Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
The Association Between Acne Vulgaris, Acne Vulgaris with Nonspecific Facial Dermatitis, and Demodex Mite Presence
Authors Paichitrojjana A , Chalermchai T
Received 18 November 2023
Accepted for publication 18 January 2024
Published 22 January 2024 Volume 2024:17 Pages 137—146
DOI https://doi.org/10.2147/CCID.S450540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Anon Paichitrojjana, Thep Chalermchai
School of Anti-Aging and Regenerative Medicine, Mae Fah Luang University, Bangkok, Thailand
Correspondence: Thep Chalermchai, Email [email protected]
Background: Demodex mites can lead to various skin disorders, from non-specific dermatitis to conditions that mimic other diseases, making it challenging to diagnose accurately. Additionally, it has been reported that Demodex mites can cause skin conditions such as perioral dermatitis, pustular folliculitis, pityriasis folliculorum, blepharitis, and rosacea. Due to conflicting studies, there is a debate regarding the link between Demodex mites and acne vulgaris. This study aims to determine the prevalence of Demodex mites on the faces of individuals with acne vulgaris, acne with nonspecific facial dermatitis, and healthy facial skin to clarify the association.
Materials and Methods: This observational case-control study involved 120 participants aged 18– 37: 40 individuals with acne vulgaris only, 40 with acne and nonspecific facial dermatitis, and 40 healthy controls. The same dermatologist examined and diagnosed all participants to ensure accuracy before being grouped. The Standardized Skin Surface Biopsy (SSSB) method was used to detect Demodex mites in all three study groups. Furthermore, additional samples were collected randomly from acne lesions using the Superficial Needle Scraping (SNS) method in the two acne groups.
Results: The study found no significant difference in Demodex prevalence and high Demodex density rate between patients with only acne vulgaris and the control group (p> 0.05). However, acne patients with nonspecific facial dermatitis had a higher rate of Demodex prevalence and high Demodex density rate than the only acne vulgaris and control group (p< 0.05). The clinical symptoms of nonspecific facial dermatitis in acne patients strongly associated with Demodex mites are patchy red, dry, scaly skin, roughness, insect bite-like papules, and flushing.
Conclusion: Demodex prevalence and high Demodex density rate are not associated with acne vulgaris. Still, it is associated with acne and nonspecific facial dermatitis, particularly in patients with patchy redness, dry, scaly skin, roughness, insect bite-like papules, and flushing.
Keywords: acne vulgaris, demodicosis, Demodex mite, Demodex folliculorum, Demodex brevis
Introduction
Demodex mites are ectoparasites belonging to the family Demodicidae of the order Acari of the class Arachnida. Only two species are found in humans, D. folliculorum in the hair follicles and D. brevis, principally in the sebaceous glands. Demodex mites were found in most people over 60 years old (84% of the tested population) and in all people over 70 (100%).1 The prevalence of Demodex mites differs in various studies based on the sensitivity of the detection method.2 Studies have found that abnormal Demodex mite proliferation is more common in individuals who are obese, have high blood sugar levels, suffer from end-stage chronic renal failure, or have a weakened immune system. Moreover, repeated application of topical steroids and other immunomodulators on the face has been reported to increase the number of Demodex mites.3
An abnormal increase in the density of Demodex mites can cause several skin disorders grouped under the term demodicosis. Clinical presentations can vary in appearance, ranging from nonspecific facial dermatitis, such as dry, itchy, and sensitive skin, to mimicking other known skin diseases, such as eczema, seborrheic dermatitis, folliculitis, and acne vulgaris.3–9 This is why it is often misdiagnosed or underdiagnosed. Additionally, it has been reported that Demodex mite proliferation is a cause of multiple skin diseases such as pityriasis folliculorum, perioral dermatitis, pustular folliculitis, blepharitis, and rosacea.1,3,9–13
The role of Demodex mites in acne vulgaris is still controversial. While many studies show a positive relationship between acne vulgaris and Demodex mite infestation, some studies deny this relationship.14–22 The studies showed significant heterogeneity due to differences in study design, sample size, population, and detection methods. Additionally, the subjects were not screened for nonspecific facial dermatitis other than acne vulgaris, which could have resulted in inaccurate findings because demodicosis can present with nonspecific facial dermatitis and coexist with acne vulgaris.23
This study aims to clarify the association between acne vulgaris and Demodex mites by determining the prevalence of Demodex mites and high Demodex density rate on the face of individuals who have only acne vulgaris and those who have acne with nonspecific facial dermatitis in comparison to participants who have healthy facial skin.
Patients and Methods
Study Population
The study group consisted of 80 patients aged 18–37 who visited our clinic seeking consultation for acne vulgaris. All patients underwent a skin examination by a dermatologist to diagnose acne and detect coexisting nonspecific facial dermatitis. The diagnosis of acne vulgaris is based on the clinical presentation of blackheads, whiteheads, inflammatory papules, nodules, and scarring. Patients who did not have comedones were excluded. Nonspecific facial dermatitis is an abnormality of the facial skin that exhibits at least one of the following symptoms: patchy red, flushing, telangiectasia, dry, scaly skin, roughness, insect bite-like papules, itching, and stinging sensation. After conducting the skin examination, acne patients were divided into two groups of 40 each. One group presented with acne vulgaris only, while the other had acne with nonspecific facial dermatitis. The control group consisted of 40 healthy participants aged 18–37. The same dermatologist examined the facial skin of all the healthy participants, as in the case of the acne group, to ensure that they had normal facial skin and did not have acne vulgaris and nonspecific facial dermatitis.
Clinical Grading of the Acne Lesions
In both acne groups, the Investigator’s Global Assessment (IGA) was used to evaluate the severity of acne vulgaris.24,25 This system is graded from 0–4 depending on the descriptive criteria of facial acne only. Grade 0 (Clear) indicates the absence of acne lesions, although some residual hyperpigmentation and erythema may still be present. Grade 1 (almost clear) means a few scattered comedones and small papules can be found. Grade 2 (mild) indicates less than half of the face is affected with comedones, papules, and pustules. If more than half of the face is affected with many comedones, papules, and pustules, along with one nodule, it is considered Grade 3 (moderate). Grade 4 (severe) is when the entire face is covered with comedones, numerous papules and pustules, and a few nodules and cysts.
Demodex Detection
All three study groups underwent Demodex mite detection using the Standardized skin surface biopsy (SSSB) method. The procedure involved collecting two samples from both cheeks by placing a 1 cm2 square marked slide coated with cyanoacrylate glue onto each cheek for 60 seconds before removal. Afterward, immersion oil was applied to a slide and covered with a cover slip for microscopic examination. After examining slides from both cheeks to count mites, the average number per square centimeter was calculated by dividing the total by two.
Additionally, in the two acne groups, samples from acne lesions were randomly collected using the Superficial needle scraping (SNS) method by gently scraping five pustules with the convex surface of a number 18 needle and smearing them onto a slide. The preparation was stained with methylene blue and examined under a light microscope at 40× and 100× magnification.
A positive result of Demodex detection is observed under a microscope by scanning for the presence of eggs, larvae, nymphs, or adults of D. folliculorum or D. brevis. High Demodex density is defined as a density of Demodex mites higher than 5 mites/cm2 observed on SSSB11 or 3 mites/5 pustules on SNS.26
Statistical Analysis
Descriptive data was reported in frequency and percentage. Chi-square or Fisher’s exact tests were used to compare the acne vulgaris only group, acne with nonspecific facial dermatitis group with normal healthy skin group (the control). Logistic regression analysis was tested to predict Demodex presence and high Demodex density based on SSSB and SNS tests.
Results
A total of 120 participants were enrolled in this study: 40 patients with acne vulgaris only, 40 patients with acne and nonspecific facial dermatitis, and 40 healthy controls. All participants were aged 18–37 years, with 69 females and 51 males. Of 80 acne patients, 21 had mild acne, 37 had moderate acne, and 22 had severe acne, according to the IGA criteria. The common features of skin disorders in acne with nonspecific facial dermatitis group are patchy redness, dryness, scaliness, roughness, insect bite-like papules, and itchy skin, respectively. The results of SSSB and SNS reveal that the prevalence rate was 34.2%, and the high Demodex density rate was 20%, as shown in Table 1.
 |
Table 1 Clinical Characteristics and Demodex Prevalence |
The results in Table 2 show that the group of acne with nonspecific facial dermatitis had a significantly higher Demodex prevalence rate (62.5%) compared to the groups with only acne vulgaris (22.5%) and the control group (17.5%) with p<0.001. No significant difference was observed between the group with only acne vulgaris and the control group (p=0.576).
 |
Table 2 Comparison of Demodex Prevalence and High Demodex Density Between Normal Healthy Skin, Acne Vulgaris Only, and Acne with Nonspecific Facial Dermatitis Group, Based on SSSB and SNS |
Consistent with the Demodex prevalence rate, the acne with nonspecific facial dermatitis group had a significantly higher high Demodex density rate (40%) compared to the groups with only acne vulgaris (12.5%) and the control group (7.5%), with p-values of 0.001 and 0.005, respectively. While no statistically significant differences existed between the group with only acne vulgaris and the control group (p=0.356).
SNS positivity was detected in 11 acne patients, of which 3 cases were from the group of patients with acne vulgaris only, and 8 cases were from the group with acne and nonspecific facial dermatitis. Among the patients with acne, 11 tested positive for SNS, out of which 10 were also positive for SSSB. Moreover, 8 cases showed high Demodex density. Only one case tested negative for SSSB.
Table 3 shows the logistic regression analysis results indicating no significant difference in the prevalence of Demodex between females (39.1%) and males (27.4%), with a p-value of 0.184. Same as the high Demodex density rate among females (24.6%) and males (13.7%) was also not significantly different, with a p-value of 0.145. When comparing different age groups, no significant difference was found in the rates of Demodex prevalence and high Demodex density rate among participants aged 18–37 (p>0.05).
 |
Table 3 Results of Logistic Regression Analysis of Demodex Prevalence and High Demodex Density |
In the group of acne with nonspecific facial dermatitis, patchy redness (p<0.001), dry and scaly skin (p<0.001), roughness (p=0.001), insect bite-like papules (p=0.006), and flushing (p=0.02) were strongly associated with Demodex prevalence (Figures 1–5). However, there was no significant statistical association found in clinical features such as telangiectasia (p=0.091), stinging (p=0.146), or itching (p=0.296).
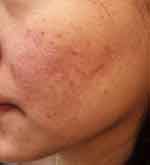 |
Figure 1 The left cheek has an erythematous patch with multiple small inflammatory papules and comedones. (SSSB = 9.5 mites/cm2, SNS = negative /5 pustules). |
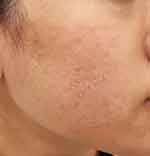 |
Figure 2 The right cheek has dry, scaly skin with multiple whitehead comedones and inflamed papules. (SSSB = 13.5 mites/cm2, SNS = negative /5 pustules). |
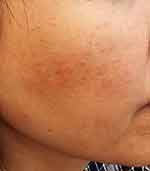 |
Figure 3 The right cheek has rough skin with comedones, and some indurated inflamed papules. (SSSB = 14 mites/cm2, SNS = 3 mites/5 pustules). |
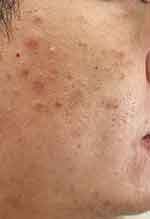 |
Figure 4 The right cheek has small papules resembling insect bites, comedones, inflamed papules, and areas of hyperpigmentation from prior acne. (SSSB = 15.5 mites/cm2, SNS = 4 mites/5 pustules). |
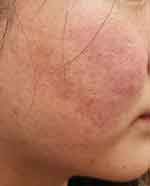 |
Figure 5 The right cheek has comedones, inflammatory papules, and flushing. (SSSB = 10 mites/cm2, SNS = negative /5 pustules). |
Discussion
Demodex infestation may cause various skin disorders, with rough, dry skin with follicular scales, papules, and pustules as common clinical features.3,4,17 Whether Demodex mites are responsible for many common skin diseases is still debated. Many studies have shown a significant association between Demodex infestation and rosacea.11,27 On the other hand, dermatologists still have controversy about the role of Demodex mites in the development of acne, as only a limited number of studies have been conducted to establish their association.14–22
This observational case-control study examined 120 participants aged 18–37: 80 patients (40 with acne vulgaris only, 40 with acne and nonspecific facial dermatitis) and 40 healthy controls.
According to this study, the prevalence rate of Demodex mites detected by SSSB in all three study groups was 33.3%. However, when SSSB and SNS were used together, the rate increased slightly to 34.2%. This outcome is consistent with previous studies that have reported the overall prevalence of Demodex mite to be around 34.8%, 40.36%, and 43%.15,17,20
It was found that 13.7% of acne patients exhibited positive results for SNS. This finding is aligned with research by Huang et al, which revealed that positive SNS was observed in 14.0% of acne patients compared to 83.0% of patients with papulopustular rosacea (P < 0.001).26 The results of SNS are lined up with those of SSSB. Of 11 SNS-positive acne patients, 10 tested positive for SSSB, and 8 had high Demodex density. Only one case tested negative for SSSB.
Previous studies have shown conflicting results regarding Demodex detection rates among males and females. Some studies show a higher prevalence of Demodex in males,15 while others show a higher prevalence in females.28 However, our study indicates no significant difference between males and females in the prevalence of Demodex and high Demodex density rate. This finding is consistent with many studies.15,18,19
In terms of age, our study and Akçınar et al found no significant difference in Demodex detection rates across age groups.19,21 However, many studies found a higher prevalence of Demodex among older individuals.17,18,22,28 These different findings may be due to the different sample sizes, mean age, and age ranges of the subjects in each study.
This study reveals a significantly higher prevalence of Demodex in the group of acne with nonspecific facial dermatitis (62.5%) compared to the group of acne vulgaris only (22.5%) and control (17.5%). Although the prevalence of Demodex in the group of only acne vulgaris is higher than in the control group, it is not significantly different.
When focusing on the high Demodex density rate, the group of acne with nonspecific facial dermatitis still had a significantly high Demodex density rate of 32.5% compared to the only acne vulgaris and control group with 12.5% and 7.5%, respectively. While no statistically significant differences were found in the acne vulgaris only group and the control group.
From this information, the prevalence of Demodex and high Demodex density rate showed a strong association with the group of acne with nonspecific facial dermatitis, while no association was found in the group of acne vulgaris only.
Compared to previous research, Okyay et al found that the prevalence of Demodex was not significantly different between patients with acne (20.7%) and those without acne (38.6%).15 A study by Manoyana et al discovered no significant difference in Demodex mite density between the acne and the control groups (P = 0.313).20 Similarly, Skrlin et al found no significant difference in Demodex prevalence between the acne and control groups, with 12% in both groups.29
However, a study by Zhao et al demonstrated that Demodex infestation was the most influential factor for developing acne vulgaris (OR = 5.565, 95% CI: 2.384–12.99 and p < 0.001).17 A study by Akçınar et al revealed a statistically significant difference in the prevalence of Demodex between the group with acne and the group without acne at 42.6% vs.12.3% (p <0.001).21
Regarding the high Demodex density rate, Maldonado-Gómez et al revealed that severe acne vulgaris was significantly associated with the high Demodex density rate (P = 0.001).30 A Meta-analysis study conducted by Zhao et al concluded that acne vulgaris is associated with Demodex infestation and suggested examining Demodex mites and using acaricidal therapies if regular acne treatments are ineffective.18
Studies about the association between Demodex mites and acne vulgaris exhibited significant heterogeneity due to variations in sample size, study design, country, population, detection method, and performing technique. This study found no significant association between Demodex mite and acne vulgaris. Although some previous studies have shown a significantly higher prevalence of Demodex in patients with acne vulgaris, this does not necessarily mean that Demodex mite is a causative factor for acne vulgaris. The reasons for the high prevalence of Demodex in acne patients remain unknown.
There are two possible explanations for this association. One possibility is that Demodex mites and acne vulgaris are causally related due to several mechanisms that suggest a connection. Demodex mites may cause acne by blocking hair follicles. The waste products and the associated bacteria may trigger a delayed hypersensitivity reaction, eventually leading to acne development.21 The other possibility is that acne vulgaris causes an abnormal proliferation of Demodex mites. While genetics is a significant factor in Demodex infestation, other potential causes exist. Bacterial infections could create an environment that promotes the growth of Demodex and weaken the immune response by decreasing the number of NK cells or lymphocytes. These cells control the mite population in the host, and their depletion could increase Demodex infestation.3,16,31,32
However, further studies are required in these regards.
Demodicosis can present in various ways and may be mistaken for other skin conditions. The appearance of the symptoms can range from non-specific facial dermatitis to more severe manifestations, depending on personal genetics, immunity, and the density of mites.2,18,31 In many cases, it may only present as nonspecific facial dermatitis, leading to underdiagnosis or misdiagnosis. This is why demodicosis has a lower incidence than it should be despite many people being infested with Demodex mites.3,18
In this study, all participants were examined by the same dermatologist to ensure accuracy. Demodex infestation can be presented with nonspecific facial dermatitis and coexist with acne vulgaris, leading to inaccurate outcomes. The clinical symptoms of nonspecific facial dermatitis in acne patients that are strongly associated with Demodex infestation, including patchy red (OR = 9.0, 95% CI: 3.0–26.9 and p < 0.001), dry, scaly skin (OR = 10.1, 95% CI: 3.0–33.9 and p < 0.001), roughness (OR = 7.7, 95% CI: 2.3–25.4 and p = 0.001), insect bite-like papules (OR = 9.4, 95% CI: 1.9–47.1 and p = 0.006), and flushing (OR = 9.4, 95% CI:1.4–62.0 and p = 0.020).
Acne patients can be infested with Demodex mites as the general population. Moreover, demodicosis can coexist with acne vulgaris, making diagnosis more challenging. If acne patients experience nonspecific facial dermatitis such as patchy red, dry, scaly skin, roughness, insect bite-like papules, or flushing, they should consider getting tested for Demodex mites because acne treatment may not improve these symptoms and can worsen demodicosis, making treatment more complicated.
There are limitations in this study that need to be taken into consideration. Firstly, the sample size is relatively small. Secondly, we only compared the prevalence and high-density rates of Demodex mites in different groups rather than the average Demodex density as in some studies. Thirdly, we did not use ether cleaning before SSSB or the two consecutive SSSB methods, which are more efficient at detecting mites than the SSSB method alone.33 This is because Thai patients with demodicosis commonly experience irritation, bleeding, and post-inflammatory hyperpigmentation after using these methods. Additionally, this study aims to use the SSSB results for comparison with previous studies that employed this method. Lastly, the study did not separate acne patients on whether they had previously received treatment, which could possibly impact the prevalence of Demodex mite observed.
Conclusion
The study revealed that patients with acne vulgaris only and the control group had no significant difference in Demodex prevalence and high Demodex density rates. Patients with acne and nonspecific facial dermatitis showed significantly higher Demodex prevalence and high Demodex density rates compared to acne vulgaris only and control groups.
Therefore, Demodex mite is not associated with acne vulgaris. However, acne with nonspecific facial dermatitis is associated with Demodex mite, particularly when symptoms such as patchy redness, dry, scaly skin, roughness, insect bite-like papules, and flushing occur.
Data Sharing Statement
Unavailable data, but the reader can personally request to access the data via the personal email of the authors.
Statement of Ethics
The present study was conducted in accordance with the World Medical Association Declaration of Helsinki. All subjects had given their written informed consent, and the study protocol was reviewed and approved by the Ethical Research Committee of Mae Fah Luang University, approval number COA 140/2023.
Acknowledgments
The authors express their gratitude to Mae Fah Luang University for providing financial support for the publication charges of this work. The research team would like to extend their appreciation to all the patients involved in the study and to the School of Antiaging and Regenerative Medicine at Mae Fah Luang University for providing the necessary research facilities.
Funding
This study did not receive any funding.
Disclosure
All authors had no conflicts of interest.
References
1. Post CF, Juhlin E. Demodex folliculorum and blepharitis. Arch Dermatol. 1963;88:298–302. doi:10.1001/archderm.1963.01590210056008
2. Rather PA, Hassan I. Human demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59(1):60–66. doi:10.4103/0019-5154.123498
3. Paichitrojjana A. Demodex: the worst enemies are the ones that used to be friends. Dermat Rep. 2022;14(3):9339. doi:10.4081/dr.2022.9339
4. Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31(8):618–626. doi:10.1111/j.1346-8138.2004.tb00567.x
5. Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170(6):1219–1225. doi:10.1111/bjd.12850
6. Forton FM, Germaux MA, Thibaut SC, et al. Demodicosis: descriptive classification and status of Rosacea, in response to prior classification proposed. J Eur Acad Dermatol Venereol. 2015;29(4):829–832. doi:10.1111/jdv.12926
7. Karincaoglu Y, Tepe B, Kalayci B, et al. Is Demodex folliculorum an aetiological factor in seborrhoeic dermatitis? Clin Exp Dermatol. 2009;34(8):e516–e520. doi:10.1111/j.1365-2230.2009.03343.x
8. Ayres S. Pityriasis folliculorum (Demodex). Arch Derm Syphilol. 1930;21(1):19–24. doi:10.1001/archderm.1930.01440070027002
9. Ayres S. Demodectic eruptions (demodicidosis) in the human. 30 years’ experience with 2 commonly unrecognized entities: pityriasis folliculorum (Demodex) and acne rosacea (Demodex type). Arch Dermatol. 1961;83:816–827. doi:10.1001/archderm.1961.01580110104016
10. Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol. 1993;28(3):443–448. doi:10.1016/0190-9622(93)70065-2
11. Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol. 1993;128(6):650–659. doi:10.1111/j.1365-2133.1993.tb00261.x
12. Dong H, Duncan LD. Cytologic findings in Demodex folliculitis: a case report and review of the literature. Diagn Cytopathol. 2006;34(3):232–234. doi:10.1002/dc.20426
13. Hsu CK, Hsu MM, Lee JY. Demodicosis: a clinicopathological study. J Am Acad Dermatol. 2009;60(3):453–462. doi:10.1016/j.jaad.2008.10.058
14. Baysal V, Aydemir M, Yorgancigil B, et al. The role of Demodexes on etiology and pathogenesis of acne vulgaris. Turkiye Parazitol Derg. 1997;21:265–268. Turkish.
15. Okyay P, Ertabaklar H, Savk E, et al. Prevalence of Demodex folliculorum in young adults: relation with sociodemographic/hygienic factors and acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20(4):474–476. doi:10.1111/j.1468-3083.2006.01470.x
16. Zeytun E, Yazıcı M. Incidence and density of demodex folliculorum and demodex brevis (Acari: demodicidae) in patients with acne in the province of Erzincan, Turkey. Int J Acarol. 2019;45:108. doi:10.1080/01647954.2018.1564790
17. Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12(12):1008–1015. doi:10.1631/jzus.B1100179
18. Zhao YE, Hu L, Wu LP, et al. A meta-analysis of association between acne vulgaris and Demodex infestation. J Zhejiang Univ Sci B. 2012;13(3):192–202. doi:10.1631/jzus.B1100285
19. Aktaş Karabay E, Çerman A A. Demodex folliculorum infestations in common facial dermatoses: acne vulgaris, rosacea, seborrheic dermatitis. An Bras Dermatol. 2020;95(2):187–193. doi:10.1016/j.abd.2019.08.023
20. Manoyana A, Chaithong U, Chiewchanvit S. Prevalence of hair follicle mites, Demodex folliculorum and Demodex brevis, on the facial skin of Chiang Mai university students, and the relationship with acne vulgaris. J Trop Med Parasitol. 2014;37:54–59.
21. Akçınar UG, Ünal E, Doğruman AF, Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type. Postepy Dermatol Alergol. 2018;35(2):174–181. doi:10.5114/ada.2018.75239
22. Erhan Z, Mustafa Y. Incidence and density of demodex folliculorum and demodex brevis (Acari: demodicidae) in patients with acne in the province of Erzincan, Turkey. Internat J Acarol. 2019. doi:10.1080/01647954.2018.1564790
23. Paichitrojjana A, Paichitrojjana A. Case series of demodicosis in acne vulgaris patients. Clin Cosmet Invest Dermatol. 2023;16:3363–3368. doi:10.2147/CCID.S441581
24. Guidance for industry acne vulgaris: developing drugs for treatment. Available from: www.fda.gov/downloads/Drugs/Guidances/UCM071292.pdf.
25. Alsulaimani H, Kokandi A, Khawandanh S, et al. Severity of acne vulgaris: comparison of two assessment methods. Clin Cosmet Invest Dermatol. 2020;13:711–716. doi:10.2147/CCID.S266320
26. Huang HP, Hsu CK, Lee JY. A new superficial needle-scraping method for assessing Demodex density in papulopustular rosacea. J Cosmet Dermatol. 2020;19(4):896–900. doi:10.1111/jocd.13082
27. Zhao YE, Wu LP, Peng Y, et al. Retrospective analysis of the association between Demodex infestation and rosacea. Arch Dermatol. 2010;146(8):896–902. doi:10.1001/archdermatol.2010.196
28. Zeytun E. Demodex (Acari: demodicidae) infestation in the elderly and its relationship with the skin parameters such as moisture, pH, and temperature: a cross-sectional study. Turk J Geriatr. 2017;20:142–150.
29. Skrlin J, Richter B, Basta-Juzbasić A, et al. Demodicosis and rosacea. Lancet. 1991;337(8743):734. doi:10.1016/0140-6736(91)90318-j
30. Maldonado-Gómez W, Guevara-Sánchez E, Guevara-Vásquez G, et al. [Association Between Demodex Infestation and Severe Acne Vulgaris: a Cross-Sectional Study of 168 Patients]. Asociación entre la infestación por el Demodex sp. y el acné vulgar grave. Estudio transversal de 168 pacientes. Actas Dermosifiliogr. 2022;113(8):758–764. Spanish. doi:10.1016/j.ad.2022.03.011
31. Akilov O, Mumcuoglu K. Association between human demodicosis and HLA class I. Clin Exp Dermatol. 2003;28(1):70–73. doi:10.1046/j.1365-2230.2003.01173.x
32. Akilov O, Mumcuoglu K. Immune response in demodicosis. J Eur Acad Dermatol Venereol. 2004;18(4):440–444. doi:10.1111/j.1468-3083.2004.00964.x
33. Forton FM, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97(2):242–248. doi:10.2340/00015555-2528
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
