment of sensory skin irritation. Contact Dermatitis. 2001;45(4):205–213. doi:10.1034/j.1600-0536.2001.450403.x
55. Dieamant GC, Velazquez PMC, Eberlin S, Nogueira C, Werka RM, Queiroz ML. Neuroimmunomodulatory compound for sensitive skin care: in vitro and clinical assessment. J Cosmet Dermatol-Us. 2008;7(2):112–119. doi:10.1111/j.1473-2165.2008.00373.x
56. Issachar N, Gall Y, Borrel MT, Poelman MC. Correlation between percutaneous penetration of methyl nicotinate and sensitive skin, using laser Doppler imaging. Contact Dermatitis. 1998;39(4):182186. doi:;39(4):182186. doi:10.1111/j.1600-0536.1998.tb05890.x
57. Sun L, Wang X, Zhang Y, Wang T, Li X, Ma Y. The evaluation of neural and vascular hyper-reactivity for sensitive skin. Skin Res Technol. 2016;22(3):381–387. doi:10.1111/srt.12278
58. Ham H, An SM, Lee EJ, Lee E, Kim HO, Koh JS. Itching sensation and neuronal sensitivity of the skin. Skin Res Technol. 2016;22(1):104–107. doi:10.1111/srt.12236
59. Lee E, An S, Lee TR, Kim HK. Development of a novel method for quantitative evaluation of sensory skin irritation inhibitors. Skin Res Technol. 2009;15(4):464–469. doi:10.1111/j.1600-0846.2009.00391.x
60. Seitz CS, Pfeuffer P, Raith P, Brocker EB, Trautmann A. Radiocontrast media-associated exanthema: identification of cross-reactivity and tolerability by allergologic testing. Eur J Radiol. 2009;72(1):167–171. doi:10.1016/j.ejrad.2008.06.010
61. Ma YF, Yuan C, Jiang WC, Wang XL, Humbert P. Reflectance confocal microscopy for the evaluation of sensitive skin. Skin Res Technol. 2017;23(2):227–234. doi:10.1111/srt.12327
62. Ma Y, Li L, Chen J, Chen T, Yuan C. Distinguishing rosacea from sensitive skin by reflectance confocal microscopy. Skin Res Technol. 2020;26(5):671–674. doi:10.1111/srt.12851
63. Luedtke MA, Papazoglou E, Neidrauer M, Kollias N. Wavelength effects on contrast observed with reflectance in vivo confocal laser scanning microscopy. Skin Res Technol. 2009;15(4):482–488. doi:10.1111/j.1600-0846.2009.00394.x
64. Zha WF, Song WM, Ai JJ, Xu AE. Mobile connected dermatoscope and confocal laser scanning microscope: a useful combination applied in facial simple sensitive skin. Int J Cosmetic Sci. 2012;34(4):318–321. doi:10.1111/j.1468-2494.2012.00726.x
65. Darvin ME, Schleusener J, Lademann J, Choe C. Current views on non-invasive in vivo determination of physiological parameters of the stratum corneum using confocal Raman microspectroscopy. Skin Pharmacol Phys. 2022;35(3):125–136. doi:10.1159/000521416
66. Mani R, Holmes J, Rerkasem K, Papanas N. Blood Vessel Density Measured Using Dynamic Optical Coherence Tomography is a Tool for Wound Healers. Int J Low Extr Wound. 2021;153473462110173. doi:10.1177/15347346211017334
67. Jiang WC, Zhang H, Xu Y, et al. Cutaneous vessel features of sensitive skin and its underlying functions. Skin Res Technol. 2020;26(3):431–437. doi:10.1111/srt.12819
68. Xu DT, Yan JN, Cui Y, Liu W. Quantifying facial skin erythema more precisely by analyzing color channels of The VISIA Red images. J Cosmet Laser Ther. 2016;18(5):296–300. doi:10.3109/14764172.2016.1157360
69. Wang XY, Liu YY, Liu YX, et al. A predictive model for differential diagnosis between rosacea and sensitive skin: a cross-sectional study. Chinese Med J-Peking. 2020;133(17):2132–2134. doi:10.1097/CM9.0000000000001001
70. Wang Y, Viennet C, Jeudy A, Fanian F, He L, Humbert P. Assessment of the efficacy of a new complex antisensitive skin cream. J Cosmet Dermatol-Us. 2018;17(6):1101–1107. doi:10.1111/jocd.12486
71. Jeong S, Lee SH, Park BD, Wu Y, Man G, Man MQ. Comparison of the Efficacy of Atopalm((R)) Multi-Lamellar Emulsion Cream and Physiogel((R)) Intensive Cream in Improving Epidermal Permeability Barrier in Sensitive Skin. Dermatology Ther. 2016;6(1):47–56. doi:10.1007/s13555-016-0097-6
72. Zhang Y, Jin Y, Humbert P, et al. An herbal cream reduces erythema of sensitive skin. J Cosmet Dermatol-Us. 2021;20(3):792–797. doi:10.1111/jocd.13610
73. Berardesca E, Bonfigli A, Cribier B, et al. A Split-Face Study Assessing the Clinical Benefit, Tolerability and Subject Satisfaction of a Dermocosmetic in Subjects with Rosacea Associated with Erythema and Sensitive Skin. Clin Cosmet Inv Derm. 2020;13:751–758. doi:10.2147/CCID.S266879
74. Fatemi S, Jafarian-Dehkordi A, Hajhashemi V, Asilian-Mahabadi A, Nasr-Esfahani MH. A comparison of the effect of certain inorganic salts on suppression acute skin irritation by human biometric assay: a randomized, double-blind clinical trial. J Res Med Sci. 2016;21:102. doi:10.4103/1735-1995.193174
75. Hawkins SS, Subramanyan K, Liu D, Bryk M. Cleansing, moisturizing, and sun-protection regimens for normal skin, self-perceived sensitive skin, and dermatologist-assessed sensitive skin. Dermatol Ther. 2004;17(Suppl 1):63–68. doi:10.1111/j.1396-0296.2004.04s1008.x
76. Berardesca E, Abril E, Serio M, Cameli N. Effects of topical gluco-oligosaccharide and collagen tripeptide F in the treatment of sensitive atopic skin. Int J Cosmetic Sci. 2009;31(4):271–277. doi:10.1111/j.1468-2494.2009.00495.x
77. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42(2):181–188. doi:10.1111/1346-8138.12720
78. Draelos ZD, Gunt H, Zeichner J, Levy S. Clinical Evaluation of a Nature-Based Bakuchiol Anti-Aging Moisturizer for Sensitive Skin. J Drugs Dermatol. 2020;19(12):1181–1183. doi:10.36849/JDD.2020.5522
79. Bornkessel A, Flach M, Arens-Corell M, Elsner P, Fluhr JW. Functional assessment of a washing emulsion for sensitive skin: mild impairment of stratum corneum hydration, pH, barrier function, lipid content, integrity and cohesion in a controlled washing test. Skin Res Technol. 2005;11(1):53–60. doi:10.1111/j.1600-0846.2005.00091.x
80. Muizzuddin N, Marenus KD, Maes DH. Factors defining sensitive skin and its treatment. Am J Contact Dermat. 1998;9(3):170–175.
81. Pierard GE, Goffin V, Hermanns-Le T, Arrese JE, Pierard-Franchimont C. Surfactant-induced dermatitis: comparison of corneosurfametry with predictive testing on human and reconstructed skin. J Am Acad Dermatol. 1995;33(3):462–469. doi:10.1016/0190-9622(95)
82. Messaraa C, Drevet J, Jameson D, Zuanazzi G, De Ponti I. CanPerformance and GentlenessBeReconciled? A Skin Care Approach for Sensitive Skin. Cosmetics-Basel. 2022;9(2):214.
83. Nisbet SJ, Dykes P, Snatchfold J. Single application of lamellar moisturizers provides significantly increased hydration of the stratum corneum for up to 24 hours in a randomized trial. J Cosmet Dermatol-Us. 2020;19(11):3091–3095. doi:10.1111/jocd.13361
84. Nojiri H, Ishida K, Yao X, Liu W, Imokawa G. Amelioration of lactic acid sensations in sensitive skin by stimulating the barrier function and improving the ceramide profile. Arch Dermatol Res. 2018;310(6):495–504. doi:10.1007/s00403-018-1833-9
85. Tan YM, Wang XM, Yuan C, et al. Skin sensitivity and intolerance in Shanghai: cumulative influence of different meteorological parameters. Cutan Ocul Toxicol. 2015;34(2):132–138. doi:10.3109/15569527.2014.914036
86. Gueniche A, Philippe D, Bastien P, et al. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef Microbes. 2014;5(2):137–145. doi:10.3920/BM2013.0001
87. Neukam K, De Spirt S, Stahl W, et al. Supplementation of flaxseed oil diminishes skin sensitivity and improves skin barrier function and condition. Skin Pharmacol Phys. 2011;24(2):67–74. doi:10.1159/000321442
88. Trojahn C, Dobos G, Blume-Peytavi U, Kottner J. The skin barrier function: differences between intrinsic and extrinsic aging. Giorn Ital Dermat V. 2015;150(6):687–692.
89. Farage MA. Vulvar susceptibility to contact irritants and allergens: a review. Arch Gynecol Obstet. 2005;272(2):167–172. doi:10.1007/s00404-005-0732-4
90. Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm-Venereol. 2003;83(6):410–413. doi:10.1080/00015550310015419
91. Jourdain R, Maibach HI, Bastien P, De Lacharrière O, Breton L. Ethnic variations in facial skin neurosensitivity assessed by capsaicin detection thresholds. Contact Dermatitis. 2009;61(6):325–331. doi:10.1111/j.1600-0536.2009.01641.x
92. Wang H, Papoiu AD, Coghill RC, Patel T, Wang N, Yosipovitch G. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Brit J Dermatol. 2010;162(5):1023–1029. doi:10.1111/j.1365-2133.2009.09628.x
93. Aramaki J, Kawana S, Effendy I, Happle R, Loffler H. Differences of skin irritation between Japanese and European women. Brit J Dermatol. 2002;146(6):1052–1056. doi:10.1046/j.1365-2133.2002.04509.x
94. Robinson MK. Population differences in acute skin irritation responses. Race, sex, age, sensitive skin and repeat subject comparisons. Contact Dermatitis. 2002;46(2):86–93. doi:10.1034/j.1600-0536.2002.460205.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
