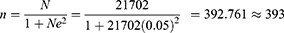Back to Journals » Research and Reports in Tropical Medicine » Volume 15
The Burden and Risk Factors of Helicobacter Pylori Infection Among Government Employees Who Clinically Complain of Indigestion but Allergic Diseases in Southeastern Ethiopia: A Multi-Institution Cross-Sectional Study
Authors Kebede T , Ashenafi H
Received 2 November 2023
Accepted for publication 13 February 2024
Published 19 February 2024 Volume 2024:15 Pages 25—49
DOI https://doi.org/10.2147/RRTM.S447203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Taye Kebede,1,2 Hagos Ashenafi2
1Department of Biomedical Sciences and Immunology, Madda Walabu University, Bale-Robe, Oromia Regional State, Ethiopia; 2Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Addis Ababa Administration City, Ethiopia
Correspondence: Taye Kebede, Department of Biomedical Sciences and Immunology, College of Natural Sciences, Madda Walabu University, Bale-Robe, Bale Zone, Oromia Regional State, Ethiopia, Tel +251 0911 355 335, Email [email protected]
Background: Helicobacter pylori (H. pylori) is believed to have spread from East Africa, but its burden is still unknown in less privileged regions of Ethiopia. Indigestion is an upset stomach, upper abdomen discomfort, heartburn, and bloating. This study evaluated the burden and risk factors for H. pylori infection among government employees who clinically complained of indigestion but allergic diseases in five public health institutions in Southeastern Ethiopia.
Methods: A health facilities-based cross-sectional survey study was conducted in Southeastern Ethiopia from March to November 2022, employing cluster sampling. Blood specimens, clinical data, and semi-structured questionnaires about risk factors were collected. Data analysis was conducted using descriptive, bivariate, and multivariable logistic regression in STATA software, Windows version 16.1.
Results: The overall prevalence of infection was found to be 77.6%. The sampled health institution (ρ-value < 0.05), engagement in sideline business (ρ-value < 0.05), sharing local spoon on meal [AOR = 39.30; CI:19.52 − 78.31; ρ-value < 0.001], admitting “Gursha” during meal [AOR = 71.48; CI:3.99 − 1279.77; ρ-value < 0.05], the toilet type [AOR = 1410.98; CI:121.16 − 16,431.19; ρ-value < 0.001], alcohol drinking [AOR = 15.15; CI:1.90 − 120.62; ρ-value < 0.05], sleeping hours length [AOR = 15.01; CI:13.48– 55.96; ρ-value < 0.001], chewing Khat [AOR = 76.73; CI:8.57– 687.07; ρ-value < 0.001], and regular hand washing before eating [AOR = 0.15; CI:0.12– 0.19; ρ-value < 0.05] were the independent predictors of H. pylori infection.
Conclusion: The prevalence rate of H. pylori infection in Southeastern Ethiopia is agonizingly high, exceeding the world average by 27.6%, the first report, and seems to be one of the neglected infectious diseases. Hence, the Oromia Region Health Bureau should reinvigorate the basic infectious disease control methods, establish routine laboratory diagnostic platforms, and intervene in selected societal practices spreading infections.
Keywords: Burden, determinants of Helicobacter pylori infection, government employees, indigestion, allergic diseases, Southeastern Ethiopia
Introduction
Helicobacter pylori is a gram-negative spiral or curved bacillus. It was discovered by two pathologists in 1982: Barry Marshall and Robin Warren, who were awarded a Nobel Prize in the indicated year.1 H. pylori has been recognized as a major human pathogen for approximately four decades.2 H. pylori infection is formally recognized as an infectious disease, an entity that is now included in the International Classification of Diseases 11th Revision. In principle, this leads to the recommendation that all infected patients receive treatment.3 Although the treatment of infected individuals reduces the transmission of infection in communities with improved socioeconomic living standards, it continues to be the most common human bacterial pathogen causing major morbidity and mortality worldwide, infecting nearly half of the world’s population.4
The frequency of infection varies significantly between and within nations, as well as within a single city and between subgroups within a community, even though a sizably high proportion of the world’s population is infected with H. pylori.5 Additionally, certain racial and ethnic minorities as well as immigrants have greater rates of H. pylori infection.6 H. pylori has been known to cause human illnesses like gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and gastric cancer for a very long time.7 The COVID-19 pandemic negatively impacted cancer diagnosis and treatment in 2020,8 leading to delays in care and increased mortality rates.9 Around 89% of all gastric cancers are attributed to H. pylori infection,10 ranking sixth in incidence and second in mortality worldwide.11 In 2020, gastric cancer was the fifth most common cancer worldwide and the fourth most common cause of cancer death.12
H. pylori infection prevalence in America is around 50%, with Asian countries having the highest variation. European countries have a high burden, with Portugal leading at 66.2%.13 Switzerland has the lowest prevalence at 18.9%.4 Low- and middle-income countries (LMICs) have a prevalence ranging from 85% to 95%.11 Africa has the highest prevalence (70%), while Oceania has the lowest (24%).4 The “African Enigma” phenomenon explains the high prevalence of H. pylori infection despite low gastric cancer incidence, and it has been hypothesized and elaborated with environmental, dietary, and genetic factors.7
H. pylori infection is typically acquired during childhood through person-to-person transmission.14 Symptoms include abdominal pain, stomach pain, bloating, heartburn, nausea, vomiting, and blood in stools.15 Duodenal ulcers cause abdominal pain several hours after eating due to stomach acid without food buffer.16 Various tests have been developed for H. pylori detection, divided into invasive and noninvasive methods. Invasive tests use gastric specimens for histology, culture, or molecular methods, while noninvasive tests use peripheral samples for antibodies, bacterial antigens, or urease activity. The choice of test depends on local experience and clinical setting.17 Routine diagnostic tests include histology, urea breath testing, culture, and serology.18
The treatment of H. pylori infection takes two weeks and includes antibiotics and proton pump inhibitor or bismuth.19 Peptic ulcer disease, stomach cancer resection, atrophic gastritis, mucosa-associated lymphoid tissue (MALT)-associated lymphoma (MALToma), and non-ulcer dyspepsia are among the associated conditions for which additional or specific treatment is indicated. As an example, long-term aspirin therapy is recommended for high-risk patients, those with a history of upper gastrointestinal bleeding, and those with gastroesophageal reflux disease.20
Ethiopia’s H. pylori infection prevalence has been studied for three decades,21 but research is rare. The disease spread from East Africa 58,000 years ago and has various strains. Inconsistent findings on burden and associated factors, such as ethnicity and socioeconomic status, are prevalent in Ethiopia’s hospitals. For example, one study prevalence report of H. pylori infection in 2017 from the Yirga Cheffe Primary Hospital, Southern Nations, Nationalities and People’s Regional State (SNNPRS) was 7.7% through stool antigen test.22 In contrast, another study in 1992 from Arba Minch Hospital in the SNNPRS of Ethiopia revealed a higher prevalence of H. pylori infection (73%) using endoscopy.21 In both hospitals; ethnicity and socioeconomic status contributed to the increase on infection rate.23
In the Bale Zone of Oromia Regional State, Goba Referral Hospital and Bale Robe General Hospital reported a high number of sick leave awardees due to indigestion among government employees. This health issue is among the top ten in the region, prompting a research project to address gastrointestinal syndrome. Additionally, to date, over 2 million residents in the Bale Zone lack access to endoscopy diagnostic machines or rapid serological diagnostic platforms, resulting in unintentional denial of the universal health coverage goal set by UN and the World Health Organization. This denial hinders access to quality health services leading to financial hardship for residents of the study area.24 The southeastern Oromia region faces high out-of-pocket expenditures due to long journeys for diagnostic services for suspected H. pylori infections, leading to catastrophic household expenses and social vulnerability. Symptoms include shortages of essential medicines and long hospital wait times. No private health facility provides accurate treatment options in this area. To the best of our knowledge, there are limited or no published studies on the burden and determinants of H. pylori infection among government employees in Ethiopia. Therefore, this study was aimed to estimate the burden of H. pylori infection and its determinants factors among government employees in the Bale Zone of southeastern Ethiopia.
Materials and Methods
Study Area
Ethiopia accounts for 1.5% of the global population. The Oromia region, the largest region in Ethiopia, is divided into 20 administrative zones (such as the Bale Zone), 30 city administrations (such as Bale Robe City), 287 rural Woredas (such as Dello Mena, Goro, and Gassera), and 46 town Woredas (such as Goba town)25 (Figure 1). In the Oromia region, the three major sectors that attract public employees are education (39.7%), health (14.1%), and agriculture (10.7%), as employees in the remaining 20 sectors account for approximately 35% of the region’s employees.26
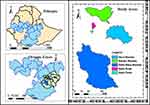 |
Figure 1 Map representing the study area. |
The land area coverage of Bale Zone (63, 555 Km2) is approximately twice that of the beautiful European country Belgium (30, 688 Km2). The zone has an estimated population of 1,854,699, of which 944,042 are males. Bale is located in the southeastern part of Oromia Regional State. Coordinate-wise, it lies between 39°59′ to 39°98′ east longitude and 7°0′ to 7°01′ north latitude.27 The official language in the Bale is Afaan Oromoo. Before it was split into two zones, there were five public hospitals, 85 health centers, 380 health posts, and more than 100 private clinics actively operating in the zone. The sampled health institutions in the current study are found at a distance range of 430 (Bale Robe City) to 555 kms (Dello Mena town) from the capital city of the Oromia region.
Study Design and Period
A multicenter health institution-based cross-sectional survey study design was employed, involving quantitative data collection methods from early March to the end of November 2022, in densely populated districts of the Bale Zone with government employees. In addition to the serological diagnostic test, a semi-structured questionnaire was employed to collect data related to the risk factors for bacterial infections through in-depth interviews by the help of trained medical laboratory technologists recruited as data collectors for the project.
Source and Study Population
The source population comprised all patients who visited the outpatient department (OPD) and inpatient department (IPD) of the public health institutions in Bale Zone with the complaint of indigestion during the data collection period but were not previously diagnosed with allergic diseases. The study population consisted of government employee patients examined at the OPD and IPD of four hospitals (Goba Tertiary Hospital, Bale Robe General Hospital, Dello Mena District Hospital, and Goro District Hospital) and Gassera Health Center with clinical indications of H. pylori infection throughout the data collection but not previously diagnosed with allergic diseases.
Sample Size Determination and Sampling Method
The sample size (n) was determined using the Taro Yamane formula28 sample size calculation formula with the assumption of a 95% confidence level. A 95% confidence interval was applied, as the assumption of normally distributed sample means and a sufficiently large sample frame (exceeding 10,000 individuals) was fulfilled and valid. The required minimum sample size (n) for this study, applying the indicated formula, was as follows:
where,
n = the calculated minimum sample size
N = population size = 21, 702, and
e = error (0.05) reliability level of 95% (level of precision set the value of 0.05).
Additionally, a 15% nonresponse rate was considered. Accordingly, the required sample size (n) for this study was 452 [393 + (393*0.15)], but we analyzed 428 subjects with complete data.
The reason for targeting selected health institutions from the zone was simply the density of government employees. Thus, the patients were sampled from all four hospitals of the Zone and Gassera Health Center (one of the districts far from the four hospitals sampled, but with large population of government employees). A cluster sampling method was employed to select study participants from the five health facilities. Among the calculated total sample sizes, 11 participants with HIV/AIDS comorbidities refused to participate. Thirteen subjects later revealed either allergic rhinitis or asthma, which lead to exclusion from the study units. Thus, only 428 patients with a complete dataset that fulfilled the inclusion criteria were finally analyzed. A pilot study was conducted on 18 patients in the Agarfa Health Center of the Bale Zone in the second half of February 2022 to attest the validity of the tools developed.
The Bale Zone has 21,702 government employees, while the five sampled districts of the zone have 12,915 permanent public employees. The zonal capital is Robe City (Bale Robe City), which is also the capital of the Robe Administration City. Bale-Robe General Hospital, and two health centers in the city render health services to residents and countless bypasses. The influx of people from rural areas to urban areas and migration into the Bale Zone from other regions of Ethiopia continues at an alarming rate, significantly jeopardizing the resources allocated and the service delivery capacity of all Bale Zone towns. Currently, patients with clinical indications for H. pylori infection are not routinely tested in all public health institutions operating in the Bale Zone. As a result, almost all patients are either placed on medication/treatment regimens based on symptomatology in the existing health facilities of the zone or referred to Yirga Cheffe General Hospital (Sidama Regional State) to seek rapid serological diagnostic tests or endoscopic examination. Referred patients from the Bale Zone are obliged to travel approximately 600 km back and forth to reach the indicated hospital.
Data Collection
The following data were collected from each participant:
Interview: Using predefined semi-structured questionnaires, trained health professionals collected data on sociodemographic, behavioural dynamics, risk, and enabling factors for H. pylori infection, including age, sex, residence, educational level, alcohol drinking and smoking status, practices of ritual jubilations, sanitation level, and salutation norms with Father of Repent. The questionnaire was first developed in English and then translated to Afaan Oromoo and tested during a pilot study at the Agarfa Health Center. It was back-transcribed into English during the data entry period into STATA statistical software.
Examination of Allergic Diseases (Allergic Rhinitis and Asthma): Both allergic rhinitis and asthma were assessed among the patients using self-report questionnaires and OPD logbooks from the surveyed medical facilities. Allergic rhinitis was defined as present if the participant reported hay fever or nasal allergies. On the other hand, asthma was considered to be present if patients reported having or had had asthma (with an explanation of the age at the onset of asthma) or reported using or having used asthma medications.
Serology: Approximately 3–5 mL of venous blood was collected from the study subjects for serological investigation of IgG antibodies. Blood samples were allowed to clot and centrifuged at 3000 rpm for 10 min; then, sera were separated and refrigerated at −20°C until testing. H. pylori infection was determined by using enzyme-linked immunosorbent assay (ELISA) using the Pyloriset® EIA-GIII kit (Orion Diagnostica, Espoo, Finland) as described elsewhere.29 Briefly, a 1:200 dilution of serum in buffer was added to H. pylori-coated microtiter wells. After 30 min of incubation, the wells were washed and peroxidase-conjugated anti-human IgG from a rabbit was incubated for an additional 30 min. After washing, tetramethylbenzidine substrate was added for 10 min, and the optical density of the solutions was measured in a 96-well microtiter plate at 450 nm using a BioTek EL 808 Automated Microtiter Plate Reader (BioTek Instruments, Winooski, Vermont). The results were expressed as units per millilitre according to a calibrator curve. A value of ≥ 20 U/mL was considered a positive result.
Inclusion and Exclusion Criteria
The inclusion criteria: Government employees aged 18 years or above working permanently in public sectors of the Bale Zone, such as health, education, agriculture, natural resources, judiciary, finance, economics, and planning, were included in the study. As far as they were presented to the selected health institutions with clinical manifestations of indigestion, government employees of the zone were included in the sample irrespective of their gender and educational level. Government employees referred for endoscopic examination to the Yirga Cheffe General Hospital, after being seen primarily in the five health facilities of the Bale Zone based on symptomatology, were also included in the current study.
The exclusion criteria: Government workers under the age of 18, those on temporary assignments, those who clinically presented to the OPD without indigestion, as well as those who self-reported having a history of allergic rhinitis, asthma, pregnancy, and chronic liver illness were all intentionally excluded from the study. Government employees who had previously undergone treatment for the aforementioned allergic conditions (allergic rhinitis and asthma) and who had received H. pylori infection treatment a month before this study instituted including the use of any antibiotics, bismuth, nonsteroidal anti-inflammatory drugs, corticosteroids, H2 receptor antagonists, or proton pump inhibitors were not included in the study. Additionally, patients who had smoked tobacco for more than a decade were not included. Thus, only 428 study participants were ultimately a part of it.
Data Management and Analyses
First, the data collectors at the sample site documented the participant’s previous history of asthma and allergic rhinitis, followed by subsequent collection of blood (3–5 mL to recover serum), and the filling-out of the semi-structured questionnaire designed for investigating the potential enabling factors of the infection. The principal investigators checked the completed questionnaire every three days to make sure it was accurate and comprehensive. For statistical analysis, STATA software Windows version 16.1 (STATA Corp., College Station, Texas, USA, 2019) was used.
Sociodemographic characteristics, patients’ behavioural phenomena and potential enabling factors for bacterial infection were reported by descriptive and inferential statistical packages. Overall, discrete variables were tested using a chi-square test, where Pearson’s chi-square (χ2) test was used to evaluate differences between proportions and a ρ-value less than 0.05 was considered statistically significant. Then, bivariate logistic regression analysis was performed to examine the presence of an association between H. pylori seropositivity and different risk factors. Subsequently, possible predictors with a ρ-value of < 0.25 in the bivariate logistic regression model were selected for further analysis with multivariable logistic regression models. A multivariable logistic regression model was used to calculate the degree of association between the response and explanatory variables with odds ratios (ORs). All presumed determinants with ρ-values less than 0.25 in the bivariate logistic regression model were entered together into the full multivariable logistic regression model. The OR at a 95% CI was used to estimate the degree of risk. A ρ-value less than 0.05 was accepted as statistically significant in the multivariable logistic regression model analysis.
Results
Sociodemographic Characteristics
In the current study, the mean magnitude of the seroprevalence of H. pylori infection using a measurement from internationally approved and nationally endorsed IgG antibody serology kits among 428 government employees who complained of indigestion and sought treatment in four hospitals and one health center in the Bale Zone, Southeastern Ethiopia, was 0.7757 (77.57%), with a standard deviation of 0.4176. Overall, nearly 22% of the government employees were found to be normal for H. pylori infections, although they had at least indigestion manifestations and sought treatment from the five health institutions operating in southeastern Ethiopia (Figure 2).
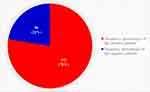 |
Figure 2 Pie chart portraying the overall seroprevalence of H. pylori infection. |
Of the 428 government employees included in the study population complaining of indigestion in the five public health facilities, almost half of them (49.77%) were sampled from two hospitals, Madda Walabu University (Goba) Tertiary Level Hospital and Bale-Robe General Hospital. The proportion of patients sampled from Goba Tertiary Level Hospital exceeded that of Bale-Robe General Hospital by 9.11%. Of all, 64.49% were urban dwellers. In terms of gender distribution, 69.39% were male patients, while the remaining 30.61% were female patients. The leading age category of the patients was in the range of 29–35.9 years (65.42%), followed by the youngest age category, which was between 21–28.9 years old (16.12%). The three leading ethnic groups of the study participants were Oromoo (73.13%), Sidama (7.01%), and Tigreway (6.78%). The highest proportion of the patient’s religious category, education level attained and marital status were Orthodox (42.52%), degree holders (55.61%), and married individuals (68.69%), respectively. More than 62% of the patients earn a monthly salary between 5001.00 and 10,000.00 Ethiopian Birr (ETB). The education sector stood first (32.48%) in hosting patients suffering from indigestion, whereas the Finance, Economics and planning sector was the least (13.79%) sampled. Overall, 48.6% of the study subjects engaged in sideline businesses to live in the study area (Table 1).
 |
Table 1 Sociodemographic Characteristics of the Indigested Government Employees (n = 428) |
The mean, minimum, and maximum age of the patients in years were 32.23 (± standard deviation of 4.95), 21, and 49, respectively. On the other hand, the mean, minimum, and maximum household sizes of the study subjects were 3 (± standard deviation of 1.47), zero, and eight, respectively (Figure 3).
 |
Figure 3 Box plot representation of the study participants age and household size. |
Forty patients were active smokers from 182 Christian religion followers, while 26 patients were currently used to smoking cigarettes among 141 Muslim religion followers (Figure 4). All patients who had smoked cigarettes for more than 10 years were not sampled in the current study.
 |
Figure 4 Frequency distribution of religions versus smoking status. |
All religions’ adherents comprised more than half of the study’s participants who engaged in active alcohol consumption. As an illustration, 71 out of the 141 Muslim patients sampled and 95 out of the 182 Orthodox Christian patients who participated in the study were currently drinking alcohol (Figure 5).
 |
Figure 5 Frequency distribution of religions versus alcohol-drinking status. |
Bivariate Logistic Regression Analysis of the Risk Factors for H. Pylori Infections
A bivariate logistic regression model analysis of the IgG test results was conducted against some of the suspected sociodemographic risk factors, patient behaviors, and enabling societal factors related to day-to-day interaction norm/culture. Subsequently, the sampled health institutions [Goba Tertiary Level Hospital: COR = 0.34; ρ-value < 0.05; Gassera Health Center: COR = 0.18; ρ-value < 0.001; and Goro District Hospital: COR = 0.15; ρ-value < 0.001], patient Residence area category [Being rural: COR = 0.6; ρ-value < 0.05], engagement in sideline business for a living [Involved in Livestock production: COR = 0.54; ρ-value < 0.05], Sharing local spoon on meal [COR = 13.5; ρ-value < 0.001], Admitted “Gursha” during meals [COR = 115.2; ρ-value < 0.001], Sharing eating utensils [COR = 11.97; ρ-value < 0.001], Eating raw/undercooked vegetables [COR = 15.4; ρ-value < 0.001], The type of toilet used to [Either absence of toilet or traditional pit latrine: COR = 671.2; ρ-value < 0.001], Active Alcohol drinker [COR = 29.5; ρ-value < 0.001], Active smoker [COR = 17.8; ρ-value < 0.001], Daily usual Sleeping hours of [5–8 hours: COR = 9.2; ρ-value < 0.001; and Greater than 8 hours: COR = 26.32; ρ-value < 0.001], Housed with domestic animals under the same roof [COR = 88.4; ρ-value < 0.001], Routinely washing their hands before eating [COR = 0.005; ρ-value < 0.001], Engaged in practices of Khat Chewing [COR = 52.7; ρ-value < 0.001], and Experienced major life events within the previous 2 years [life partner death, political abandonment (lack of political tranquility), famine (drought), family displacement, significant economic inflations, and victim of civil war] [COR = 6.48; ρ-value < 0.001] were found a risk factors for H. pylori infections. “Gursha” is a mouthful and morsel of food, which one places carefully in another’s mouth with a bare hand, usually as a gesture of affection. It is when husband and wife, visitors, relatives, elders and friends at the meal feed one another with a delicious bite of porridge, injera and sauce, and other dignified meals to display respect. “Kissing-Cross” is an act of kissing a cross commonly made from wood, light metals, semiprecious metal or even sometimes precious metal held by the priest or the bishop of Ethiopian Orthodox Tewahido Church, by apostles indoors or outdoors. Khat (Catha edulis) is a green, luxuriant plant whose buds and leaves are chewed for stimulating and euphoric effects, which is most frequently used by a cohort of young people.
Unlike those variables discussed above, sex, patient age category, ethnic group, religion, educational level, marital status, monthly income category earned, employee working sector, common eating spicy foods, regularly eating salty foods, access to in-home water service, eating from vendors or restaurants, and repeatedly eating peppery foods were not found to be risk factors for H. pylori infections among government employees sampled from Southeastern Ethiopia (Table 2) by the bivariate logistic regression model analysis.
 |
Table 2 Bivariate Logistic Regression Analysis of Plausible Risk Factors for IgG Test Results |
Multivariable Logistic Regression Model Fit Analysis of the Potential Risk Factors of H. Pylori Infections
After fulfilling the candidacy test (which was a ρ-value less than 0.25), we carried out by one-on-one variable analysis between the dependent and independent variables by the bivariate logistic regression model, and the following variables again fit the multivariable logistics regression model analysis by independently determining the H. pylori infection in patients recovered from five public health institutions in the Bale Zone, Southeastern Ethiopia. Those variables include the sampled health institutions [Goba Tertiary Level Hospital: AOR = 0.29; ρ-value < 0.05; Gassera Health Center: AOR = 0.18; ρ-value < 0.05; and Goro District Hospital: AOR = 0.13; ρ-value < 0.001], engagement in sideline business for a living [Involved in Livestock production: AOR = 0.45; ρ-value < 0.05], Sharing local spoon on meal [AOR = 39.30; ρ-value < 0.001], Admitted “Gursha” during meal [AOR = 71.48; ρ-value < 0.05], The type of toilet used to [Either absence of toilet or traditional pit latrine [AOR = 1410.98; ρ-value < 0.001], Currently active Alcohol drinker [AOR = 15.15; ρ-value < 0.05], Daily usual Sleeping hours of [5–8 hours: AOR = 4.61; ρ-value < 0.001; and Greater than 8 hours: AOR = 15.01; ρ-value < 0.001], Routinely washing their hands before eating [AOR = 0.15; ρ-value < 0.05], and Engaged in practices of Khat Chewing [AOR = 76.73; ρ-value < 0.001], as depicted in the following Table
On the other hand, the following variables, which showed a ρ-value of less than 0.25 in the bivariate logistic regression model analysis, such as residence category, ethnic group, religion, highest education level attained, current employee organization, history of kissing cross, regularly eating salty foods, having in-home water service, sharing eating utensils, eating raw/uncooked vegetables, active smoking status, housing with domestic animals under the same roof, and experienced major life events (over the last 24 months), did not independently predict the infection status of H. pylori in government employees seeking treatment in five health institutions in the Bale Zone, southeastern Ethiopia (Table 3).
 |
Table 3 Multivariable Logistic Regression Analysis Model Fit of Plausible Risk Factors with Infection |
Determinant Factors of H. Pylori Infections as Retrieved from Multivariate Logistic Regression Analysis
The sampled health institution (ρ-value < 0.05), engagement in sideline business for a living (ρ-value < 0.05), sharing local spoon on meal [AOR = 39.30; CI: 19.52 −78.31; ρ-value < 0.001], admitted “Gursha” during meal [AOR = 71.48; CI: 3.99 −1279.77; ρ-value < 0.05], the type of toilet used to [AOR = 1410.98; CI: 121.16 −16,431.19; ρ-value < 0.001], currently active Alcohol drinker [AOR = 15.15; CI: 1.90 −120.62; ρ-value < 0.05], common sleeping hours (on daily basis) [AOR = 15.01; CI: 13.48–55.96; ρ-value <0.001], regular hand washing before eating [AOR = 0.15; CI: 0.12–0.19; ρ-value <0.05], and usual involvement in Khat chewing [AOR = 76.73; CI: 8.57–687.07; ρ-value <0.001] were the independent predictors of H. pylori infection in the patients from Bale Zone. In contrast, a history of kissing cross from the hands of the father of repent [AOR = 1.13; CI: 0.25–4.84; ρ-value ≥ 0.05] was an independent predictor of H. pylori infection in the patients (Table 4).
 |
Table 4 Independent Predictors of H. Pylori Infection Among Indigested Workers |
Discussion
Numerous assays have been developed to identify H. pylori, each with specific advantages and disadvantages. The choice of a certain test for a given patient is influenced by the tests’ objectives, local knowledge, and the therapeutic setting.17 One of the noninvasive diagnostic procedures used in the current study’s evaluation of H. pylori infection was serology. Additionally, some of the study participants referred to the neighbouring Sidama Regional State Hospital, Yirga Cheffe General Hospital, for endoscopic check-up.
Serology is best suited for large epidemiological investigations. Many patients receiving hospital-based care are tested with endoscopy, which might be followed by an invasive test for H. pylori. However, fecal antigen assays for children provide a way to determine the presence of H. pylori without requiring an endoscopy or vena puncture.30 As there was a large sample size in our study and conducted on blood samples collected from people older than 18 years, serology is economically feasible for surveillance study to determine the presence of H. pylori infection. Up until the conclusion of the current study, patients with dyspepsia in southeastern Ethiopia did not have access to endoscopic examination in Bale Zone and Robe City.
The preferred diagnostic test in this investigation was the H. pylori blood IgG antibody test against the bacterial antigen. According to the study’s findings, four hospitals and a health center serving southeast Ethiopia’s patients with dyspepsia syndrome had an average prevalence of H. pylori infection of about 78%. The prevalence rate of H. pylori in this study is significantly lower than that in Nigeria, a developed African nation, where it was estimated to be 87.7%.31 Once more, Vietnam reported a higher prevalence rate than the current study, with an overall prevalence of H. pylori that was similar to the Nigerian report’s figure of 87.7%.32 Additionally, it is lower than that of the report from the Southern Ethiopia study on H. pylori infection, which indicated an overall infection prevalence rate of 83.3%.33
Unlike the present study findings, a cross-sectional study conducted in Ningxia between 2017 and 2022 reported a decreasing infection burden from 60.3% in 2017 to 43.6% in 2022.34 Again, the current study is favourably higher than the study conducted in two districts of Cameroon, where the prevalence was found to be 52.27%.35 Similarly, our study’s prevalence rate is lower than a study reported from West Cameroon, which revealed a 43.4% seropositivity in patients.36 In addition, a lower prevalence rate was reported by other studies as compared to the present study results; including an infection rate of 43.45% among adults in China,37 30.8% of infection cases in children of Northeast Romania,38 32.5% prevalence rate among patients with dyspepsia and other gastrointestinal diseases in Saudi Arabia,39 and pooled H. pylori prevalence of 44.2% in mainland China from the meta-analysis.40 The discrepancies between the current study and the studies with higher or lower prevalence rates might emanate from the differences in countries’ human development index, sample size, target cohort age, public awareness, sociocultural status, personal hygiene awareness, and environmental sanitation level, and study design.
Regarding the risk factors, according to this study, the study participants’ level of education was not an independent predictor of H. pylori infection, in contrast to the study results reported from Cameroon.41 The possible reason for difference might be the number of study participants. Additionally, in the present report, the history of kissing crosses from the hands of the father of Repent was not an independent determinant of H. pylori infection in the patients. However, a previous study from a closer analogy revealed that mouth-to-mouth kissing was an independent predictor of H. pylori infection in patients,42 as the difference might be due to the smaller sample size of Orthodox Tewahido Christians in the present study. In addition, our study findings disagree with the previous study report from islands of Indonesia, which showed that ethnicity is an independent risk factor for H. pylori infection.43
In this study, almost very uniquely unlike the previous studies from other regions of Ethiopia and abroad, there were statistically significant differences among the sampled health institutions, patients’ engagement in sideline business for a living, sharing local spoons while serving meal, admitting “Gursha” during get-together meal, and frequent practices of chewing a khat. The only published study that supports the latter risk factor in the present study was shown in one study, where khat chewing was reported as a risk factor for H. pylori infection in resource-limited settings of northwest Ethiopia.44
Similarly, the type of toilet used by the patients and currently being an active alcohol drinker were independent predictors of H. pylori infection in the patients from this study. In line with the preceding determinant of H. pylori infection in southeastern Ethiopia in this study, such findings are partly consistent with the previous report from the West African country, Benin, where the risk of H. pylori infection was resolved by improving the living conditions of the population at risk of the infection.45 Another previous study from Cuba also implicated drinking water from water delivery trucks as a protective factor against H. pylori infection.46 In another former hospital-based cross-sectional study on the prevalence and risk factors for H. pylori from dyspeptic patients in Northwest Ethiopia, there were also statistically significant differences in the prevalence of H. pylori with alcohol consumption.47
The type of toilet used by the patients was found to be significantly associated with H. pylori infection in the present study as opposed to results from a previous study that aimed to assess the magnitude of H. pylori and associated risk factors among symptomatic patients attending Jasmin internal medicine and paediatrics specialized private clinics in Addis Ababa city of Ethiopia, as it revealed that the type of toilet patients used and source of drinking water was not significantly associated with H. pylori infection.48 In addition, a previous cross-sectional study on the epidemiology and risk factors for H. pylori infection in Timergara City of Pakistan reported that the odds of developing H. pylori infection were significantly higher in males, smokers, snuff addiction, no regular toothpaste users, and regular soft drinks takers.49 However, a study on Uganda’s comfortability supported the present study findings, which indicated that the lack of sanitary facilities was an independent explanatory variable for H. pylori infection.50
Reciprocal to the present study, a study targeting the seroprevalence of H. pylori infection and associated factors among adults in Mizan Aman town in Southwest Ethiopia reported that independent risk factors for the seroprevalence of H. pylori infection were the presence of domestic animals, sources of drinking water, toilet type, shared beds with siblings, family size, storing and reusing water and occupational status.51 Again, a systematic review finding revealed that health professionals were at a higher risk of H. pylori infection than the general population, especially among those working at gastrointestinal units, stating it as an occupational disease.52 In addition, a comparative study conducted in Assosa General Hospital in West Ethiopia among dyspepsia and non-dyspepsia adults opposed our study conclusion regarding alcohol drinking and khat chewing, stating that they were not independent risk factors for H. pylori infection.53
Among the variables that were not measured in the current study, a study on the prevalence and associated risk factors for H. pylori infection in northwestern China reported that individuals with a habit of eating quickly and individuals who consumed more fruit and vegetables were determinant factors for H. pylori infection,54 and the study reported from Iraq stressed that oral diseases such as periodontal diseases and caries are important risk factors for H. pylori colonization.55
The category of daily sleeping hours was also linked to H. pylori infection in the subjects sampled for the current investigation. Similar to patients from Southeastern Ethiopia, routine hand washing before meals was a reliable indicator of H. pylori infection. There were also statistically significant variations in the prevalence of H. pylori with the habit of washing hands before a meal in a previous hospital-based cross-sectional investigation on the prevalence and risk factors for H. pylori from dyspeptic patients in Northwest Ethiopia.47 Assosa General Hospital in West Ethiopia conducted a comparative cross-sectional study among individuals with and without dyspepsia, and the results suggested that smoking did not significantly increase the risk of H. pylori infection.53
By employing a stratified sampling method, a previous research report from the Hainan Province of China about the potential risk factors of H. pylori infection prevalence revealed a negative association with hand washing before meals.56 Also, a cross-sectional study from two rural villages in Northwest China) stated hand washing before meals is not significantly associated with an increase in the infection intensity of H. pylori infection57 and agrees with that of the report from Hainan Province of China. However, in another population-based randomized intervention study from rural China, infrequent handwashing before meals was found an independent risk factor for an increased prevalence of H. pylori.58 This later study result supports our findings that regular hand washing before a meal is a significant contributor to H. pylori infection. The differences and similarities between those previous findings from China with the current study might be due to sampling methods and sample size difference.
In an attempt to verify the treatment status of all the IgG antibody-positive patients from all sampled health institutions, a full dosage regimen of omeprazole antibiotics was prescribed as per the Oromia Health Bureau essential drug formulary guidelines, as a full dose effective antibiotic is recommended for the eradication of the microbe. This narration and treatment indication is supported by other studies elsewhere, such as59 where treatment for H. pylori infection is recommended in patients with an active or past history of peptic ulcer, chronic dyspepsia, chronic nonsteroidal anti-inflammatory drugs or aspirin use, precancerous gastric lesions, gastric cancer, MALT lymphoma, family history of gastric cancer, family history of peptic ulcers, a household family member having active H. pylori infection, iron deficiency anaemia, idiopathic thrombocytopenic purpura, or vitamin B12 deficiency. However, treatment failure is a common phenomenon of this bacterial infection due to point mutations in the 23S rRNA gene of H. pylori.60
The current study has some limitations; including its focus on indigestion syndrome complaining patients, and serological assays’ inability to differentiate between active and past infections. The study also excluded government employees under 18 years old, limiting its generalizability to all government workers. Additionally, the study was conducted in a less privileged southeastern part of Ethiopia, which may have a higher prevalence of H. pylori infection compared to other economically better countries. Other risk factors, such as access to information, eating habits, and fruit and vegetable consumption, were not considered. The study’s cross-sectional survey design allowed for limited follow-up and assessment of patient prognosis over time.
Nevertheless, this study provides a novel finding on H. pylori infection in southeastern Ethiopia, a first of its kind in the region. The large sample size and thorough analyses make it applicable to similar LMICs. The study population was sampled from five public health institutions, ensuring representativeness. The results can be generalizable to similar areas in East African countries. The study excludes patients with documented allergic diseases; allergic rhinitis, and asthma.
Conclusions
Amid many reports of antimicrobial resistance elsewhere, the priority given for H. pylori bacterial infection in the current less privileged study area of southeast Ethiopia is less where the magnitude of its infection is alarmingly high (77.6%). On Bale Land, it seems to spread submissively by a variety of interconnected determinant factors and warranting an urgent intervention regarding the basic hygienic principles, sanitation, societal economic well-being and a few societal get-together practices. Again, the total lack of single endoscopy machines in southeastern Ethiopia makes the bacterial diagnosis a nightmare. Hence, as the region is free from conflict now, Ethiopia with its international development partner has to strengthen its healthcare diagnostics delivery in southeastern Ethiopia.
Abbreviations
AAU, Addis Ababa University; AOR, Adjusted Odds Ratio; CI, Confidence Interval; COR, Crude Odds Ratio; ELISA, Enzyme-linked immunosorbent assay; LMICs, Low- and Middle-income Countries; IgG, Immunoglobulin G; IPD, In-Patient Department; MALT, Mucosa-associated lymphoid tissue; mL, Milliliter; MWU, Madda Walabu University; nM, Nanometer; OPD, Out-Patient Department; SNNPRS, Southern Nations, Nationalities and People’s Regional State; STATA, Data Analysis and Statistical Software; U, Unit; UN, United Nations.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study project obtained an ethical approval letter from the Research and Development Directorate of Madda Walabu University (Bale, Ethiopia); the ethical approval letter code was MWU-BAC-1054-12-2021. All participants in the study provided their written informed consent before the collection of data, and participation was fully voluntarily. The Helsinki Declaration on Biomedical Ethics served as the guide for conducting this study. The data gathered from the study subjects was kept private. Mini-compensation for individuals who tested positive for H. pylori infection were made and monitored by the clinical staff (doctors or health officers) in charge of the hospital or health center until the completion of the full-dosage of the administered antibiotics. Those who tested positive for the bacteria were given the current treatment regimen recommended by the Yirga Cheffe General Hospital.
Acknowledgments
The laboratory department of Yirga Cheffe General Hospital, all leadership from the sampled hospitals and Gassera Health Center, the data collectors, and, for the most part, the study participants, are acknowledged by the authors. Also, the authors are grateful to Mr Tesfaye Faye (from the department of Geography and Environmental Studies of MWU) for delineating the map of the study area.
Funding
The study was funded by Madda Walabu University (MWU) and Addis Ababa University (AAU). However, both Universities, MWU and AAU, have no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sue K. Park Extradigestive Manifestations of Helicobacter Pylori Infection: An Overview. Roesler BM, ed.. ExLi4EvA;2016. doi:10.5772/62971
2. Kusters JG, Van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–490. doi:10.1128/CMR.00054-05
3. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71(9):1724–1762. doi:10.1136/gutjnl-2022-327745
4. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi:10.1053/j.gastro.2017.04.022
5. World Gastroenterology Organisation. Guideline. Vol. 2021. Milwaukee, USA: World Gastroenterology Organisation; 2021. doi:10.2169/naika.91.547
6. Burucoa C, Axon A. Epidemiology of helicobacter pylori infection. Helicobacter. 2017;22:1–5. doi:10.1111/hel.12403
7. Smith S, Fowora M, Pellicano R. Infections with helicobacter pylori and challenges encountered in Africa. World J Gastroenterol. 2019;25(25):3183–3195. doi:10.3748/wjg.v25.i25.3183
8. Yabroff KR, Wu XC, Negoita S, et al. Association of the COVID-19 pandemic with patterns of statewide cancer services. J Natl Cancer Inst. 2022;114(6):907–909. doi:10.1093/jnci/djab122
9. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
10. Yeh JM, Kuntz KM, Ezzati M, Goldie SJ. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer. 2009;124(1):157–166. doi:10.1002/ijc.23864
11. Khoder G, Sualeh Muhammad J, Mahmoud I, Soliman SSM, Burucoa C. Prevalence of helicobacter pylori and its associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens. 2019;8(2):44. doi:10.3390/pathogens8020044
12. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
13. Borka Balas R, Meliț LE, Mărginean CO. Worldwide prevalence and risk factors of helicobacter pylori infection in children. Children. 2022;9(9). doi:10.3390/children9091359
14. Abadi ATB, Kusters JG. Management of Helicobacter pylori infections. BMC Gastroenterol. 2016;16(1):2–5. doi:10.1186/s12876-016-0496-2
15. Sheila E. Helicobacter pylori Infection. N Engl J Med. 2019;380(12):1158–1165. doi:10.1056/NEJMcp1710945
16. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390(10094):613–624. doi:10.1016/S0140-6736(16)32404-7
17. Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, et al. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38(1):55–66. doi:10.1007/s10096-018-3414-4
18. Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - Recent developments in diagnosis. World J Gastroenterol. 2014;20(28):9299–9313. doi:10.3748/wjg.v20.i28.9299
19. Graham DY, Lu H, Shiotani A. Vonoprazan-containing helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol. 2021;36(5):1159–1163. doi:10.1111/jgh.15252
20. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24(10):1587–1600. doi:10.1111/j.1440-1746.2009.05982.x
21. Tedla Z. Helicobacter pylori infection in patients with upper gastrointestinal symptoms in Arba Minch Hospital: southwestern Ethiopia. Ethiop Med J. 1992;30(1):43–49.
22. Ayele B, Molla E. Dyspepsia and associated risk factors at yirga cheffe primary hospital, Southern Ethiopia. Clin Microbiol Open Access. 2017;06(03). doi:10.4172/2327-5073.1000282
23. Natuzzi E. Neglected tropical diseases: is it time to add Helicobacter pylori to the list? Glob Health Promot. 2013;20(3):47–48. doi:10.1177/1757975913499037
24. Ifeagwu SC, Yang JC, Parkes-Ratanshi R, Brayne C. Health financing for universal health coverage in Sub-Saharan Africa: a systematic review. Glob Heal Res Policy. 2021;6(1). doi:10.1186/s41256-021-00190-7
25. Central Statistical Agency (CSA). Summary and Statistical Report of the 2007: Population and Housing Census Results. Addis Ababa, Ethiopia; 2008.
26. Nigussie Daba. Human resource dynamics in oromia regional state, ethiopia: trends and challenges. Eur J Bus Manag. 2016;8(25):74–81.
27. Central Statistical Authority (CSA). 2007 Population and Housing Census of Ethiopia: Administrative Report. Ethiopia: Addis Ababa; 2012.
28. Harper & Row Publishers. STATISTICS: An Introductory Analysis. Tokyo, Japan: Harper & Row Publishers; 1973.
29. González CA, Megraud F, Buissonniere A, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012;23(5):1320–1324. doi:10.1093/annonc/mdr384
30. Shaikh S, Khaled MA, Islam A, Kurpad AV, Mahalanabis D. Evaluation of stool antigen test for Helicobacter pylori infection in asymptomatic children from a developing country using 13C-urea breath test as a standard. J Pediatr Gastroenterol Nutr. 2005;40(5):552–554. doi:10.1097/01.MPG.0000150093.12457.0D
31. Lee YC, Dore MP, Graham DY. Diagnosis and Treatment of Helicobacter pylori Infection. Annu Rev Med. 2022;73:183–195. doi:10.1146/annurev-med-042220-020814
32. Che TH, Nguyen TC, Ngo DTT, et al. High prevalence of helicobacter pylori infection among school-aged children in Ho Chi Minh City, Vietnam. Int J Public Health. 2022;67:1–8. doi:10.3389/ijph.2022.1605354
33. Tadesse E, Daka D, Yemane D, Shimelis T. Seroprevalence of Helicobacter pylori infection and its related risk factors in symptomatic patients in southern Ethiopia. BMC Res Notes. 2014;7(1):1–5. doi:10.1186/1756-0500-7-834
34. Zhou Y, Deng Y, You Y, et al. Prevalence and risk factors of Helicobacter pylori infection in Ningxia, China: comparison of two cross-sectional studies from 2017 and 2022. Am J Transl Res. 2022;14(9):6647–6658.
35. Ndip RN, Malange AE, Akoachere JFT, MacKay WG, Titanji VPK, Weaver LT. Helicobacter pylori antigens in the faeces of asymptomatic children in the Buea and Limbe health districts of Cameroon: a pilot study. Trop Med Int Heal. 2004;9(9):1036–1040. doi:10.1111/j.1365-3156.2004.01299.x
36. Agbor NE, Esemu SN, Ndip LM, Tanih NF, Smith SI, Ndip RN. Helicobacter pylori in patients with gastritis in West Cameroon: prevalence and risk factors for infection. BMC Res Notes. 2018;11(1):1–6. doi:10.1186/s13104-018-3662-5
37. Zhou XZ, Lyu NH, Zhu HY, et al. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: the time to change practice for related disease prevention. Gut. 2023:1–15. doi:10.1136/gutjnl-2022-328965
38. Lupu A, Miron IC, Cernomaz AT, et al. Epidemiological characteristics of helicobacter pylori infection in children in Northeast Romania. Diagnostics. 2023;13(3):1–12. doi:10.3390/diagnostics13030408
39. Chowdhury S, Chakraborty P. Universal health coverage ‑ There is more to it than meets the eye. J Fam Med Prim Care. 2017;6(2):169–170.
40. Ren S, Cai P, Liu Y, et al. Prevalence of helicobacter pylori infection in China: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(3):464–470. doi:10.1111/jgh.15751
41. AbongwaL MS, Sanda A, Signang EM. Knowledge, practice and prevalence of helicobacter pylori infection in the North West Region of Cameroon. Clin Biotechnol Microbiol. 2017;1(4):135–143.
42. Hailu A, Sileshi B, Panari H. Prevalence of helicobacter pylori infection and associated factors among gastritis patents in yekatit 12 teaching hospital Addis Ababa Ethiopia. Res Sq. 2020;1:1–17.
43. Syam AF, Miftahussurur M, Makmun D, et al. Risk factors and prevalence of Helicobacter pylori in five largest islands of Indonesia: a preliminary study. PLoS One. 2015;10(11):1–14. doi:10.1371/journal.pone.0140186
44. Negash M, Baynes HW, Geremew D. Helicobacter pylori infection and its risk factors: a prospective cross-sectional study in resource-limited settings of Northwest Ethiopia. Can J Infect Dis Med Microbiol. 2018;2018:3. doi:10.1155/2018/9463710
45. Aguemon BD, Struelens MJ, Massougbodji A, Ouendo EM. Prevalence and risk-factors for Helicobacter pylori infection in urban and rural Beninese populations. Clin Microbiol Infect. 2005;11(8):611–617. doi:10.1111/j.1469-0691.2005.01189.x
46. Venero-Fernández SJ, Ávila-Ochoa I, Menocal-Herredia L, et al. Prevalence of and factors associated with Helicobacter pylori infection in preschoolers in Havana, Cuba: a population-based study. Rev Gastroenterol Mex. 2020;85(2):151–159. doi:10.1016/j.rgmx.2019.03.010
47. Abebaw W, Kibret M, Abera B. Prevalence and risk factors of H. pylori from dyspeptic patients in Northwest Ethiopia: a hospital based cross-sectional study. Asian Pacific J Cancer Prev. 2014;15(11):4459–4463. doi:10.7314/APJCP.2014.15.11.4459
48. Shiferaw G, Abera D. Magnitude of Helicobacter pylori and associated risk factors among symptomatic patients attending at Jasmin internal medicine and pediatrics specialized private clinic in Addis Ababa city, Ethiopia. BMC Infect Dis. 2019;19(1):1–6. doi:10.1186/s12879-019-3753-5
49. Hussain Shah SR, Almugadam BS, Hussain A, Ahmad T, Ahmed S, Sadiqui S. Epidemiology and risk factors of Helicobacter pylori infection in Timergara city of Pakistan: a cross-sectional study. Clin Epidemiol Glob Heal. 2021;12:100909. doi:10.1016/j.cegh.2021.100909
50. Aitila P, Mutyaba M, Okeny S, et al. Prevalence and risk factors of helicobacter pylori infection among children aged 1 to 15 years at holy innocents children’s hospital, Mbarara, South Western Uganda. J Trop Med. 2019;2019:1–7. doi:10.1155/2019/9303072
51. Belay AS, Abateneh DD, Yehualashet SS. Seroprevalence of helicobacter pylori infection and associated factors among adult dyspeptic patients in public health facilities, mizan Aman Town, Southwest, Ethiopia: institutional-based cross-sectional study. Int J Gen Med. 2020;13:577–585. doi:10.2147/IJGM.S273523
52. Kheyre H, Morais S, Ferro A, et al. The occupational risk of Helicobacter pylori infection: a systematic review. Int Arch Occup Environ Health. 2018;91(6):657–674. doi:10.1007/s00420-018-1315-6
53. Dilnessa T, Amentie M. Prevalence of Helicobacter pylori and risk factors among dyspepsia and non-dyspepsia adults at Assosa General Hospital, West Ethiopia: a comparative study. Ethiop J Heal Dev. 2017;31(1):4–12.
54. Zhang F, Pu K, Wu Z, et al. Prevalence and associated risk factors of Helicobacter pylori infection in the Wuwei cohort of north-western China. Trop Med Int Heal. 2021;26(3):290–300. doi:10.1111/tmi.13517
55. Almashhadany DA, Zefenkey ZF, Zaki AM. Dental risk factors associated with oral Helicobacter pylori infection: a cross-sectional study based on saliva antigen test. J Infect Dev Ctries. 2022;16(3):516–521. doi:10.3855/jidc.15420
56. Chen RX, Zhang DY, Zhang X, et al. A survey on Helicobacter pylori infection rate in Hainan Province and analysis of related risk factors. BMC Gastroenterol. 2023;23(1):1–9. doi:10.1186/s12876-023-02973-3
57. She X, Zhao J, Cheng S, Shi H, Dong L, Zhao P. Prevalence of and risk factors for Helicobacter pylori infection in rural areas of Northwest China: a cross-sectional study in two villages of Yan’an city. Clin Epidemiol Glob Heal. 2023;21(2022):101294. doi:10.1016/j.cegh.2023.101294
58. Brown LM, Thomas TL, Ma JL, et al. Helicobacter pylori infection in rural China: demographic, lifestyle and environmental factors. Int J Epidemiol. 2002;31(3):638–646. doi:10.1093/ije/31.3.638
59. Aumpan N, Mahachai V, Vilaichone R. Management of Helicobacter pylori infection. JGH Open. 2023;7(1):3–15. doi:10.1002/jgh3.12843
60. Yakoob J, Jafri W, Abbas Z, et al. Risk factors associated with Helicobacter pylori infection treatment failure in a high prevalence area. Epidemiol Infect. 2011;139(4):581–590. doi:10.1017/S0950268810001226
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.