Back to Journals » Risk Management and Healthcare Policy » Volume 16
The Association of Albumin-Bilirubin (ALBI) Grade with Mortality Risk in Trauma Patients with Liver Injuries
Authors Chou SE, Rau CS, Su WT, Tsai CH, Hsu SY, Hsieh CH
Received 11 November 2022
Accepted for publication 21 February 2023
Published 24 February 2023 Volume 2023:16 Pages 279—286
DOI https://doi.org/10.2147/RMHP.S397210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Sheng-En Chou,1 Cheng-Shyuan Rau,2 Wei-Ti Su,1 Ching-Hua Tsai,1 Shiun-Yuan Hsu,1 Ching-Hua Hsieh1
1Department of Trauma Surgery, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 2Department of Neurosurgery, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
Correspondence: Ching-Hua Hsieh, Tel +886-7-7327476, Email [email protected]
Introduction: The albumin-bilirubin (ALBI) grade objectively assesses liver function with better performance than the Child-Pugh and end-stage liver disease scores. However, the evidence is lacking on the ALBI grade in trauma cases. This study aimed to identify the association between the ALBI grade and mortality outcomes in trauma patients with liver injury.
Methods: Data from 259 patients with traumatic liver injury at a level I trauma center between January 1, 2009, and December 31, 2021 were retrospectively analyzed. Independent risk factors for predicting mortality were identified using multiple logistic regression analysis. Participants were characterized by ALBI score into grade 1 (≤ − 2.60, n = 50), grade 2 (− 2.60 < and ≤ − 1.39, n = 180), and grade 3 (> − 1.39, n = 29).
Results: Compared to survival (n = 239), death (n = 20) was associated with a significantly lower ALBI score (2.8± 0.4 vs 3.4± 0.7, p < 0.001). The ALBI score was a significant independent risk factor for mortality (OR, 2.79; 95% CI, 1.27– 8.05; p = 0.038). Compared with grade 1 patients, grade 3 patients had a significantly higher mortality rate (24.1% vs 0.0%, p < 0.001) and a longer hospital stay (37.5 days vs 13.5 days, p < 0.001).
Discussion: This study showed that ALBI grade is a significant independent risk factor and an useful clinical tool to discover liver injury patients who are more susceptible to death.
Keywords: trauma, liver injury, mortality, albumin-bilirubin grade, ALBI grade, liver function
Introduction
Accurate measurement of the liver functional reserve is difficult. To evaluate liver function, Pugh et al introduced the Child-Pugh score in 1973 as a prognostic indicator for liver cirrhosis.1 The Child-Pugh score comprises five factors (albumin, bilirubin, prothrombin/international normalized ratio (INR), magnitude of ascites, and stage of hepatic encephalopathy) and is one of the most commonly used systems for grading liver function in patients with hepatocellular carcinoma.2,3 However, critics of the Child-Pugh score reference the non-standardization of clinical parameters used for ascites and encephalopathy, as well as the simultaneous use of the interrelated variables, ascites and serum albumin level.4,5
The model for end-stage liver disease (MELD) score is a validated severity scoring system for predicting the survival of patients with chronic liver disease based on serum levels of bilirubin, creatinine, and the INR.3 However, the INR level variability between different laboratories significantly impacts the accuracy and consistency of the MELD score.6–8 The interlaboratory variability in the INR has the largest impact on the MELD score, with a mean difference of around 5 MELD points in most studies.6 This interlaboratory variability is also seen for serum creatinine levels and has disadvantages for certain patient populations, especially women.7
Indocyanine green clearance has been reported to assess liver functional reserve; however, it is time-consuming and expensive.9,10 Furthermore, although some serological tests for liver enzymes are commonly used in the clinical setting, they may reflect the degree of liver damage or dysfunction, but not the liver function reserve.2
In 2015, Johnson proposed the albumin-bilirubin (ALBI) grade to objectively assess liver function.11 This evidence-based model is calculated using serum levels of albumin and bilirubin with the following formula: 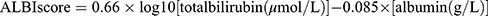 . Based on the ALBI score, patients could be stratified into classes with clearly different prognoses: grade 1 (≤ −2.60), grade 2 (−2.60 < and ≤ −1.39), and grade 3 (> −1.39) [10]. Compared with the Child-Pugh score, the ALBI grade has better predictive performance in evaluating liver function in patients with hepatocellular carcinoma.12–14 The ALBI grade also functions as a useful and valuable predictor for outcomes in early stage and intermediate-stage hepatocellular, as well as in many systemic interventions or treatments for hepatocellular carcinoma.15–20 It also functions as a crucial biomarker for the development of liver disease, reflecting the potential for hepatic failure and mortality from liver-related causes.21–27 Patients with cholangiocarcinoma,28 intrahepatic cholangiocarcinoma,29 pancreatic cancer with liver metastases,30 colorectal cancer with liver metastases,31 and primary biliary cholangitis32 can all benefit from knowing their ALBI grade as a prognostic indicator. Additionally, other non-hepatological disorders, including aortic dissection,33 heart failure,34,35 acute pancreatitis,36 lung cancer,24,37,38 esophageal cancer,39 gastric cancer,40 lung cancer,41 and medulloblastoma,42 have shown a high correlation between ALBI and death.
. Based on the ALBI score, patients could be stratified into classes with clearly different prognoses: grade 1 (≤ −2.60), grade 2 (−2.60 < and ≤ −1.39), and grade 3 (> −1.39) [10]. Compared with the Child-Pugh score, the ALBI grade has better predictive performance in evaluating liver function in patients with hepatocellular carcinoma.12–14 The ALBI grade also functions as a useful and valuable predictor for outcomes in early stage and intermediate-stage hepatocellular, as well as in many systemic interventions or treatments for hepatocellular carcinoma.15–20 It also functions as a crucial biomarker for the development of liver disease, reflecting the potential for hepatic failure and mortality from liver-related causes.21–27 Patients with cholangiocarcinoma,28 intrahepatic cholangiocarcinoma,29 pancreatic cancer with liver metastases,30 colorectal cancer with liver metastases,31 and primary biliary cholangitis32 can all benefit from knowing their ALBI grade as a prognostic indicator. Additionally, other non-hepatological disorders, including aortic dissection,33 heart failure,34,35 acute pancreatitis,36 lung cancer,24,37,38 esophageal cancer,39 gastric cancer,40 lung cancer,41 and medulloblastoma,42 have shown a high correlation between ALBI and death.
Currently, there are no studies on the usefulness of ALBI grade in trauma patients. Therefore, this study aimed to identify the association between the ALBI grade and mortality outcomes in trauma patients with liver injury. This retrospective study was performed using data collected from a registered trauma database from a level I trauma center.
Materials and Methods
Institutional Review Board Statement
The Chang Gung Memorial Hospital’s Institutional Review Board granted ethics approval (202201380B0), which complies with the Helsinki Declaration. Patients were not required to provide informed consent for this study as anonymous retrospective data was gathered after the acceptance of treatment with written consent from each patient.
Patient Inclusion and Clinical Variables
Of the 46, 808 registered trauma patients enrolled between January 1, 2009, and December 31, 2021 (Figure 1), 720 trauma patients had sustained liver injury (ICD-9-CM diagnosis code 864). After excluding patients aged less than 20 years (n = 157) and those with incomplete data for albumin or bilirubin (n = 304), 259 adult trauma patients with liver injuries were included in the study population. The ALBI score was calculated using the following formula: 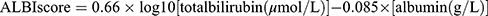 ; the study population was categorized into grade 1 (score ≤ −2.60, n = 50), grade 2 (−2.60 < score ≤ −1.39, n = 180), and grade 3 (score > −1.39, n = 29), respectively, according to the ALBI score.11 Medical information of the study population was retrieved from the registered trauma database of the hospital.43–45 We collected the following data: age; sex; body mass index (BMI), vital signs upon arrival at emergency room, trauma mechanism (blunt or penetration injury), level of albumin, bilirubin, glutamic oxaloacetic transaminase (GOT), and glutamate-pyruvate transaminase (GPT) at admission; preexisting comorbidities, including coronary artery disease (CAD), diabetes mellitus (DM), hypertension (HTN), end-stage renal disease (ESRD), Glasgow Coma Scale (GCS), injury severity score (ISS), length of hospital stay (LOS), and in-hospital mortality.
; the study population was categorized into grade 1 (score ≤ −2.60, n = 50), grade 2 (−2.60 < score ≤ −1.39, n = 180), and grade 3 (score > −1.39, n = 29), respectively, according to the ALBI score.11 Medical information of the study population was retrieved from the registered trauma database of the hospital.43–45 We collected the following data: age; sex; body mass index (BMI), vital signs upon arrival at emergency room, trauma mechanism (blunt or penetration injury), level of albumin, bilirubin, glutamic oxaloacetic transaminase (GOT), and glutamate-pyruvate transaminase (GPT) at admission; preexisting comorbidities, including coronary artery disease (CAD), diabetes mellitus (DM), hypertension (HTN), end-stage renal disease (ESRD), Glasgow Coma Scale (GCS), injury severity score (ISS), length of hospital stay (LOS), and in-hospital mortality.
 |
Figure 1 Flow chart illustrating the participant selection process and characterization into albumin-bilirubin (ALBI) grade 1 to 3. |
Statistical Analyses
We used SPSS version 23.0 for Windows (IBM Inc., Chicago, IL) for all statistical analyses. The two-sided Fisher’s exact test or Pearson’s χ2 test was used to compare categorical data. The Kolmogorov–Smirnov test was used to test the normalization of the distributed continuous variables. Unpaired Student’s t-test was performed to analyze normally distributed continuous data, whereas non-normally distributed continuous data were analyzed using the Mann–Whitney U-test. Normally and non-normally distributed continuous data were expressed as mean ± standard deviation and median with interquartile range (IQR, Q1–Q3), respectively. Multivariate logistic regression analysis was performed to identify the independent effects of univariate predictive variables on mortality in the study population, with the presentation of odds ratios (ORs) and 95% confidence intervals (CIs) of the risk factors associated with mortality. Statistical significance was set at p < 0.05.
Results
Comparison of Patient and Injury Characteristics in Death and Survival
As shown in Table 1, there were 20 deaths and 239 survivors in the study population. No significant difference in age, sex, and trauma mechanisms in blunt or penetration injuries was found between the patients who died and those who survived. The death patients had a significantly higher BMI (28.0±4.0 vs 24.5±4.4, p < 0.001) and heart rate (109.3±28.9 vs 95.1±19.6 beats/min, p = 0.003) upon arrival at emergency room than the survival patients. There was a significantly higher rate of patients with preexisting CAD, ESRD, and HTN in the death group than in the survival group. There were significantly lower levels of albumin and GPT in the death group than in the survivors; however, there were no significant differences in the levels of total bilirubin and GOT between the groups. Patients who died had a significantly lower ALBI score than those who survived (2.8±0.4 vs 3.4±0.7, p < 0.001). Patients who died had a significantly lower GCS score (median [Q1-Q3]; 8 [3–13] vs 15 [14–15], p < 0.001) but higher ISS (median [Q1-Q3]; 33 [24–40] vs 20 [13–29], p < 0.001) than the surviving patients.
 |
Table 1 Patient and Injury Characteristics Based on Mortality Outcomes in Patients with Traumatic Liver Injury |
Risk Factors for Mortality
As shown in Table 2, univariate logistic regression analysis revealed that pre-existing CAD, ESRD, HTN, BMI, heart rate upon arrival at emergency room, GCS, ISS, GPT level, and ALBI score were significant risk factors for mortality in adult trauma patients with liver injuries. Multivariate logistic regression analysis revealed that heart rate, GCS, ISS, GPT level, and ALBI score were significant independent risk factors for mortality. The increase of each ALBI score was associated with around 2.8- fold of mortality risk in the patients (OR, 2.79; 95% CI, 1.27–8.05; p = 0.038).
 |
Table 2 Univariate and Multivariate Analysis to Identify the Risk Factors for Mortality in the Trauma Patients with Liver Injury |
Comparison of Patient and Injury Characteristics Based on ABLI Grade
There was no significant difference in age or sex predominance among the three groups of patients (Table 3). There were no significant intergroup differences in the prevalence of preexisting comorbidities, except for a significantly higher rate of CAD in grade 3 patients than in grade 1 patients. The albumin levels of grade 2 and grade 3 patients were significantly lower, while the total bilirubin levels were significantly higher than those in Grade 1 patients. There were no significant intergroup differences in the GOT and GPT levels. A significantly higher ISS was observed in grade 3 patients than in grade 1 patients. Grade 3 patients had a significantly longer LOS in hospital (37.5 days vs 13.5 days, p < 0.001) and higher mortality rate (24.1% vs 0.0%, p < 0.001) than grade 1 patients.
 |
Table 3 Outcomes and Characteristics of the Trauma Patients with Liver Injury According to ALBI Grade |
Discussion
This study revealed that the ALBI score was a significant independent risk factor for mortality in traumatic liver injury. In our study, death was significantly associated with a lower ALBI score compared to survival. In addition, grade 3 patients had a significantly higher mortality rate and a longer length of hospital stay than grade 1 patients. ALBI grade could be a valuable tool for stratifying the mortality risk of adult trauma patients with liver injury.
In this study, the percentage of patients with grade 1, grade 2 and grade 3 ALBI scores was 7.7% (20/259), 69.5% (180/259), and 11.2% (29/259), respectively. These characteristics are similar to that of a study that initially established the ALBI grade based on the training set of the Japanese patient population,14 where 25%, 65%, and 10% of patients were classified as grade 1, grade 2, and grade 3, respectively.15 In addition, in the cancer patients undergoing liver resection, higher ALBI grades were associated with 2.06 odds of poor overall survival.46 In this study, in addition to commonly recognized risk factors for mortality in trauma patients such as increased heart rate, lower conscious level, and injury severity, the multivariate logistic regression analysis revealed that the ALBI score had a 2.79 odds of mortality risk in trauma patients with liver injury. The ALBI grade as a proxy for liver function explains mortality risk not only in patients with mild or early stage liver diseases receiving liver resection,10 but also in trauma patients with liver injury.
An increase in the serum level of GOT indicates parenchymal liver illness with liver-specific dysfunction because GPT is found predominantly in the cytosol of hepatocytes.47,48 Notably, this study investigated subjects with liver injury and revealed that there were significantly lower levels of GPT in patients who died than in those who survived, and multivariate logistic regression analysis also revealed that GPT level was a significant independent risk factor for mortality (OR, 0.97; 95% CI, 0.97–0.99). In addition, in the comparison among patients with different ABLI grades, although there was a significantly different risk for mortality among these groups, there were no significant intergroup differences in the levels of GPT. This result implies that the ABLI grade may have a better prognostic value than the serum level of GPT.
This study has some limitations that should be recognized. First, because patients declared dead on arrival at the emergency room were not registered in the trauma database, only in-hospital mortality was evaluated in this study, thus leading to some selection bias in the outcome measurement. Second, the number of patients included in the study population was relatively small. Third, there were unknown and uncontrolled conditions such as resuscitation, massive blood transfusion, and liver surgery in the retrospective design of this study, leading to bias in outcome measurement. We can only assume that the outcomes of the intervention or treatment were uniform across the studied groups. Fourth, this study excluded many patients who had no data on albumin or bilirubin levels, which may have led to a selection bias. Furthermore, because the study is limited to a single level I trauma center, additional verification is required before generalizing the study’s findings to other medical facilities. Furthermore, the ALBI was developed based on a study of the adult population; there were no comparable references in the children’s literature. As a result, additional research was required to determine whether the ALBI score is beneficial for pediatric trauma patients with liver injury. Finally, we examined the utility of ALBI grades in a single urban trauma center in southern Taiwan, and inter-country validation has not yet been confirmed.
Conclusions
This study demonstrated that ALBI grade is a significant independent risk factor and a valuable screening tool to identify patients with liver injury with a higher risk for mortality.
Acknowledgments
We appreciate the assistance of the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Funding
This research was funded by Chang Gung Memorial Hospital, grant number CMRPG8M0621.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi:10.1002/bjs.1800600817
2. Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13(4):361–370. doi:10.1016/s1499-3872(14)60267-8
3. Strahl S, Maier KP. [Risk-classification in liver cirrhosis]. Risikoklassifikationen bei Leberzirrhose. Praxis. 2006;95(34):1275–1281. German. doi:10.1024/0369-8394.95.34.1275
4. Li L, Li X, Li W, et al. Prognostic models for outcome prediction in patients with advanced hepatocellular carcinoma treated by systemic therapy: a systematic review and critical appraisal. BMC Cancer. 2022;22(1):750. doi:10.1186/s12885-022-09841-5
5. Peng Y, Wei Q, He Y, et al. ALBI versus Child-Pugh in predicting outcome of patients with HCC: a systematic review. Expert Rev Gastroenterol Hepatol. 2020;14(5):383–400. doi:10.1080/17474124.2020.1748010
6. Porte RJ, Lisman T, Tripodi A, Caldwell SH, Trotter JF. The International Normalized Ratio (INR) in the MELD score: problems and solutions. Am J Transplant. 2010;10(6):1349–1353. doi:10.1111/j.1600-6143.2010.03064.x
7. Sacleux SC, Samuel D, Critical A. Review of MELD as a reliable tool for transplant prioritization. Semin Liver Dis. 2019;39(4):403–413. doi:10.1055/s-0039-1688750
8. Lisman T, van Leeuwen Y, Adelmeijer J, et al. Interlaboratory variability in assessment of the model of end-stage liver disease score. Liver Int. 2008;28(10):1344–1351. doi:10.1111/j.1478-3231.2008.01783.x
9. Wang YY, Zhao XH, Ma L, et al. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018;118(3):440–445. doi:10.1002/jso.25184
10. Marasco G, Alemanni LV, Colecchia A, et al. Prognostic value of the albumin-bilirubin grade for the prediction of post-hepatectomy liver failure: a systematic review and meta-analysis. J Clin Med. 2021;10(9):2011. doi:10.3390/jcm10092011
11. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/jco.2014.57.9151
12. Wang Z, Fan Q, Wang M, Wang E, Li H, Liu L. Comparison between Child-Pugh Score and albumin-bilirubin grade in patients treated with the combination therapy of transarterial chemoembolization and sorafenib for hepatocellular carcinoma. Ann Transl Med. 2020;8(8):537. doi:10.21037/atm.2020.02.114
13. Zhao S, Wang M, Yang Z, et al. Comparison between Child-Pugh score and Albumin-Bilirubin grade in the prognosis of patients with HCC after liver resection using time-dependent ROC. Ann Transl Med. 2020;8(8):539. doi:10.21037/atm.2020.02.85
14. Zhao S, Zhang T, Li H, et al. Comparison of albumin-bilirubin grade versus Child-Pugh score in predicting the outcome of transarterial chemoembolization for hepatocellular carcinoma using time-dependent ROC. Ann Transl Med. 2020;8(8):538. doi:10.21037/atm.2020.02.124
15. Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3(5):100347. doi:10.1016/j.jhepr.2021.100347
16. Khalid MA, Achakzai IK, Hanif FM, Ahmed S, Majid Z, Luck NH. To determine the prognostic value of the albumin-bilirubin grade (ALBI) in patients underwent transarterial chemoembolization for unresectable hepatocellular carcinoma. Gastroenterol Hepatol Bed Bench. 2019;12(2):110–115.
17. Ma T, Li QS, Wang Y, et al. Value of pretransplant albumin-bilirubin score in predicting outcomes after liver transplantation. World J Gastroenterol. 2019;25(15):1879–1889. doi:10.3748/wjg.v25.i15.1879
18. Shimose S, Iwamoto H, Tanaka M, et al. Association between adverse events and prognosis in patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Cancers. 2022;14(17). doi:10.3390/cancers14174284
19. Su TS, Yang HM, Zhou Y, et al. Albumin - bilirubin (ALBI) versus Child-Turcotte-Pugh (CTP) in prognosis of HCC after stereotactic body radiation therapy. Radiat Oncol. 2019;14(1):50. doi:10.1186/s13014-019-1251-y
20. Xu L, Wu J, Lu W, Yang C, Liu H. Application of the albumin-bilirubin grade in predicting the prognosis of patients with hepatocellular carcinoma: a systematic review and meta-analysis. Transplant Proc. 2019;51(10):3338–3346. doi:10.1016/j.transproceed.2019.08.027
21. Campani C, Bamba-Funck J, Campion B, et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab. Liver Int. 2022. doi:10.1111/liv.15487
22. Chen CW, Kuo CJ, Lee CW, et al. Albumin-bilirubin grade as a novel predictor of the development and short-term survival of post-banding ulcer bleeding following endoscopic variceal ligation in cirrhotic patients. Medicina. 2022;58(12):1836. doi:10.3390/medicina58121836
23. Kim TH, Kim BH, Park JW, et al. Proton beam therapy for treatment-naïve hepatocellular carcinoma and prognostic significance of albumin-bilirubin (ALBI) grade. Cancers. 2022;14(18). doi:10.3390/cancers14184445
24. Shi XR, Xu XY, Zhang GL, Jiang JY, Cao DD. The prognostic role of albumin-bilirubin grade in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Eur Rev Med Pharmacol Sci. 2022;26(20):7687–7694. doi:10.26355/eurrev_202210_30045
25. Wang R, Katz D, Lin HM, et al. A retrospective study of the role of perioperative serum albumin and the albumin-bilirubin grade in predicting post-liver transplant length of stay. Semin Cardiothorac Vasc Anesth. 2022;10892532221141138. doi:10.1177/10892532221141138
26. Xu FQ, Ye TW, Wang DD, et al. Association of preoperative albumin-bilirubin with surgical textbook outcomes following laparoscopic hepatectomy for hepatocellular carcinoma. Front Oncol. 2022;12:964614. doi:10.3389/fonc.2022.964614
27. Toyoda H, Johnson PJ. The ALBI score: from liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022;4(10):100557. doi:10.1016/j.jhepr.2022.100557
28. Tsilimigras DI, Hyer JM, Moris D, et al. Prognostic utility of albumin-bilirubin grade for short- and long-term outcomes following hepatic resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 706 patients. J Surg Oncol. 2019;120(2):206–213. doi:10.1002/jso.25486
29. Ni JY, An C, Zhang TQ, Huang ZM, Jiang XY, Huang JH. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int J Hyperthermia. 2019;36(1):328–336. doi:10.1080/02656736.2019.1567834
30. Sakin A, Sahin S, Sakin A, et al. Assessment of pretreatment albumin-bilirubin grade in pancreatic cancer patients with liver metastasis. J Buon. 2020;25(4):1941–1946.
31. Abdel-Rahman O. Prognostic value of baseline ALBI score among patients with colorectal liver metastases: a pooled analysis of two randomized trials. Clin Colorectal Cancer. 2019;18(1):e61–e68. doi:10.1016/j.clcc.2018.09.008
32. Ito T, Ishigami M, Morooka H, et al. The albumin-bilirubin score as a predictor of outcomes in Japanese patients with PBC: an analysis using time-dependent ROC. Sci Rep. 2020;10(1):17812. doi:10.1038/s41598-020-74732-3
33. Liu J, Wu M, Xie E, et al. Assessment of liver function for evaluation of short- and long-term outcomes in type B aortic dissection patients undergoing thoracic endovascular aortic repair. Front Cardiovasc Med. 2021;8:643127. doi:10.3389/fcvm.2021.643127
34. Kawata T, Ikeda A, Masuda H, Komatsu S. Association between albumin-bilirubin score at admission and in-hospital mortality in patients with acute heart failure. Int Heart J. 2021;62(4):829–836. doi:10.1536/ihj.21-080
35. Han S, Wang C, Tong F, et al. Prognostic impact of albumin-bilirubin score on the prediction of in-hospital mortality in patients with heart failure: a retrospective cohort study. BMJ Open. 2022;12(1):e049325. doi:10.1136/bmjopen-2021-049325
36. Shi L, Zhang D, Zhang J. Albumin-bilirubin score is associated with in-hospital mortality in critically ill patients with acute pancreatitis. Eur J Gastroenterol Hepatol. 2020;32(8):963–970. doi:10.1097/meg.0000000000001753
37. Kinoshita F, Yamashita T, Oku Y, et al. Prognostic impact of albumin-bilirubin (ALBI) grade on non-small lung cell carcinoma: a propensity-score matched analysis. Anticancer Res. 2021;41(3):1621–1628. doi:10.21873/anticanres.14924
38. Matsukane R, Watanabe H, Hata K, et al. Prognostic significance of pre-treatment ALBI grade in advanced non-small cell lung cancer receiving immune checkpoint therapy. Sci Rep. 2021;11(1):15057. doi:10.1038/s41598-021-94336-9
39. Aoyama T, Ju M, Machida D, et al. Clinical impact of preoperative albumin-bilirubin status in esophageal cancer patients who receive curative treatment. In Vivo (Brooklyn). 2022;36(3):1424–1431. doi:10.21873/invivo.12847
40. Kanda M, Tanaka C, Kobayashi D, et al. Preoperative albumin-bilirubin grade predicts recurrences after radical gastrectomy in patients with pT2-4 gastric cancer. World J Surg. 2018;42(3):773–781. doi:10.1007/s00268-017-4234-x
41. Zhang J, Xu Q, Zhang H, et al. High preoperative albumin-bilirubin score predicts poor survival in patients with newly diagnosed high-grade gliomas. Transl Oncol. 2021;14(4):101038. doi:10.1016/j.tranon.2021.101038
42. Zhu S, Cheng Z, Hu Y, et al. Prognostic value of the systemic immune-inflammation index and prognostic nutritional index in patients with medulloblastoma undergoing surgical resection. Front Nutr. 2021;8:754958. doi:10.3389/fnut.2021.754958
43. Hsieh CH, Hsu SY, Hsieh HY, Chen YC. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed J. 2017;40(2):113–120. doi:10.1016/j.bj.2016.10.005
44. Hsieh CH, Liu HT, Hsu SY, Hsieh HY, Chen YC. Motorcycle-related hospitalizations of the elderly. Biomed J. 2017;40(2):121–128. doi:10.1016/j.bj.2016.10.006
45. Hsieh CH, Chen YC, Hsu SY, Hsieh HY, Chien PC. Defining polytrauma by abbreviated injury scale >/= 3 for a least two body regions is insufficient in terms of short-term outcome: a cross-sectional study at a level I trauma center. Biomed J. 2018;41(5):321–327. doi:10.1016/j.bj.2018.08.007
46. Geng L, Zong R, Shi Y, Xu K. Prognostic role of preoperative albumin-bilirubin grade on patients with hepatocellular carcinoma after surgical resection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(7):769–778. doi:10.1097/meg.0000000000001618
47. Lao TT. Implications of abnormal liver function in pregnancy and non-alcoholic fatty liver disease. Best Pract Res Clin Obstet Gynaecol. 2020;68:2–11. doi:10.1016/j.bpobgyn.2020.02.011
48. Khalesi S, Johnson DW, Campbell K, et al. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. Eur J Nutr. 2018;57(6):2037–2053. doi:10.1007/s00394-017-1568-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
