Back to Journals » Infection and Drug Resistance » Volume 15
The Association Between the Albumin and Viral Negative Conversion Rate in Patients Infected with Novel Coronavirus Disease 2019 (COVID-19)
Authors Lang L, Zhu Z, Xu Z, Zhu S, Meng P, Wang H, Song Z, Wang Y, Bi J
Received 22 December 2021
Accepted for publication 3 March 2022
Published 8 April 2022 Volume 2022:15 Pages 1687—1694
DOI https://doi.org/10.2147/IDR.S353091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Li-wei Lang,1,* Zhen-zhen Zhu,1,* Zhe Xu,2,* Shan-wei Zhu,1 Peng Meng,3 Hong-yan Wang,1 Zhan-dong Song,1 Ying Wang,4 Jing-feng Bi5
1Clinical Trial Research Center, Department of Pharmacy, Medical Supplies Center of PLA General Hospital, Beijing, 100039, People’s Republic of China; 2Department of Infectious Diseases, The Fifth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Infectious Diseases, Beijing, 100039, People’s Republic of China; 3Department of Outpatient, Zi-Bo Central Hospital of Shan Dong, Shan Dong, 255036, People’s Republic of China; 4Respiratory Department No. 960 Hospital, The People’s Liberation Army, Jinan, 250014, People’s Republic of China; 5Clinical Trial Research Center, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100039, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing-feng Bi, Clinical Trial Research Center, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100039, People’s Republic of China, Email [email protected] Ying Wang, Respiratory Department No. 960 Hospital, The People’s Liberation Army, Jinan, 250014, People’s Republic of China, Email [email protected]
Purpose: The novel coronavirus disease 2019 (COVID-19) epidemic is the severe global pandemic with large numbers of infected cases and deaths in recent decades. The previous studies were all about the influence of albumin (ALB) for the severity and mortality of in-patients infected with COVID-19. But few studies exist about the influence factors to achieve viral negative conversion. Therefore, this study conducted an exploratory study to investigate the effect of albumin on negative conversion rate.
Methods: Among the 190 hospitalized patients with moderate COVID-19 who had a course of disease longer than 30 days, 102 achieved viral negative conversion in 30– 45 days and 88 not after 45 days. Taking other variables as concomitant variable, Cox proportional hazard regression model was applied to explore the influence of albumin to negative conversion rate under various factors.
Results: By comparing patients who could and could not achieve the finally viral negative conversion, a possible nonlinear relationship between the continuous variables and clinical outcomes was examined by a restricted cubic spline regression model. An association was found between albumin levels and hazard ratio of viral negative conversion rate (P = 0.027). The increase of albumin was accompanied with decreases of hazard ratio of viral negative conversion rate (the value of albumin < 38 g/L). But when the value of albumin was higher than 38 g/L, the hazard ratio of viral negative conversion rate approached 1, it means that albumin is not a risk factor for the viral negative conversion rate of COVID-19 disease.
Conclusion: For patients with COVID-19, albumin is a common and observed laboratory parameter. It is associated with final viral negative conversion rate although its underlying mechanism and relationship with the viral negative conversion rate still need to be clarified.
Keywords: COVID-19, albumin, negative conversion rate
Introduction
The novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread around the word rapidly.1 As of Oct 30th, 2020, there were more than 44.6 million confirmed cases and 1,175,000 deaths (2.6%) in more than 219 countries.2 According to the clinical classification method, the patients were divided into four types: mild type, moderate type, severe type and critically ill type according to the severity of the disease.3,4
Most of the current studies indicated that albumin had effect on the severity of pulmonary disease in patients with COVID-19.5–16 Here, a paper was reported the prevalence of low levels of albumin in a cohort of 207 patients with COVID-19 and its relationship with disease severity and clinical outcome. A negative association was found between albumin levels and severity of COVID-19 disease and death in this study.14 Meantime, a systematic review and meta-analysis study showed that four studies assessed the low levels of albumin and severity of COVID-19 and increased risk was demonstrated in other paper.5 In addition, there are many other studies on the albumin levels and the severity of COVID-19 diseases.2,6,10,12,14–17 In these articles, an increase in the severity of COVID-19 pneumonia was positively associated with lower levels of albumin. Therefore, based on previous literature, we believe that albumin is associated with the severity of COVID-19 disease.
In the process of fighting COVID-19 disease of Huo Shen Shan hospital of Wuhan, Hubei, China, 2020, the clinical observation of COVID-19 diseases showed that a part of patients with the low levels of albumin had the increasing tendency of negative conversion time. Therefore, we aimed to develop a retrospective study of patients in Huo Shen Shan hospital in Wuhan who had not turned negative for a long time (more than 30 days), to clarify the association between albumin levels and negative conversion rate.
Materials and Methods
Patients
In this retrospective study, we included confirmed mild cases of COVID-19 from February 4, 2020, to April 14, 2020 in Huo Shen Shan hospital in Wuhan, Hubei, China.
Diagnostic Standard
COVID-19 was diagnosed according to the WHO interim guidance and confirmed by at least two positive real-time reverse transcriptase polymerase-chain-reaction (RT-PCR) tests for viral nucleic acids.7,8,18 Throat swab samples were collected for determination SARS-Cov-2 RNA from patients. The real time RT-PCR assay was conducted using a SARSCov-2 nucleic acid detection kit according to the factory’s instruction (Guangzhou DaAn Gene Limited Company). The RT-PCR assay was conducted under the following conditions: incubation at 50°C for 15 minutes and 95°C for 15 minutes, 45 cycles of the denaturation at 94°C for 15 seconds, and extending and collecting fluorescence signal at 55°C for 45 seconds. A cycle threshold value equal and less than 40 was defined as a positive test result, and a cycle threshold value more than 40 was defined as a negative test. Two target genes—open reading frame 1ab (ORF1ab) and nucleocapsid protein (N)— were simultaneously amplified and assayed during the real time RT-PCR assay.8
Criteria for Negative Conversion
All patients were observed negative for nucleic acid tests. The standard of criterion is nucleic acid tests negative twice consecutively on respiratory tract samples such as sputum and nasopharyngeal swabs at least 24 h apart.
Inclusion Criteria
(1) all adult patients enrolled in this study were diagnosed according to the criteria of diagnosis and treatment protocol for novel coronavirus pneumonia; (2) all the subjects were mild cases since these patients showing fever and respiratory symptoms with radiological findings of pneumonia; (3) positive time of nucleic acid test ≥ 30 days; (4) all patients proceed nucleic acid test every 1–3 days after 30 days to determine whether the nucleic acid test turning negative.
Excluded Criteria
(1) patients with malignant tumors and malignant hematological diseases; (2) patients with acquired immunodeficiency syndrome (AIDS); (3) the blood routine examination or blood biochemical examination was not carried on the range of ±4 days for onsets of COVID-19 disease; (4) liver failure (total bilirubin 10 mg/dL and/or severe coagulation disorders); (5) renal function failure (urine 0.5 m*kg−1*h−1, creatinine (Cr) or blood urea nitrogen (BUN) 1.5× upper limits of normal (ULN) although there is adequate circulating blood and cardiac output).
Clinical Data
The following data were retrieved from clinical charts at presentation: (1) demographic data (sex, age, smoking and alcohol history); (2) concomitant diseases (such as with or without diabetes, hypertension, cardiovascular disease, cerebrovascular disease, respiratory disease, chronic liver disease and so on); (3) therapeutic medication situation (specification, dosage, usage and time); (4) time of nucleic acid test turning negative; (5) the routine blood examinations and blood biochemical tests, which was turning negative at the range of 30–45 days (±4 days), and not turning negative after 45 days (±4 days); 6) treatments strategies of COVID-19 infection (thymus peptide, interferon, convalescent plasma, hormones, immunoglobulin and so on).
Ethics Approval
The study was approved by the Ethics Committee of No. 960 Hospital (RE2020-70), the People’s Liberation Army, Jinan, China, and informed consent was signed.
Statistical Analyses
The quantitative data was expressed as Mean±SD when accorded with normality, when the variance was homogeneous or not, t test or correction t test was used for group comparison, respectively. The quantitative data was expressed as Median (IQR) when did not accord with normality and was compared using Wilcoxon’s rank-sum. The categorical data was expressed as percentage and was compared using chi-square test. First, Cox proportional hazard regression model was used for single factor analysis with negative conversion rate as dependent variable and its influence single factor as independent variables. Then variables with the p value less than 0.2 was taken as concomitant variable, albumin as independent variable. Cox proportional hazard regression model was applied to explore various factors to negative conversion rate. A possible nonlinear relationship between the continuous variables and clinical outcomes was examined by a restricted cubic spline regression model.19 Statistical analysis was performed using SAS9.4. All the tests were two-sided test (α=0.05).
Results
In total, the study population included 232 patients with clinically confirmed COVID-19 (more than 30 days). All patients were selected by excluded criteria. Finally, 190 patients were recruited and 42 patients were excluded. The flow chart is shown in Figure 1. 190 patients had an overall course beyond 30 days from February 4 to April 14, 2020. Among these, 102 were to achieve viral negative conversion in 30–45 days and 88 were not after 45 days.
 |
Figure 1 Study flow chart. Abbreviations: BUN, blood urea nitrogen; ULN, upper limit of normal. |
Table 1 lists the baseline characteristics of the two groups. The frequencies or proportions of patients in the two groups were given for all the categorical variables in the table. Among those variables, significant difference between the two groups was found in the following categories: albumin (ALB)(P=0.010), total protein (TP) (P=0.006), globulin (GLO) (P=0.020), Immunoglobulin (P=0.0154) (All the P value <0.05). There were no statistically obvious differences for others.
 |
Table 1 Clinical Characteristics of Patients with 190 COVID-19 Between the Non-Negative Conversion and Negative Conversion of Group (n = 190) |
Table 2 lists results of its single factor to negative conversion rate. Cox proportional hazard regression model method was used for single factor analysis. The result showed that the values of albumin those who came out of hospital was associated with viral negative conversion rate (the P value was 0.009 (<0.05)).
 |
Table 2 The Single Factor Analysis Results of Cox Proportional Hazard Regression Model |
Meantime, taking variables that the P value was less than 0.2 as concomitant variable (variables come from Table 2), albumin as independent variable. Cox proportional hazard regression model was applied to explore albumin to negative conversion rate under the various factors. The result also showed that the values of albumin was associated with viral negative conversion rate (the P value was 0.027). Figure 2 showed that a negative association was found between albumin levels and hazard ratio of viral negative conversion rate. When the value of albumin was less than 38 g/L, the increase of albumin levels was accompanied with decreases of hazard ratio of viral negative conversion rate. But when the value of albumin was about more than 38 g/L, the hazard ratio of viral negative conversion rate approached 1, it shows that albumin is not a risk factor for the viral negative conversion rate of COVID-19 disease.
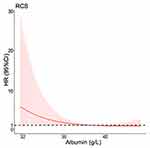 |
Figure 2 Hazard ratio function for albumin to negative conversion rate. Abbreviations: RCS, restricted cubic splines; HR, hazard ratio; CI, confidence interval. |
Discussion
The previous studies are all about influence of albumin on the severity and mortality of in patients infected with COVID-19. The previous literatures also demonstrated that the decrease of immunity of the organism could be induced by the hypoalbuminemia.22 Therefore, we paid more attention to the albumin levels and nutritional status of critically ill type patients on clinical. But few studies exist about the influence factors to achieve viral negative conversion rate. Also, the underlying mechanisms have not been clarified, yet. Here, we made explorative research on the relationship between albumin levels and viral negative conversion rate in a cohort of 190 patients with COVID-19 and its mechanism of action.
In our study, we observed a negative association between albumin levels and hazard ratio of viral negative conversion rate (the value of albumin was less than 38 g/L). Meantime, the result of various factors analysis showed that there are the significant statistical differences in the effect of albumin levels for viral negative conversion rate (P<0.05). It further illustrates that albumin is a risk factor for the viral negative conversion rate of COVID-19 disease when the value of albumin was less than 38 g/L. But when the value of albumin was about more than 38 g/L, the hazard ratio of viral negative conversion rate approached 1, which means that albumin is no longer a risk factor for the viral negative conversion rate.
As previously demonstrated, the viral negative conversion rate strongly is correlated with low levels of serum albumin at admission. Several mechanisms may be involved in the albumin levels on the viral negative conversion rate with COVID-19 disease. (a) Albumin plays a key role in the homeostasis of vascular endothelium, offering protection from inflammation and remaining the primary determinant of colloid osmotic pressure (COP). It appears that albumin administration may affect vascular permeability, cell signaling, neutrophil adhesion, redox homeostasis. It also has anticoagulant and antithrombotic properties. Consequently, albumin play an important role in the recovery of diseases.20 (b) Albumin also has immunomodulatory and anti-inflammatory effects through binding of bacterial products, modulation of antigen-presenting cell function, modulation of cytokine production.15,21
Based on the results of our research when the value of albumin was less than 38 g/L, the level of albumin is a risk factor for the viral negative conversion rate, and the normal range of albumin is 40–55 g/L in the healthy body. The low level of albumin can affect its protection role from inflammation situation, antigen-presenting cell function and cytokine production, and hence, impact the effect of virus clearance and the viral negative conversion rate. Therefore, we should also pay attention to the albumin levels of mild type patients. The value of albumin should be maintained a high levels, such as more than 38 g/L, which is beneficial to SARS-CoV-2 RNA turned negative. It is speculated that a high albumin level is also needed for critically ill type and mild type patients, which has great clinical significance of viral negative conversion rate and prognosis.
There are several limitations in our study. First, this is also a small cohort study. Second, some unmeasured factors which not included in the analysis may potentially bias the result. Third, the nucleic acid detection intervals are different among individuals. Hence, detection of virus negative conversion might be delayed than estimated. Further large randomized prospective studies are still needed to confirm the reliability of the results.
Conclusion
Albumin which is a common and observed laboratory parameter is strictly associated with finally viral negative conversion rate in patients with COVID-19. In our study, when the value of albumin was less than 38 g/L, we observed a negative association between albumin level and hazard ratio of viral negative conversion rate. It was believed that albumin is a risk factor for the viral negative conversion rate of COVID-19 disease. Nevertheless, when the albumin levels recovered to normal (more than 38 g/L), albumin is no longer a risk factor for the viral negative conversion rate.
Funding
This study was supported by the National Key Research and Development Program of China (grant no 2020YFC0860900).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu D, Wu T, Liu Q, et al. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi:10.1016/j.ijid.2020.03.004
2. vanZyl JS, Alam A, Felius J, et al. ALLY in fighting COVID-19: magnitude of albumin decline and lymphopenia (ALLY) predict progression to critical disease. J Investig Med. 2021;69(3):710–718. doi:10.1136/jim-2020-001525
3. National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. (trial version 7); 2020. Available from: https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf. Accessed March 30, 2022.
4. World Health Organization Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected. Interim Guidance; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
5. Aziz M, Fatima R, Lee-Smith W, et al. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24(1):255–259. doi:10.1186/s13054-020-02995-3
6. Bi XJ, Su ZX, Yan HX, et al. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to albumin ratio and platelet count. Platelets. 2020;31(5):674–679. doi:10.1080/09537104.2020.1760230
7. Chen NS, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/s0140-6736(20)30211-7
8. Chen T, Wu D, Chen HL, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1091
9. Kermali M, Khalsa RK, Pillai K, et al. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020;254:117788. doi:10.1016/j.lfs.2020.117788
10. Liu SQ, Luo HY, Wang YC, et al. Clinical characteristics and risk factors of patients with severe COVID-19 in Jiangsu province, China: a retrospective multicentre cohort study. BMC Infect Dis. 2020;20(1):584–593. doi:10.1186/s12879-020-05314-x
11. Moutchia J, Pokharel P, Kerri A, et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One. 2020;15(10):e0239802. doi:10.1371/journal.pone.0239802
12. Artigas A, Wernerman J, Arroyo V, et al. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62–70. doi:10.1016/j.jcrc.2015.12.019
13. Bao JF, Li CX, Zhang K, et al. Comparative analysis of laboratory indexes of severe and non-severe patients infected with COVID-19. Clin Chim Acta. 2020;509:180–194. doi:10.1016/j.cca.2020.06.009
14. Bassoli C, Oreni L, Ballone E, et al. Role of serum albumin and proteinuria in patients with SARS-CoV-2 pneumonia. Int J Clin Pract. 2021;75(4):e13946. doi:10.1111/ijcp.13946
15. Huang W, Li C, Wang ZQ, et al. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2623 hospitalized cases. Sci China Life Sci. 2020;63(11):1678–1687. doi:10.1007/s11427-020-1733-4
16. Li JY, Li M, Zheng SS, et al. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark Med. 2020;14(10):827–837. doi:10.2217/bmm-2020-0254
17. Liu FF, Ji CC, Luo JJ, et al. Clinical characteristics and corticosteroids application of different clinical types in patients with Corona virus disease 2019. Sci Rep. 2020;10(1):13689. doi:10.1038/s41598-020-70387-2
18. World Health Organization Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance.
19. Harre FE, Lee L, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–1202. doi:10.1093/jnci/80.15.1198
20. Polito C, Martin GS. Albumin: physiologic and clinical effects on lung function. Minerva Anestesiol. 2013;79(10):1180–1186.
21. Aldecoa C, Llau JV, Nuvials X, et al. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020;10(1):85. doi:10.1186/s13613-020-00697-1
22. Ali AM, Kunugi H. Hypoproteinemia predicts disease severity and mortality in COVID-19: a call for action. Diagn Pathol. 2021;16(1):31. doi:10.1186/s13000-021-01092-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
