Back to Journals » Clinical Ophthalmology » Volume 14
The Baerveldt Glaucoma Drainage Device: Efficacy, Safety, and Place in Therapy
Authors Poelman HJ, Wolfs RCW , Ramdas WD
Received 9 June 2020
Accepted for publication 27 August 2020
Published 24 September 2020 Volume 2020:14 Pages 2789—2797
DOI https://doi.org/10.2147/OPTH.S219271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Huub J Poelman, Roger CW Wolfs, Wishal D Ramdas
Department of Ophthalmology, Erasmus Medical Center, Rotterdam, the Netherlands
Correspondence: Wishal D Ramdas
Department of Ophthalmology, Erasmus Medical Center, Rotterdam, ‘S Gravendijkwal 230, CE 3015, Rotterdam, The Netherlands
Email [email protected]
Objective: This review summarizes published findings concerning the Baerveldt-350 glaucoma drainage device (GDD). Most studies focus on the comparison between different treatments; in this review, the primary focus is efficacy, safety, and place in therapy for the Baerveldt implant.
Methods: A systematic review was performed using the PubMed database for literature on March 13th, 2020. Efficacy was estimated by performing multiple meta-analyses to calculate the weighted mean difference in intraocular pressure (IOP) and IOP-lowering medication after surgery. In order to get an indication of the safety of the Baerveldt implant, all recorded peri- and postoperative complication were summarized.
Results: A total of 21 studies, including 12 randomized controlled trials, were included with a follow-up up to 5 years, covering a mix of glaucoma types. At the last follow-up point, at 5 years postoperative, the mean (95% confidence interval) reduction in IOP was 15.57 mmHg (14.43– 16.71) and the mean (95% confidence interval) reduction in IOP-lowering medication after surgery was 1.81 (1.61– 2.01). Most frequently observed postoperative complications were corneal edema (2– 34%) and tube complications (4– 33%). Rates of required re-intervention ranged from 0% to 51% across all included studies.
Conclusion: The efficacy of the Baerveldt implant is a significant reduction in IOP in the long term. The safety profile of the Baerveldt implant in terms of complication incidence is similar to those reported for other GDD’s. For treatment of secondary glaucoma, we suggest the Baerveldt (or any other similar GDD) as the choice of treatment in patients where highest IOP reduction is desired.
Keywords: Baerveldt implant, glaucoma drainage device, glaucoma, intraocular pressure
Introduction
Glaucoma is a neurodegenerative eye disease for which only one modifiable risk factor has been identified till date: (increased) intraocular pressure (IOP). For several decades glaucoma belongs to the most common causes of irreversible blindness worldwide. Although normal-tension glaucoma exists, usually the IOP is increased above 21mmHg which causes optic nerve strain. Rapid increase or continuous high IOP leads to damage of the optic nerve, causing irreversible visual field loss.1
Glaucoma can be caused by various factors. Primary open angle glaucoma is the most common form of glaucoma and is caused by obstruction of the trabecular meshwork. Less frequent, glaucoma can also primarily be caused by closure of the anterior chamber angle or secondary, by e.g. uveitis, pigment dispersion, or exfoliation. In any case, treatment of glaucoma is always oriented around decreasing the IOP, which can be achieved by medical treatment, laser treatment, or surgery.2 The first step often consists of topical medication, resulting in decreased production and/or increased outflow of aqueous humor. Alternatively, laser treatment can be used in various ways, either to decrease production of aqueous humour, or to increase aqueous outflow.3 Finally, surgical intervention is available as another viable treatment option. Two commonly used surgical methods are trabeculectomy and the implantation of a glaucoma drainage device (GDD) such as the Baerveldt implant.
In February 1990, the Baerveldt implant was introduced in California (USA) and is used worldwide ever since. The Baerveldt-350 consists of a non-valved silicone tube (0.63 mm external diameter, 0.30 mm inner diameter). It is attached to a medical-grade silicon plate with a large surface area of 350mm2, which is placed with its ends beneath the recti muscles, commonly in the superotemporal quadrant.4
This review summarizes published findings from randomized controlled trials (RCT) and non-randomized studies concerning the Baerveldt implant. Most studies focused on the comparison between different treatments. In this review the primary focus is efficacy, safety, and place in therapy for the Baerveldt implant.
Methods
Data Search and Study Selection
A systematic review was performed using the guidelines implied by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA; Liberati et al 2009). The PubMed database was searched for studies describing the Baerveldt implant published until March 13th, 2020. The following MeSH terms were used: (baerveldt[All Fields] AND implant[All Fields]) OR (baerveldt[All Fields] AND glaucoma[All Fields]) OR (baerveldt[All Fields] AND device[All Fields]). The search query was limited to include the following article types: clinical study, clinical trial, comparative study, meta-analysis, observational study, RCT, review, and systematic reviews. All eligible articles had to have an available abstract in English language, had to be performed in humans or on human tissue, and had to be peer-reviewed. From the retrieved studies, titles and abstracts were scanned. Next, the full-text was read and the reference lists from all identified studies were scanned in the same way to find other allegeable studies.
Data Extraction and Quality Assessment
All retrieved studies had to report on either postoperative IOP, postoperative IOP-lowering medication or peri/postoperative complications. The peri/postoperative complications had to be listed with a percentage indicating the incidence during follow-up for the Baerveldt implant. Also, only complications reported by studies from the last 20 years were included, because of technical improvements last decades. Identical or very similar complications were grouped. Complications were categorized as “perioperative”, “early postoperative” (≤3 months), and “cumulative postoperative” (≤5 years).
Quality of the included articles was assessed based on the dictated level of evidence according to Levels of Evidence For Primary Research Question, adopted by the North American Spine Society. Finally, for the postoperative IOP and postoperative IOP-lowering medication analysis, only high level of evidence randomized controlled trials were selected. For the complication assessment, RCT and non-RCT studies were analyzed separately.
Data Analysis
Not all articles reported data at every follow-up moment. For each time interval, the mean from all included studies was calculated in regard to the included eye at that time interval. Next, the mean and standard deviation were used in the meta-analysis to calculate the weighted mean differences (WMDs) with their corresponding 95% confidence interval (CI) for each time interval. Heterogeneity was evaluated by calculating theI2-statistics.
Using this method, the WMD of postoperative IOP across the retrieved studies was calculated for the Baerveldt implant at 1 day, 1 week, 1 month, 6 months, 1 year, 35 years of follow-up. This method was also used to analyze the change in number of IOP-lowering medications. Data analysis was performed using RevMan 5 for Windows (The Cochrane Collaboration, Oxford, UK) and Microsoft Excel for Windows (Microsoft, Redmond, Washington, US).
Results
The initial literature search on Pubmed resulted in a total of 402 articles. After applying search limits and additional filters, 88 articles remained. After reading the full text for inclusion criteria, a total of 21 studies, including 12 randomized controlled trials were included.5–25 (Figure 1). All RCT studies had a follow-up ranging from 1 to 5 years, covered a mix of glaucoma types and compared the superotemporal placed, perioperatively occluded Baerveldt 350mm implant to other interventions (Table S1).
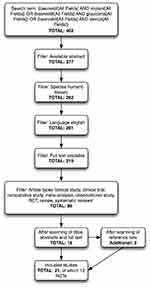 |
Figure 1 Flowchart of Pubmed search. Abbreviation: RCT, randomized controlled trial |
Multiple RCT articles resulted from the three large Baerveldt studies: the ‘Tube Versus Trabeculectomy Study’, the ‘Ahmed Versus Baerveldt Study’ and the ‘Ahmed Baerveldt comparison study’. Other (non-RCT) studies had a follow-up ranging from 0.5 to 5 years, covered a mix of glaucoma types and generally compared the superotemporal placed, perioperatively occluded Baerveldt 350mm (or 250mm) implant to other interventions.
Postoperative IOP Reduction
Of the included studies, 8 articles presented the postoperative IOP reduction in mmHg with standard deviation or 95% CI interval (Figure 2). At 1 day postoperative, the IOP reduction (WMD 95% CI) was 6.90 mmHg (5.65–8.15), which remained stable till 1 month. Thereafter the IOP reduction increased strongly to 11.42 mmHg (10.64–12.19) at 6 months postoperative. The reduction in IOP then became somewhat less but kept increasing the following years. At 1 and 3 years postoperative both the IOP reduction (WMD 95% CI) were 12.66 mmHg (11.93–13.39) and 12.69 mmHg (11.91–13.47), respectively. At the last follow-up point, at 5 years postoperative, the WMD (95% CI) reduction in IOP was 15.57 mmHg (14.43–16.71; Figure 3).
Postoperative IOP-Lowering Medication Reduction
A reduction in postoperative IOP-lowering medication was presented by 7 of the included studies (Figure 4). At 1 day postoperative, the reduction in number of IOP-lowering medication (WMD [95% CI]) was 1.90 (1.53–2.27), however, only one study reported this. At 1 week postoperative, the medication reduction increased to 2.09 (1.92–2.25). From 1 month postoperative till the last follow-up point, at 5 years postoperative, the WMD (95% CI) reduction of IOP-lowering medication remained stable 1.86 (1.69–2.03) and 1.81 (1.61–2.01; Figure 3), respectively.
Perioperative and Postoperative Complications
The peri- and postoperative complications from RCT and non-RCT studies were listed with their corresponding ranges of incidence (Table S2). Perioperative complications were reported by two of the RCT studies. Hyphema (1–3%) and scleral perforation (3%) were the most frequently recorded perioperative complications. Early postoperative complications were reported by two RCT and three non-RCT studies for the first 3 months postoperative.
For the postoperative complications, corneal edema had the highest incidence range. In 12–34% of all recorded patients this complication occurred with according to one article, 22% occurring in the first 3 months. Tube complications had the next highest incidence range of up to 33% (16% in the first 3 months). Tube complications consisted mainly out of (unintended) tube occlusion, tube erosion, and tube malposition. Other complications that were frequently recorded were hyphema (2–19%), diplopia or motility disorders (2–17%). Cataract progression was excluded by most articles from their complication analysis, two studies however reported cataract progression in 41% of their operated patients. In total, the rates of required re-intervention ranged from 22–51% across all analyzed RCT studies.
The incidence of more severe complications was notably less with endophthalmitis or blebitis at 1–2%, retinal detachment at 1–2% and phthisis bulbi at 2–6% of all eyes. In 6% of all operated eyes (a progression to) no light perception occurred.
For the additional nine (non-RCT) studies, incidence ranges are relatively similar although somewhat higher. Notable exceptions are diplopia/motility disorder and corneal edema, which are recorded less frequently at only 4–9% and 2–19% respectively. Also tube complications (4–13%) and cataract progression (4–6%) were reported less in the non-RCT studies. In total, re-intervention was required at 0–19% of all performed Baerveldt implantations reported in the additional non-RCT studies.
Discussion
This review summarizes published findings concerning the Baerveldt-350 GDD. At the last follow-up point, at 5 years postoperative, the mean (95% CI) reduction in IOP was 15.57 mmHg (14.43–16.71) and the mean (95% CI) reduction in IOP-lowering medication after surgery was 1.81 (1.61–2.01). Most frequently observed postoperative complications were corneal edema (2–34%) and tube complications (4–33%), both early and late/persisting in follow-up. In 0–51% of all recorded follow-up patients re-intervention was required.
Efficacy
At one month after surgery, the Baerveldt implant showed a relatively small IOP reduction of 7.0 mmHg. After approximately 6–8 weeks the suture tied around the tube resolves and the flow through the tube starts, resulting in a higher decrease in IOP. At 6 months after surgery the IOP reduction greatly improves, with a final reduction of 15.57 mmHg from baseline at 5 years follow-up. For the RCT articles, this corresponds with an IOP reduction from baseline between 41–55% at 5 years follow-up. However, the increased IOP reduction at 5 years follow-up (Figure 3) is likely caused by currently unavailable 5 year data from the Primary Tube Versus Trabeculectomy study.5,6 According to Gedde et al,26 the Primary Tube Versus Trabeculectomy study has a lower baseline IOP resulting in lower IOP reduction, causing a lower mean IOP reduction on other follow-up points in the meta-analysis (Figure 2).
The reduction in IOP-lowering medication fluctuates during the first month, but remains relatively constant with a mean reduction of 1.81 IOP-lowering medication from baseline at 5 years of follow-up.
When compared to other common treatment options, results were similar. In the Ahmed Baerveldt Comparison study, it is reported that for the first month the Ahmed FP7 delivered a greater reduction in IOP and IOP-lowering medication, but the Baerveldt achieved a greater decrease afterwards.13,14 At 5 years of follow-up they report an approximately 2 mmHg-lower IOP for the Baerveldt group. In the Ahmed Versus Baerveldt study by Christakis et al,11,12 slightly better IOP and medication reduction were reported for the Baerveldt group. When compared to trabeculectomy in the Tube versus trabeculectomy study,7,10 they reported a difference in IOP reduction of approximately 2 mmHg in favor of trabeculectomy.
In the long term, the IOP-lowering potential of the Baerveldt implant is even somewhat better than that of the other frequently used Ahmed FP7, which might be explained by the fact that the implant endplate of the Baerveldt implant is bigger than that of the Ahmed implant. Studies between different Baerveldt sizes and between Molteno implant variations show that higher success rates and greater long-term IOP control are found when devices with larger endplates are implanted.16,27,28 Another possible explanation could be that with the Baerveldt implant, the initially tied tube results in less exposure of the bleb to inflammatory cells, cytokines, and proteins post-surgery, resulting in less scarring of the fibrous capsule surrounding the end plate when compared to the Ahmed implant.27
Safety
Overall, relatively high rates with wide ranges of postoperative complications were reported.
However, when looking at other common treatment options, complications are often reported at similar or just slightly lower incidences. The Ahmed versus Baerveldt study reported a slightly lower complication rate for the Ahmed FP7 but an identical percentage of postoperative interventions.12 A larger number of complications attributed to an early onset hypotony were seen in the Baerveldt group, likely due to the absence of a built-in flow restriction. In the Ahmed Baerveldt Comparison study, a higher percentage of serious complications leading to postoperative surgical interventions was reported in the Bearveldt group. Surgical re-intervention was required 5% more with the Baerveldt implant than with the Ahmed implant, mainly due to hypotony (related) difficulties.15 Other frequent complications however, such as corneal edema and diplopia, were seen just as often in both groups. When compared to trabeculectomy in the Tube versus trabeculectomy study, they report a large number of complications for both interventions albeit mostly transient and self-limiting.10 Early postoperative complications were reported 16% more frequent following trabeculectomy than in the Baerveldt group. Wound leak in particular was more common after trabeculectomy resulting from the immediate filtration. Because of the smaller surgical opening and the fact that this opening is commonly covered by a patch (donor sclera or other tissue), the incidence of wound leakage is much lower. At 5 year follow-up however, further complications, re-interventions and secondary cataract progression were similar for both groups.
Place in Therapy
Of all commonly used GDD’s the IOP-lowering effect of the Ahmed FP7 has the fastest effect in the short term (similar to trabeculectomy).7,8,13,14 The Ahmed FP7 decreases IOP to a greater degree in the early postoperative period compared to the Baerveldt-350.29 Nevertheless, in the long term the Baerveldt-350 had a greater IOP-reduction and a similar incidence of glaucoma reoperations than the Ahmed FP7 after 5 years of follow-up.12,14,15 According to the Tube versus trabeculectomy study, the trabeculectomy resulted in a statistically significant lower IOP than Baerveldt-350.8,10 Whether this difference of 2 mmHg is clinically significant remains debatable. Therefore, according to current scientific evidence, the trabeculectomy might be in favor as surgical therapy of primary open-angle glaucoma. However, for secondary causes of glaucoma (e.g. uveitic or traumatic glaucoma) the fibrosis or scarring can be challenging. Also trabeculectomy can be more demanding in terms of follow-up appointments, especially in the early postoperative period. More checkups are required due to e.g. risk of wound leakage, over- or underfiltration or removal of sutures, resulting in more load on both doctor and patient when compared to the GDD’s with a somewhat more predictable postoperative course. Moreover, as the difference in efficacy between the Baerveldt-350 and the trabeculectomy is very low and the safety of the Baerveldt-350 might be better in terms of fibrosis due to e.g. uveitis, the Baerveldt (or any other GDD) would be in favor in these cases.29 Unfortunately, RCT studies on trabeculectomy vs Ahmed FP7 are lacking, making it hard to choose a specific GDD.
Conclusion
In conclusion, the Baerveldt-350 glaucoma implant device has been available for a relatively long time. It is effective in lowering IOP and has the highest long-term IOP-lowering potential of all assessed GDD’s. Furthermore, its complication incidence seems similar to other GDD’s and shows all-round good performance. For treatment of secondary open-angle glaucoma tube implants are first choice, stated characteristics make the Baerveldt implant the GDD of choice in patients where the highest IOP reduction is desired. However, the relatively small number of randomized controlled trials still results in the desire for more trials. Moreover, the high rates of complications warrant comparisons to newer minimally invasive glaucoma implants.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hertzog LH, Albrecht KG, LaBree L, Lee PP. Glaucoma care and conformance with preferred practice patterns: examination of the private community-based ophthalmologist. Ophthalmol. 1996;103(7):1009–1013.
2. Yadav KS, Sharma S. Implantable drainage devices in glaucoma: quo vadis? Eur J Pharm Sci. 2019;133:1–7.
3. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505–1516.
4. Hodkin MJ, Goldblatt WS, Burgoyne CF, Ball SF, Insler MS. Early clinical experience with the Baerveldt implant in complicated glaucomas. Am J Ophthalmol. 1995;120(1):32–40.
5. Gedde SJ, Feuer WJ, Shi W, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Graefes Arch Clin Exp Ophthalmol. 2018;125(5):650–663.
6. Gedde SJ, Feuer WJ, Lim KS, et al. Treatment outcomes in the Primary Tube Versus Trabeculectomy (PTVT) study after 3 years of follow-up. Ophthalmology. 2019.
7. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143(1):9–22.
8. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e2.
9. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Surgical complications in the Tube Versus Trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2007;143(1):23–31.
10. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–14.e1.
11. Christakis PG, Kalenak JW, Zurakowski D, et al. The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology. 2011;118(11):2180–2189.
12. Christakis PG, Kalenak JW, Tsai JC, et al. The ahmed versus baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093–2102.
13. Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt comparison study after 1 year of follow-up. Ophthalmology. 2011;118(3):443–452.
14. Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–316.
15. Budenz DL, Feuer WJ, Barton K, et al. Postoperative complications in the Ahmed Baerveldt comparison study during five years of follow-up. Am J Ophthalmol. 2016;163(7582):e3.
16. Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology. 1999;106(12):2312–2318.
17. Iwasaki K, Arimura S, Takihara Y, Takamura Y, Inatani M. Prospective cohort study of corneal endothelial cell loss after Baerveldt glaucoma implantation. Acta Ophthalmol. 2018;13(7):e0201342.
18. Chansangpetch S, Surukrattanaskul S, Tapaneeyangkul P, Tantisevi V. Hypertensive phase and its association with surgical outcomes in Baerveldt implantation. Int Ophthalmol. 2018;38(4):1717–1725.
19. Rai AS, Shoham-Hazon N, Christakis PG, Rai AS, Ahmed IIK. Comparison of the Ahmed and Baerveldt glaucoma shunts with combined cataract extraction. Can J Ophthalmol. 2018;53(2):124–130.
20. Resende AF, Moster MR, Patel NS, et al. Ahmed versus baerveldt glaucoma drainage implantation in patients with markedly elevated intraocular pressure (>/=30 mm Hg). Graefes Arch Clin Exp Ophthalmol. 2016;25(9):738–743.
21. Panarelli JF, Banitt MR, Gedde SJ, Shi W, Schiffman JC, Feuer WJ. A retrospective comparison of primary baerveldt implantation versus trabeculectomy with mitomycin C. Ophthalmology. 2016;123(4):789–795.
22. Iverson SM, Bhardwaj N, Shi W, et al. Surgical outcomes of inflammatory glaucoma: a comparison of trabeculectomy and glaucoma-drainage-device implantation. Jpn J Ophthalmol. 2015;59(3):179–186.
23. Allan EJ, Khaimi MA, Jones JM, Ding K, Skuta GL. Long-term efficacy of the Baerveldt 250 mm2 compared with the Baerveldt 350 mm2 implant. Ophthalmology. 2015;122(3):486–493.
24. Wang JC, See JL, Chew PT. Experience with the use of Baerveldt and Ahmed glaucoma drainage implants in an Asian population. Ophthalmology. 2004;111(7):1383–1388.
25. Seah SK, Gazzard G, Aung T. Intermediate-term outcome of Baerveldt glaucoma implants in Asian eyes. Ophthalmology. 2003;110(5):888–894.
26. Gedde SJ, Feuer WJ, Chen PP, Heuer DK, Singh K, Wright MM. Comparing treatment outcomes from the tube versus trabeculectomy and primary tube versus trabeculectomy studies. Ophthalmology. 2020.
27. Gedde SJ, Panarelli JF, Banitt MR, Lee RK. Evidenced-based comparison of aqueous shunts. Curr Opin Ophthalmol. 2013;24:87–95.
28. Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99:1512–1519.
29. Ramdas WD, Pals J, Rothova A, Wolfs RCW. Efficacy of glaucoma drainage devices in uveitic glaucoma and a meta-analysis of the literature. Graefes Arch Clin Exp Ophthalmol. 2019;257(1):143–151.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



