Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
The Association of Telangiectasias with Other Peripheral Vascular Lesions of Systemic Sclerosis
Authors Bobeica C , Niculet E , Musat CL, Iancu L, Craescu M, Luca AM, Stefanescu BI , Gheorghe E, Debita M , Vasile CI , Balan G, Busila C, Tatu AL
Received 1 October 2023
Accepted for publication 25 December 2023
Published 26 January 2024 Volume 2024:17 Pages 211—218
DOI https://doi.org/10.2147/CCID.S432422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Carmen Bobeica,1,* Elena Niculet,2,3,* Carmina Liana Musat,2,* Lina Iancu,4 Mihaela Craescu,2,3 Andreea Mioara Luca,5 Bogdan Ioan Stefanescu,6 Emma Gheorghe,7 Mihaela Debita,8 Claudiu-Ionut Vasile,8 Gabriela Balan,8– 10 Camelia Busila,8 Alin Laurentiu Tatu3,8,11
1Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, 800008, Romania; 2Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, 800008, Romania; 3Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID), “Dunărea de Jos” University, Galați, Romania; 4Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, 800008, Romania; 5Department of Plastic Surgery, “Sf. Ioan” Clinical Emergency Hospital for Children, Galați, 800487, Romania; 6Clinical Surgical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 7Department No. 1 (Preclinical), Faculty of Medicine and Pharmacy, “Ovidius” University, Constanța, 900527, Romania; 8Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, 800008, Romania; 9Department of Gastroenterology, “Sf. Apostol Andrei” County Emergency Clinical Hospital, Galaţi, 800578, Romania; 10Research Center in the Field of Medical and Pharmaceutical Sciences, “Dunărea de Jos” University, Galaţi, 800008, Romania; 11Dermatology Department, “Sf. Cuvioasa Parascheva” Clinical Hospital of Infectious Diseases, Galați, 800179, Romania
*These authors contributed equally to this work
Correspondence: Elena Niculet; Carmina Liana Musat, Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University of Galați, 35 Alexandru Ioan Cuza Street, Galați, 800216, Romania, Tel +40741398895 ; +40723338438, Email [email protected]; [email protected]
Purpose: Systemic sclerosis (SSc) is a relatively rare collagenosis manifested as microvasculopathy, excessive cutaneous and visceral fibrosis in a background of autoimmune alteration. Autoimmune vasculopathy in SSc occurs early and begins with endothelial cell activation followed by blood vessel intimal proliferation in a context of defective angiogenesis. The alteration of peripheral micro and macrocirculation in SSc is evident through vascular lesions, such as Raynaud’s phenomenon, telangiectasias, acrocyanosis, digital ulcers, gangrene, peripheral pulse deficiency. Our paper details the results of the study on the association between telangiectasias and other types of immune-mediated peripheral vascular lesions that can be identified in SSc. The presence of these peripheral vascular lesions can provide information about the magnitude of the peripheral vasculopathy.
Patients and Methods: A total of 37 patients diagnosed with SSc, recruited from a university clinic in Bucharest between February 2019 and March 2020, were enrolled in an observational study. We evaluated the presence of telangiectasias, as a stigma of autoimmune microvasculopathy, and their association with other immune-mediated peripheral vascular lesions that may be present in SSc.
Results: The presence of telangiectasias was identified in the absence, but especially in the presence of acrocyanosis and digital ulcerations, and patients with peripheral pulse deficiency almost always had telangiectasias. Less than a quarter of the patients with digital ulcers progressed unfavorably to gangrene, and only one required amputation, telangiectasias being present not only in the patient with amputation but in all patients with gangrene.
Conclusion: We appreciate that telangiectasias may be the clinical expression of peripheral vasculopathy characteristic of SSc, they can often be present in association with other peripheral vascular lesions and may represent a valuable indicator for the gangrene risk of digital ulcerations in SSc.
Keywords: systemic sclerosis, vascular lesions, phenomenon Raynaud’s, telangiectasias, acrocianosis, digital ulcers
Introduction
Systemic sclerosis (SSc) is a chronic inflammatory disease with autoimmune determinism included in the collagenoses group. Vasculopathy and skin and visceral fibrosis are the two links in the pathogenic chain of SSc.1 Due to defective angiogenesis, the vascular alteration in SSc is on one hand proliferative-obliterative in nature; it occurs by arteriolar occlusion followed by fibrous proliferation, but at the same time it is also destructive because arterioles and capillaries are lost. Vasculopathy is characteristic of the pathogenesis in SSc along with skin and visceral fibrosis and begins at the peripheral level. Peripheral vasculopathy induces fibrosis. Peripheral vasculopathy in SSc is the expression of inflammation of the vascular wall in the context of autoimmune alteration. Inflammation of the vascular wall causes vascular occlusion and peripheral vasospasm.2 Raynaud’s phenomenon represents the recurrent vasospasm of the digital arteries and is triggered by exposure to cold or emotions. Characteristic for SSc is the secondary Raynaud’s phenomenon, which associates structural changes of the vascular wall compared to the primary one, which is only a functional vasospasm.3 The autoimmune process-vascular lesion interdependency maintains the chronic inflammation and determines the self-propagation of the autoimmune disorder.4–7 Thus, the altered immune response induces the development of anti-endothelial antibodies which mediate microvascular injury. In turn, damaged endothelial cells maintain the autoimmune process by providing molecules known as damage-associated molecular patterns (DAMPs).4,7,8 DAMPs are molecules characteristic for endothelial damage and have a chemotactic role for immune cells. Early in this process, the adhesion molecules on the cell surface are altered and allow perivascular infiltration with LyTh and other immune cells such as monocytes and macrophages. Immune cells release cytokines with pro-inflammatory potential, especially IL-6, which is an indicator for disease severity.4,9–12 The abundance of immune cells determines increased serum levels of cytokines and chemokines which are involved in fibrogenesis.4,13–16 The role of Ly Th2 is essential in the pathogenesis of SSc. LyTh2 secretes IL-4 and IL-13 involved in LyB proliferation as a source of IgG-type antibodies and adhesion molecule.4,17–20 Under LyTh2 control, fibroblasts and macrophages release TGFβ, the key element that induces fibroblast activation involved in excessive fibrosis.11–13,21 Some authors noted low levels of IL-35 in patients with SSc, while for TGFβ, IL-10 and IL −17 increased plasma levels were recorded.22
Although the pathogenesis of SSc is partially known, it is still not clear how this disease begins.4,23–25 Peripheral microvasculopathy is the result of defective angiogenesis that induces cellular hypoxia by reducing blood flow. Vascular endothelial growth factor (VEGF) is responsible for altering angiogenesis.1,12,26,27
The plasma level of VEGF is not always increased in the conditions of tissue ischemia in SSc.1,28,29 Some authors found low level of VEGF in patients with active digital ulcers and increased levels of VEGF in those with healed ulcers.1,12,30
Raynaud’s phenomenon is often the first clinical manifestation of microangiopathy in SSc and it is almost always present in SSc.4,31 Studies have shown that the vasospasm characteristic of Raynaud’s phenomenon is present not only at the peripheral blood circulation level, but also at the level of the visceral microcirculation: lungs, kidneys, heart.1,32 Peripheral microangiopathy involves the prioritized damage to arterioles and capillaries. Over time, the endothelial injury is followed by ectasia and rupture until the loss of capillaries through hypoxia and the generation of reactive oxygen species.4,33,34
The smooth muscle fibers in the walls of the blood vessels proliferate, the intima of the vessels thickens, and the basal membrane doubles in thickness. It is assumed that the endothelial-mesenchymal transition has a crucial role in the pathogenesis of SSc.4,33
Telangiectasia represents one of the five clinical elements of the CREST syndrome. According to multiple studies, telangiectasia is present not only in CREST syndrome, but also in the two subsets of SSc, limited and diffuse.35,36 Telangiectasias are dilatations of the blood capillaries present on the mucous or skin on the face, hands and even chest caused by inflammation of the vascular wall. These are the expression of autoimmune peripheral microvasculopathy in SSc and their presence in large numbers indicates a risk of pulmonary arterial hypertension.37
Telangiectasias are also present in other diseases. Thus, multiple telangiectasias in rosacea reflect vascular changes and can lead to changes in the distribution of the skin microbiome.32
Digital ulcerations and other peripheral microvascular and macrovascular lesions, such as acrocyanosis, telangiectasias and peripheral pulse deficit, but also visceral vascular lesions are the clinical expression of autoimmune microvasculopathy in SSc. Acrocyanosis is the blue coloration of one or more distal phalanges of the hands and is a characteristic element for SSc that expresses vascular occlusion or peripheral vasospasm.38,39
Considering the unclear aspects of autoimmune vasculopathy, we evaluated the presence of telangiectasias and their association with other immune-mediated peripheral vascular lesions in SSc in order to establish possible correlations.
Materials and Methods
In our study, 37 patients diagnosed with SSc were enrolled according to the criteria issued in 2013 by the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR).40 The study was approved by the Ethics Council of the Hospital “St. Maria” from Bucharest (approval number 5213 of 04.04.2019). The patients were hospitalized in the rheumatology and internal medicine departments of the “Sf Maria” Bucharest Clinical Hospital between February 2019 and March 2020.
The criteria for inclusion in the study were based on the criteria for the positive diagnosis of SSc. Patients diagnosed with the two subsets of SSc, limited and diffuse, as well as patients with overlap syndromes between SSc and other autoimmune diseases were included in the study. Cases of SSc “sine scleroderma” were not excluded from the study, but no case was registered.
The data were obtained from the patients’ clinical files and during the clinical examination after obtaining their informed consent. The study procedures were in accordance with the Declaration of Helsinki. In this observational study, we evaluated the presence of peripheral vascular lesions in relation to SSc subsets and the association between telangiectasias, as a stigma of affected capillaries, and other types of vascular lesions.
The statistical data were processed through SPSS 24.0 (IBM Corp.) and Microsoft Excel was used. The qualitative data were represented by the distribution frequencies together with the contingent table, and the quantitative data were expressed by descriptive statistics. In order to compare samples, the Chi-square test was used. The p-values had 2 tails and a significant value was considered at p < 0.05.
In this observational study, we evaluated the presence of telangiectasias, and their association with other immune-mediated peripheral vascular lesions that may be present in SSc.
Results
In our study, we analyzed the association of telangiectasias (as a sign of damage to small blood vessels) with other peripheral vascular lesions and complications characteristic of SSc, such as Raynaud’s phenomenon, acrocyanosis, diminution or absence of peripheral pulse, digital ulcerations with unfavorable evolution towards gangrene and even amputation.
We also studied the frequency distribution of telangiectasias by disease subsets, limited and diffuse SSc. Since SSc is included in the group of rare diseases,41 a small number of only 37 patients aged between 28 and 76 years were enrolled in this observational study. The investigated group consists mainly of women (81.1%) from the urban environment (64.9%) and comes from the south-eastern region of Romania.
With regards to all of the investigated elements, higher proportions were observed in patients with telangiectasias compared to those without telangiectasias, the discrepancies being sometimes even very pronounced. However, the statistical significance of these discrepancies is limited, the main reason being the small number of cases that could be investigated. Thus, we found the following:
Among patients with telangiectasias, 68.0% are with diffuse SSc and 32.0% with limited SSc, compared to patients without telangiectasias, who belong equally to the subsets of diffuse and limited SSc. Thus, it can be stated that telangiectasias are predominantly present in patients with diffuse SSc, although the differences found between patients with telangiectasias and those without telangiectasias do not reach the threshold of statistical significance (Table 1).
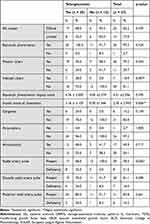 |
Table 1 Telangiectasias and Other Peripheral Vascular Lesions - Correlations and Associations |
Telangiectasias are not present in all patients with SSc. We looked for the association of telangiectasia with other lesions suggestive of digital vasculopathy to identify the magnitude of peripheral microvasculopathy.
Although telangiectasias are characteristic for CREST syndrome and limited SSc, our study identified them especially in diffuse SSc for which they have no specificity. The presence of telangiectasias in diffuse SSc was also reported by other authors35 and suggests that vascular ectasias could be an indicator for the more severe vascular alteration in diffuse SSc, known to have a dark prognosis compared to limited SSc.42
All patients, with one exception, have Raynaud’s phenomenon, and the patient without Raynaud’s phenomenon belonged to the limited SSc subset. Among patients with Raynaud’s phenomenon, over 69.4% have telangiectasias, and the patient without Raynaud’s phenomenon does not have telangiectasias (Table 1). On the other hand, Raynaud’s phenomenon was present in patients without telangiectasias in a very high percentage (91.7%), but we cannot discuss about a statistically significant difference between patients with or without telangiectasias regarding the occurrence of Raynaud’s phenomenon (Table 1).
The Raynaud’s phenomenon impact score is 4.76 ± 2.650 in patients with telangiectasias, with a range of 1 to 9 and a median value of 5.00, while the similar impact score in patients without telangiectasias is slightly lower (4.00 ± 2.374), with a maximum value of 8 and also lower median (4.00); although patients with telangiectasias have a higher impact score than the others, this difference cannot be characterized as statistically significant (Table 1).
Regarding the situation of digital ulcerations, it can be certainly stated that they are more frequent and in more severe forms in patients with telangiectasias, as compared to others, although even in this case the statistical significance was not identified, except in isolated situations. Thus, 76.0% of patients with telangiectasias presented with ulcerations, as compared to only 58.3% of the others, but due to the limited case series, statistical significance could not be established.
A particular situation is represented by infected ulcerations, identified in almost a third (28.0%) of patients with telangiectasias and none of the others; this time, the difference between the two categories of patients is important and statistically significant (Table 1).
The impact score of ulcerations in patients with telangiectasias is 3.16 ± 3.105, with a range of variation between 0 and 9 and a median value equal to 3.00, this time being significantly higher compared to the impact score of ulcerations in patients without telangiectasias, with a mean value of 0.50 ± 1.446 and a maximum of 5. The statistical significance observed in this case therefore allows us to state that the impact of ulcerations is much more pronounced in patients with telangiectasias compared to the others (Table 1).
Regarding complications, they are again more common in patients with telangiectasias. Among patients with telangiectasias, 24.0% presented digital ulcers that progressed unfavorably towards gangrene, and in the group of patients without telangiectasias, no cases of gangrene were registered. Although the difference is obvious, statistical significance is not reached.
Of the 6 patients with gangrene, only one required amputation. Thus, we appreciate that telangiectasias were present in all patients with gangrene and implicitly, in the patient with amputation. In relation to the cases of telangiectasias, amputation was performed in only one of the 25 patients with telangiectasias, ie 4.0%, and again in none of those without telangiectasias (Table 1). This result indicates the presence of a risk of gangrene with amputation in patients with telangiectasias in the context of systemic sclerosis.
Telangiectasias have been identified in association with acrocyanosis, another peripheral vascular lesion. Acrocyanosis was present more frequently than gangrene, being observed in 48.0% of patients with telangiectasias and in 41.7% of those without telangiectasias (Table 1).
Peripheral pulse detection is diminished in patients with telangiectasias compared to the others. Thus, a radial pulse deficit was identified in 32.0% of the patients with telangiectasias, while no patient without telangiectasias had a radial pulse deficit, the difference being statistically significant. Be analyzing the pulse at the dorsalis pedis artery, it is observed that 24.0% of the patients with telangiectasias have a pulse deficit at the dorsalis pedis artery, compared to 8.3% of the others without telangiectasias (Table 1).
In the case of anterior tibial pulse, a similar balance is observed. Only 20.0% of patients with telangiectasias have anterior tibial pulse deficiency, compared to 8.3% of patients without telangiectasias.
Discussion
The present study analyzed the clinical elements of SSc that reflect the peripheral vascular alteration. Taking telangiectasias as a benchmark, we analyzed its associations with other lesions and vascular complications in order to optimize the quantification of vascular alteration. Although without reaching statistical significance, telangiectasias are more frequent in the subset of diffuse SSc, known as a form of SSc with multiple visceralizations and with a dismal prognosis.43 This result suggests that telangiectasies can probably be present only in association with Raynaud’s phenomenon. It seems that the peripheral vasospasm from Raynaud’s phenomenon occurs first and then the vascular dilations characteristic of telangiectasias appear. Since Raynaud’s phenomenon was present in almost all patients with SSc, we highlight that telangiectasias were not present in the patient without Raynaud's phenomenon, but to conclude that telangiectasias are present only in association with Raynaud’s phenomenon, an extended study on larger groups of patients is necessary. Also, the presence of telangiectasias suggests a greater degree of vascular alteration that predisposes patients to the development of active, persistent digital ulcers, with unfavorable evolution towards bacterial infections, gangrene and even amputation. This observation is supported by the presence of telangiectasias in all patients with gangrene and amputation. We appreciated that telangiectasias can represent an indicator for the unfavorable evolution of digital ulcerations by the presence of vasculopathy. Also, we believe that active, unhealed digital ulcerations with a risk of superinfection are maintained by the vascular alteration highlighted by telangiectasias presence. In a somewhat similar way, studies have shown that digital ulcerations with unfavorable evolution towards infection, osteomyelitis and gangrene reflect a dark prognosis of the disease due to the presence of vascular alteration.44 Telangiectasia itself is the expression of peripheral vascular alteration evident at the skin level and is a clinical diagnostic criterion for SSc.40 We can say that the presence of telangiectasias can be an indication for the persistence and unfavorable evolution of digital ulcers towards infections and even gangrene.
Most cases of acrocyanosis and peripheral pulse deficiency are associated with the presence of telangiectasias. Therefore, we appreciate that the peripheral vascular alteration in SSc is manifested complexly by vascular dilatations and persistent vasospasm characteristic of acrocyanosis. Moreover, we noted that the radial pulse deficit can only be present in patients with telangiectasias. Regarding the peripheral pulse, the detection of a pulse deficit is more important in patients with telangiectasias compared to those without telangiectasias, although the discrepancies are small.
All these results classify telangiectasias as a good indicator for peripheral vascular alteration with some degree of severity and opens the opportunity for future studies on peripheral vascular lesions in SSc. Although there are several therapeutic targets, it is essential to identify new and truly effective therapies that improve the autoimmune vascular alteration.
Conclusion
The pathogenesis of SSc is not completely known. Although there is no treatment to cure the disease, new molecules are under study to improve the therapeutic management of SSc. In this context, establishing new correlations between the different vascular elements of SSc is valuable for improving the management and prognosis of the disease since multiple telangiectasias represent a risk factor for pulmonary arterial hypertension.37 Numerous studies are needed to identify the etiology and entire pathogenic chain of vasculopathy, but also different associations of peripheral vascular lesions in order to anticipate the risk of complications. The evaluation of peripheral vascular lesions in patients with SSc is essential for the direction and optimization of therapeutic management.
Acknowledgments
The authors wish to acknowledge that the present study was academically supported by the “Dunărea de Jos” University of Galați, Romania, through the research center – Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The article publishing charge was paid by the “Dunărea de Jos” University of Galati, Romania.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Waszczykowska A, Goś R, Waszczykowska E, Dziankowska-Bartkowiak B, Podgórski M, Jurowski P. The role of angiogenesis factors in the formation of vascular changes in scleroderma by assessment of the concentrations of VEGF and sVEGFR2 in blood serum and tear fluid. Mediators Inflamm. 2020;2020:7649480. doi:10.1155/2020/7649480
2. Asano Y, Sato S. Vasculopathy in scleroderma. Semin Immunopathol. 2015;37(5):489–500. doi:10.1007/s00281-015-0505-5
3. Ruaro B, Smith V, Sulli A, et al. Innovations in the assessment of primary and secondary Raynaud’s phenomenon. Front Pharmacol. 2019;10:360. doi:10.3389/fphar.2019.00360
4. Rosendahl AH, Schönborn K, Krieg T. Pathophysiology of systemic sclerosis (scleroderma). Kaohsiung J Med Sci. 2022;38(3):187–195. doi:10.1002/kjm2.12505
5. Hasegawa M, Sato S, Ihn H, Takehara K. Enhanced production of interleukin-6 (IL-6), oncostatin M and soluble IL-6 receptor by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Rheumatology. 1999;38(7):612–617. doi:10.1093/rheumatology/38.7.612
6. Branisteanu DE, Pirvulescu RA, Spinu AE, et al. Metabolic comorbidities of psoriasis (Review). Exp Ther Med. 2022;23(2):179. doi:10.3892/etm.2021.11102
7. Toledo DM, Pioli PA. Macrophages in systemic sclerosis: novel insights and therapeutic implications. Curr Rheumatol Rep. 2019;21(7):31. doi:10.1007/s11926-019-0831-z
8. Cristodor PL, Nechifor A, Fotea S, et al. New antifibroblastic medication in dermatology: could nintedanib treat scarring? Int J Gen Med. 2022;15:7169–7172. doi:10.2147/IJGM.S377073
9. Roumm AD, Whiteside TL, Medsger TA, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27(6):645–653. doi:10.1002/art.1780270607
10. Niculet E, Chioncel V, Elisei AM, et al. Multifactorial expression of IL-6 with update on COVID-19 and the therapeutic strategies of its blockade (Review). Exp Ther Med. 2021;21(3):263. doi:10.3892/etm.2021.9693
11. Tatu AL, Nadasdy T, Arbune A, et al. Interrelationship and sequencing of interleukins 4, 13, 31 and 33 – an integrated systematic review: dermatological and multidisciplinary perspectives. J Inflamma Res. 2022;15:5163–5184. doi:10.2147/JIR.S374060
12. Bobeica C, Niculet E, Craescu M, et al. Hearing loss secondary to systemic sclerosis vasculopathy: case study with a short review. Clin Cosmet Invest Dermatol. 2022;15:967–973. doi:10.2147/CCID.S356818
13. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727–737. doi:10.1084/jem.170.3.727
14. Vasile CI, Vasile MC, Zlati ML, et al. Post COVID-19 Infection Psychosis: could SARS-CoV-2 Virus Infection Be a Neuropsychiatric Condition That Triggers Psychotic Disorders? - A Case-Based Short Review. Infect Drug Resist. 2022;15:4697–4705. doi:10.2147/IDR.S373578
15. Tatu AL, Nwabudike LC. Male genital lichen sclerosus — a permanent therapeutic challenge. J Am Acad Dermatol. 2018;79(3Suppl1):AB185. doi:10.1016/j.jaad.2018.05.750
16. Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24(2):328–332.
17. Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15(1):19–26. doi:10.1016/0167-5699(94)90021-3
18. Doucet C, Brouty-Boyé D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecules and inflammatory cytokine expression in human lung fibroblasts. Int Immunol. 1998;10(10):1421–1433. doi:10.1093/intimm/10.10.1421
19. Bobeica C, Niculet E, Halip AI, et al. Predictive value of immunological markers in systemic sclerosis. Exp Ther Med. 2021;22(3):994. doi:10.3892/etm.2021.10426
20. Tatu AL, Nwabudike LC. Bullous reactions associated with COX-2 inhibitors. Am J Ther. 2017;24(4):e477–e480. doi:10.1097/MJT.0000000000000569
21. Sharad J. Hyaluronic acid filler injection for localized scleroderma - Case report and review of literature on filler injections for localized scleroderma. Clin Cosmet Invest Dermatol. 2022;15:1627–1637. doi:10.2147/CCID.S356641
22. Osuna-Gómez R, Castellví I, Mulet M, Ortiz MÀ. Impaired regulation by IL-35 in systemic sclerosis. Int J Mol Sci. 2023;24(13):10567. doi:10.3390/ijms241310567
23. Massagué J. TGFβ signaling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630. doi:10.1038/nrm3434
24. Higashi-Kuwata N, Jinnin M, Makino T, et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther. 2010;12(4):R128. doi:10.1186/ar3066
25. Niculet E, Bobeica C, Tatu AL. Glucocorticoid-induced skin atrophy: the old and the new. Clin Cosmet Invest Dermatol. 2020;13:1041–1050. doi:10.2147/CCID.S224211
26. Sahin H, Wasmuth HE. Chemokines in tissue fibrosis. Biochim Biophys Acta. 2013;1832(7):1041–1048. doi:10.1016/j.bbadis.2012.11.004
27. Smith G, McLeod D, Foreman D, Boulton M. Immunolocalization of the VEGF receptors FLT-1, KDR, and FLT-4 in diabetic retinopathy. Br J Ophthalmol. 1999;83(4):486–494. doi:10.1136/bjo.83.4.486
28. Choi JJ, Min DJ, Cho ML, et al. Elevated vascular endothelial growth factor in systemic sclerosis. J Rheumatol. 2003;30(7):1529–1533.
29. Ozcelik O, Haytac MC, Ergin M, Antmen B, Seydaoglu G. The immunohistochemical analysis of vascular endothelial growth factors A and C and microvessel density in gingival tissues of systemic sclerosis patients: their possible effects on gingival inflammation. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 2008;105(4):481–485. doi:10.1016/j.tripleo.2007.07.021
30. Distler O, Del Rosso A, Giacomelli R, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002;4(6):R11. doi:10.1186/ar596
31. Waszczykowska A, Goś R, Waszczykowska E, Dziankowska-Bartkowiak B, Jurowski P. Assessment of skin microcirculation by laser Doppler flowmetry in systemic sclerosis patients. Postepy Dermatol Allergol. 2014;31(1):6–11. doi:10.5114/pdia.2014.40653
32. Tatu AL, Ionescu MA, Cristea VC. Demodex folliculorum associated Bacillus pumilus in lesional areas in rosacea. Indian J Dermatol Venereol Leprol. 2017;83(5):610–611. doi:10.4103/ijdvl.IJDVL_921_16
33. Suliman YA, Distler O. Novel aspects in the pathophysiology of peripheral vasculopathy in systemic sclerosis. Curr Rheumatol Rev. 2013;9(4):237–244. doi:10.2174/157339710904140417123932
34. Nwabudike LC, Tatu AL. Magistral prescription with silver nitrate and Peru Balsam in difficult-to-heal diabetic foot ulcers. Am J Ther. 2018;25(6):e679–e680. doi:10.1097/MJT.0000000000000622
35. Johnson SR, van den Hoogen F, Devakandan K, Matucci-Cerinic M, Pope JE. Systemic sclerosis: to subset or not to subset, that is the question. Eur J Rheumatol. 2020;7(Suppl 3):S222–S227. doi:10.5152/eurjrheum.2020.19116
36. Bobeica C, Niculet E, Craescu M, et al. CREST syndrome in systemic sclerosis patients - is dystrophic calcinosis a key element to a positive diagnosis? J Inflam Res. 2022;15:3387–3394. doi:10.2147/JIR.S361667
37. Adigun R, Goyal A, Hariz A. Systemic Sclerosis. Treasure Island (FL): SteatPearls Publishing; 2023.
38. Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013;65(8):1953–1962. doi:10.1002/art.37988
39. Lupu MN, Miulescu M, Stefanopol IA, et al. Effect of 2,6-diisopropylphenol and 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy) propane as Anesthetic. Rev.Chim. 2019;70(5):1888–1892.
40. Masi AT, Medsger TA Jr. Progress in the evolution of systemic sclerosis classification criteria and recommendation for additional comparative specificity studies. J Rheumatol. 2015;42(1):8–10. doi:10.3899/jrheum.141020
41. Tyndal AJ, Bannert B, Vonk M, et al. Causes and risk factors of death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi:10.1136/ard.2009.114264
42. Haustein UF. Systemic sclerosis-scleroderma. Dermatol Online J. 2002;8(1):3.
43. LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–205.
44. Giuggioli D, Magnani L, Spinella A, et al. Infections of scleroderma digital ulcers: a single center cohort retrospective study. Dermatol Reports. 2021;13(3):9075. doi:10.4081/dr.2021.9075
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
