Back to Journals » Vascular Health and Risk Management » Volume 18
The Association Between Sublingual Varices and Cardiovascular Risk Factors
Authors Bergh H , Albrektson M, Kastberg C, Baigi A, Hedström L
Received 14 December 2021
Accepted for publication 30 March 2022
Published 23 April 2022 Volume 2022:18 Pages 319—327
DOI https://doi.org/10.2147/VHRM.S354021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Takashi Kajiya
Håkan Bergh,1,2 Margit Albrektson,3 Clovis Kastberg,4 Amir Baigi,1,2 Lennart Hedström3
1School of Public Health and Community Medicine, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden; 2Department of Research & Development Unit, Hospital Varberg, Region Halland, Sweden; 3Public Dental Services, Västra Vall, Varberg, Sweden; 4Tre Tandläkare, Varberg, Sweden
Correspondence: Håkan Bergh, School of Public Health and Community Medicine, Institute of Medicine, P.O. Box 463, Gothenburg, 405 30, Sweden, Email [email protected] Lennart Hedström, Public Dental Service, Västra Vallgatan 14, Varberg, S-43244, Region Halland, Sweden, Email [email protected]
Objective: To study the association between sublingual varices (SV) and cardiovascular (CVD) risk factors.
Methods: A total of 989 consecutive dental patients aged 55– 84 years participated in the study, which applied a survey about risk factors, diseases and medications. Digital photos of the lateral borders of the tongue, height, weight, waist, hip and blood pressure were registered, and blood samples were analyzed. Those with SV were compared with those without SV (nSV).
Results: Those with SV had more hypertension 41.8% vs 27.0% (p< 0.0001), a higher systolic blood pressure (BP) 139.5 (SD 18.6) mmHg vs 134.3 (SD 18.8) mmHg (95% CI − 7.73 ─ − 2.72), more diabetes type 2 (DM-2) 7.4% vs 3.8% (p=0.014), a higher fasting plasma glucose 5.9 (SD 1.5) mmol/L vs 5.7 (SD 1.0) mmol/L (95% CI − 0.42 ─ − 0.05), more dyslipidemia 24.1% vs 17.7% (p=0.018), lower HDL 1.6 vs 1.7 (p=0.003), a greater waist circumference 97.0 cm vs 93.9 cm (95% CI − 4.66 ─ − 1.46), a greater waist/hip ratio 0.92 cm/cm vs 0.90 cm/cm (95% CI − 0.03 ─ − 0.01), and a higher BMI 26.6 kg/m2 vs 26.0 kg/m2 (95% CI − 1.11 ─ − 0.03). The following associations with SV were found in multivariate analysis: hypertension OR=1.6 (95% CI 1.19 ─2.13), a high systolic BP OR =1.5 (95% CI 1.11 ─2.13), a high fP-glucose OR= 1.8 (95% CI 1.03 ─3.21), a low HDL OR= 1.8 (95% CI 1.15 ─2.92), a greater waist circumference OR= 1.68 (95% CI 1.10 ─2.58), a greater waist/hip ratio OR=2.21 (95% CI 1.36 ─3.58), and a higher BMI OR=1.05 (95% CI 1.02 ─1.09).
Conclusion: This study shows an association between SV and a high BP, a high fP-glucose, hypertension, diabetes mellitus type 2, dyslipidemia, abdominal obesity, older age and smoking.
Keywords: sublingual varices, cardiovascular riskfactors
Introduction
Varicosities, benign venous lesions often found under the lateral borders of the tongue, are a condition called sublingual varices (SV). The prevalence is approximately 30% depending on the age of the population.1–4 The pathogenesis of SV is unknown and it is generally considered an effect of the aging process, but more recent research has found an association with cardiovascular diseases (CVD),1,2,4 hypertension,1,3,5 and smoking,2–4 and thus, there is a growing interest in SV. Besides these relationships, other surveys have indicated a possible connection with diabetes mellitus type 2 (DM-2).1,5 The possible relationship between SV and CVD risk factors is poorly explored. The relationship between SV and hypertension was shown in two studies, where blood pressure was measured,3,5 while the relationship between SV and DM2 was based on past recall answers in a selected study population.1,5 No previous study has investigated the association between SV and other CVD risk factors such as high blood glucose, dyslipidemia and abdominal obesity.
The aim of this study was to investigate whether there is an association between SV and risk factors for CVD with a focus on high blood pressure, high blood glucose, dyslipidemia, and abdominal obesity.
Methods
Setting
This study was performed at two dental clinics (one public dental clinic and one private clinic) with a total clientele of 13,000 patients. Both clinics are situated in the center of a small town on the Swedish west coast.
Study Population
In connection with their annual dental examination, patients aged 55─84 years were consecutively invited (postal letter and waiting-room poster) to participate in this study. The exclusion criteria were atrial fibrillation and renal dialysis. During the inclusion period (May 2018 to March 2020), 989 patients agreed to participate in the study.
Data Collection
All patients received both verbal and written information about the study and those who agreed to participate provided written consent. The patients answered a questionnaire either before or during their visit to the clinic. The questionnaire covered information about tobacco use and a family history of myocardial infarction, any diagnosed diseases (hypertension, DM-2, dyslipidemia, heart disease, vascular disease), and medications prescribed (for hypertension, DM-2, dyslipidemia).
Digital photos were taken, one of each side of the lateral border of the tongue. Height (cm) and weight (kg) without shoes was registered. Waist circumference (cm) (at the midpoint between the lowest rib and the iliac crest), and the hip circumference (cm) (at the greater trochanter) was measured, both in a standing position.
After ≥ 5 minutes of rest, the blood pressure (BP) mmHg was measured in a sitting position twice in each upper arm with an automatic blood pressure device (Omron M6 Comfort, Omron Health Care Ltd., Kyoto, Japan). The cuff was adapted to the size of the upper arm, 22─42 cm. A dental nurse or a dental hygienist, both educated and well trained in blood pressure measurements, took all blood pressure measurements. Patients with an average BP ≥140/90 mm Hg were instructed in how to measure their home blood pressure (Omron M6 Comfort) (morning and evening, twice at each occasion with 1–2 minutes apart, for one week), and then an average home BP was calculated (based on the BP values from Day 2─7).6 Fasting blood samples (P-glucose, HbA1c, P-cholesterol, P-high density lipoprotein (HDL), P-low density lipoprotein (LDL), P-triglycerides, CRP and creatinine) were taken within the next few days at a nearby primary health care center (PHC) and were analyzed at an ISO15189 accredited laboratory. The data collection is illustrated in Figure 1. All participants received a written report about their blood tests from the survey´s responsible doctor with advice to contact the PHC if necessary.
Data Adaptation
Age was registered in whole years. The questions about smoking and snuff use were formulated as “Do you smoke?” and “Do you use snuff?” with the following answers: Yes daily; Yes sometimes; No. These answers were dichotomized to Yes (daily/sometimes)/No. The question about diseases was formulated as follows: “Do you have any of these diseases diagnosed by a physician?” with the following answers: Yes; No; I don´t know. The answers were dichotomized to Yes/No, where “I don´t know” was counted as “No”. The question about medication had the alternatives: Yes; No; I don´t know. The answers were dichotomized in the same way as for diseases.
BMI was calculated as body weight (kg) divided by squared height (m2). An average clinic systolic and diastolic BP was calculated from the four measured BPs (mmHg).
In logistic regression analysis, systolic BP, fP-glucose, fP-lipids, BMI, waist circumference, and waist/hip ratio were analyzed as categorical variables with the following categorization: systolic BP <130, 130–139, ≥140 mmHg; fP-glucose <5.6, 5.6─6.9, ≥7.0 mmol/L; P-cholesterol q1<4.8, q2 4.8─5.4, q3 5.5─6.1, q4 >6.1 mmol/L; P-HDL q1<1.4, q21.4─1.6, q31.7─2.0, q4>2.0 mmol/L; waist circumference q1<87, q2 87─94, q3 95─102, q4>102 cm; waist/hip ratio q1<0.853, q2 0.853─0.913, q3 >0.913─0.962, q4>0.962 cm/cm.
Two experienced dentists examined the digital photos (blinded from each other´s result and from the patient´s questionnaire answers, measurements and blood sample data) and classified the sublingual lateral veins as none/few visible SV (nSV)(grade 0) (Figure 2) or medium/severe SV (grade 1) (Figure 3) in accordance with the method used in a previous study.3 A consensus was reached in cases where the assessment differed between observers. The classification process is illustrated in Figure 1.
 |
Figure 2 None/few sublingual varices (grade 0). Written consent obtained from patient. |
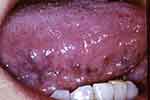 |
Figure 3 Medium/severe sublingual varices (grade 1). Written consent obtained from patient. |
Statistical Analysis
The study population was divided into two subgroups (SV or nSV). For comparisons between groups, Chi-squared test, Student´s t-test and Mann–Whitney U-test were applied. For studies of possible associations between SV and the independent variables (hypertension, systolic blood pressure, DM-2, fP-glucose, dyslipidemia, fP-cholesterol, fP-HDL, waist circumference, waist-hip ratio) multiple logistic regression was performed using the enter method where the estimated values were adjusted for sex and age (Model 1) or for sex, age and smoking (Model 2).
Multicollinearity was tested with multiple linear regression analysis. The tolerance limit was set to VIF=3.0. Cohen’s kappa was used for a study on the coherence of the sublingual images (the interrater reliability). All tests were double-sided, and the significance level was set at p 0.05. SPSS 27.0 was used for the analyses.
Ethics
This study was approved by the Regional Research Ethics Committee at the University of Lund (EPN 2018/60) and was conducted in accordance with the Helsinki Declaration.
Results
The external dropout frequency was not registered, but very few patients declined to participate. The internal dropout rate was 11.7% (116/989), mostly represented by those who did not have blood samples taken. The male/female ratio was 45.5%/54.5%, and the mean age was 66.5 years.
Those with SV were, to a greater extent, men, had a higher mean age, were more often smokers, and they reported more hypertension, more DM-2, and more dyslipidemia than those with nSV (Table 1). Multicollinearity tests were performed where the tolerance limit was set to VIF=3.0, showing that all variables in the models had a VIF <2.0.
Blood Pressure
Those with SV had a higher mean systolic BP of 139.5 mmHg (SD 18.6) versus 134.3 mmHg (SD 18.8) (95% CI −7.73─ −2.72), Table 1. After excluding those diagnosed with hypertension (n313), the difference in systolic BP between the two groups remained (95% CI −8.25 ─ −1.99). After adjusting for the covariates sex and age, the relationship remained between the diagnosis of hypertension and SV (OR 1.6, 95% CI 1.19 ─ 2.13) and between a high systolic BP and SV (OR 1.5, 95% CI 1.11 ─ 2.13) (Table 2).
When adjusting the model for age, sex, and smoking, the relationship was not significantly affected (Table 2).
DM-2/Glucose Value
fP-glucose was higher among those with SV 5.9 mmol/L (SD 1.5) vs nSV 5.7 mmol/L (SD 1.0) (95% CI −0.42 ─ 0.05) (Table 1). The difference in fP-glucose remained after the exclusion of patients diagnosed with DM-2 (n=42) (95% CI −0.31─ −0.01)(data not shown). A high fp-glucose value (≥7.0 mmol/L) was associated with SV (OR 1.8, 95% CI 1.03─3.21) when adjusted for age and sex.
Dyslipidemia
The patients with SV had a lower fP-cholesterol (95% CI 0.08─ 0.38), a lower fP-HDL (95% CI 0.04─ 0.17), and a lower fP-LDL (95% CI 0.05─ 0.34) compared with nSV (Table 1). The differences between the groups remained after excluding those (n=178) diagnosed with dyslipidemia; fP-cholesterol (95% CI −0.01─ 0.30), fP-HDL (95% CI 0.01─ 0.17) and fP-LDL (95% CI −0.04─ 0.25)(data not shown). The relationship between the independent variables cholesterol and HDL (as categorical variables), and the dependent variable SV/nSV remained after adjusting for the covariates age and sex (Table 3). The strength of the relationship changed insignificantly when the model was adjusted for the covariates age, sex and smoking (Table 3).
Abdominal Obesity, BMI
Patients with SV had a higher BMI 26.6 (SD 4.1) vs 26.0 (SD 4.0) (95% CI −1.11─ −0.03), a greater waist circumference 97.0 (SD 12.0) vs 93.9 cm (SD 12.0) (95% CI −4.66 ─ −1.46), and a larger waist/hip ratio cm/cm (95% CI −0.03 ─ −0.01) compared to those with nSV (Table 1). After adjusting for the covariates sex and age the relationship between SV and a greater waist circumference remained significant OR 1.7 (95% CI 1.10 ─ 2.58), and for a larger waist/hip ratio OR 2.2 (95% CI 1.36 ─ 3.58) (Table 4). The strength of the relationship changed insignificantly when the model was adjusted for the covariates age, sex and smoking (Table 4).
Interrater Agreement
The two observers evaluated the sublingual veins and agreed for 787 (80%) participants. The Cohen´s kappa coefficient was 0.57. An analysis of the group with full agreement (n787) showed that all significant differences between the two groups (SV and nSV) in Table 1 remained, except for BMI, which disappeared, and systolic home BP was added (95% CI −7.14 ─ −0.01). The following differences between the groups (SV/nSV) were reinforced: systolic BP (95% CI −8.44 ─ −2.81), fP-glucose (95% CI −0.49 ─ −0.05), fP-cholesterol (95% CI 0.10 ─ 0.44), fP-HDL (CI 0.05 ─ 0.20), fP-LDL (CI 0.07 ─ 0.40), waist circumference (95% CI −5.32 ─ −1.72) and waist/hip ratio (95% CI −0.04 ─ −0.02).
Discussion
The results of this study indicate an association between SV and the following cardiovascular risk factors: hypertension, DM-2, dyslipidemia, high systolic BP, high fP-glucose, low fP-HDL, greater waist circumference, larger waist-hip ratio, male sex, older age and smoking. A relationship between SV and a diagnosis of hypertension,1,3,5 and a high BP3 were previously found. The results from this study verified the association between SV, a diagnosis of hypertension and a high systolic BP. An association between SV and a high systolic BP was also found among those without a formal diagnosis of hypertension. The relationship between SV and a high fP-glucose is a new finding and is reinforced by the relationship between SV and DM-2. One former study found an association between SV and diabetes mellitus (unclear if it was DM-1 or DM-2 or both) in a population attending a radiology department.1 The relationship between SV and dyslipidemia has not previously been studied. The strongest association was between SV and a low HDL. The association between SV and abdominal obesity is also a new finding and was not previously studied. A greater waist circumference, a larger waist/hip ratio and a higher BMI were all significantly associated with SV.
Sublingual varices were associated with a high systolic BP, a high fP-glucose, a low HDL and abdominal obesity. These four factors are all risk factors for CVD and are components of metabolic syndrome. Other factors associated with SV, shown in this study and in others, are older age1–3,5 and smoking,1–3,5,7 which are both risk factors for CVD. An association between SV and CVD was previously reported in studies based on recall information about CVD and in two studies where hypertension was included as CVD.1–4,8
The pathophysiological mechanism behind SV is unknown. If a high BP in the arterioles could affect the venules, a prerequisite of arteriovenous shunts is necessary, in a previous study, there is speculation about this but not concluded.9 The associations in this study between SV and a high systolic BP, a high fP-glucose, and a low HDL were found among those without formal respective diagnoses of hypertension, DM-2 and dyslipidemia is especially interesting, as this may indicate that the vessels under the tongue could be affected by a systemic pathological process behind the CVD, such as metabolic syndrome. If there is a mutual pathophysiology between SV and CVD, other possibilities for diagnostics would appear as SVs are easily accessible for visual inspection. As these results were not previously shown, additional studies are required for verification, and studies with CVD as an endpoint are required.
Methodological Considerations
The consecutive selection of this study population included both healthy and diseased individuals at different ages. This study population could be considered a close representative selection of the population, as approximately 60% of the inhabitants aged >24 years attend routine dental care each year,10 and 77% over a three years period.11 One methodological weakness is the subjective classification of the sublingual veins as SV or nSV. The blinded classification process resulted in a Cohen´s kappa coefficient of 0.57, which indicates that the distinction between SV and nSV is not clear. The transformation of the sublingual vessels from nSV to SV is likely to occur gradually. The two assessors classified the vessels under the tongue on both the left and right sides dichotomously (0/1) (a total of four assessments of each patient).
The total sum score of the sublingual vessels was from 0 to 4, where 0 and 4 were a complete consensus. The association between SV and systolic BP, fP-glucose, fP-HDL and abdominal obesity were all reinforced when only those patients classified with a complete consensus (SV sum score either 0 or 4) were included in the analysis. This would argue against the assessment bias having a significant impact on the results.
The questionnaire was not validated, but the questions were formulated in a simple manner with a limited number of possible answers.
The study had an internal dropout rate (11.7%), which mostly consisted of those who did not provide blood samples. The external dropout lacks numbers, but according to those who invited the patients to the study, very few refused to participate.
We consider that the aforementioned weaknesses have neither biased the study´s results nor validity in any crucial way.
Conclusion
Sublingual varices were found to be associated with all CVD risk factors included in metabolic syndrome as well as older age, male sex and smoking. These associations in combination with the unknown pathophysiological mechanism for the development of SV raises the question of whether there may be a common origin with CVD. If the sublingual vessels are influenced by the same pathophysiological mechanism as in CVD, this association could be of great importance for an early diagnosis, as these vessels are easily visible.
Acknowledgment
We would like to express our thanks to the staff at the Public Dental Clinic, Västra Vall, Varberg and at the private clinic Tre Tandläkare, Varberg, and to all participating patients.
Funding
This study was supported by grants from Sparbanksstiftelsen Varberg, the County of Halland and Praktikertjänst.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Akkaya N, Olmez D, Ozkan G. Evaluation of the factors associated with sublingual varices: a descriptive clinical study. Folia Morphol (Warsz). 2019;78(2):325–330. doi:10.5603/FM.a2018.0101
2. Al-Shayyab MH, Baqain ZH. Sublingual varices in relation to smoking, cardiovascular diseases, denture wearing, and consuming vitamin rich foods. Saudi Med J. 2015;36(3):310–315. doi:10.15537/smj.2015.3.10429
3. Hedstrom L, Albrektsson M, Bergh H. Is there a connection between sublingual varices and hypertension? BMC Oral Health. 2015;15:78. doi:10.1186/s12903-015-0054-2
4. Hedstrom L, Bergh H. Sublingual varices in relation to smoking and cardiovascular diseases. Br J Oral Maxillofac Surg. 2010;48(2):136–138. doi:10.1016/j.bjoms.2009.05.005
5. Accardo A, Pascazio L, Costantinides F, Gorza F, Silveri G. Influence of hypertension and other risk factors on the onset of sublingual varices. BMC Oral Health. 2021;21(1):235. doi:10.1186/s12903-021-01604-1
6. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357. doi:10.1097/01.hjh.0000431740.32696.cc
7. Barzideh N, Alaee A, Azizi A. The relationship between smoking and sublingual varices in the elderly. Oman Med J. 2021;36(4):e288. doi:10.5001/omj.2021.94
8. Lynge Pedersen AM, Nauntofte B, Smidt D, Torpet LA. Oral mucosal lesions in older people: relation to salivary secretion, systemic diseases and medications. Oral Dis. 2015;21(6):721–729. doi:10.1111/odi.12337
9. Southham JC, Ettinger RL. A histological study of sublingual varices. Oral Surg Oral Med Oral Pathol. 1974;38(6):879–886. doi:10.1016/0030-4220(74)90340-5
10. The national board of health and welfare. [Statistics on dental health 2020] Statistik om tandhälsa 2020 [Internet]. Stockholm: Socialstyrelsen. Report No.: 2021-9-7565; 2021. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2021-9-7565.pdf.
11. The national board of health and welfare. [Conditions and development in health care and dental care - Progress report 2020] Tillstånd och utveckling inom hälso- och sjukvård samt tandvård - Lägesrapport 2020 [Internet]. Stockholm: Socialstyrelsen. Report No.: 2020-3-6667; 2020. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2020-3-6667.pdf.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





