Back to Journals » International Journal of Nanomedicine » Volume 19
The Application of Nanovaccines in Autoimmune Diseases
Received 6 November 2023
Accepted for publication 5 January 2024
Published 12 January 2024 Volume 2024:19 Pages 367—388
DOI https://doi.org/10.2147/IJN.S440612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Yuhong Tang,* Lili Li*
Department of Dermatology, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lili Li, Department of Dermatology, People’s Hospital of Guangxi Zhuang Autonomous Region, 6 Taoyuan Road, Nanning, 530021, People’s Republic of China, Tel +8618777190925, Email [email protected]
Abstract: Autoimmune diseases are diseases caused by the body’s chronic immune responses to self-antigens and attacks on the host’s own cells, tissues and organs. The dysfunction of innate immunity and adaptive immunity leads to the destruction of autoimmune tolerance, which is the most basic factor leading to pathogenesis. The optimal strategy for autoimmune diseases is to modify the host immune system to restore tolerance. The ideal effect of therapeutic autoimmune diseases is to eliminate the autoantigen-specific spontaneous immune response without interfering with the immune response against other antigens. Therapeutic nanovaccines that produce immune tolerance conform to this principle. Nanomaterials provide a platform for antigen loading and modification due to their unique physical and chemical properties. Nanovaccines based on nanomaterial technology can simultaneously enable antigens and adjuvants to be absorbed by immune cells and induce rapid and durable immunity. Nanovaccines have the advantages of being able to be designed and loaded and of better protecting antigens from premature degradation. Nanovaccines also have the ability to target specific tissues or cells through optimized design. We review the latest research progress of nanovaccines for autoimmune diseases and the design strategies of nanovaccines to promote the development of more effective nanovaccines for autoimmune diseases.
Keywords: nanovaccines, autoimmune diseases, immune tolerance
Introduction
Autoimmune diseases are diseases caused by the body’s chronic immune responses to self-antigens and attacks on the host’s own cells, tissues and organs, such as multiple sclerosis (MS), type 1 diabetes (T1D) and systemic lupus erythematosus (SLE).1 The pathogenesis of autoimmune diseases is very complex. Under the influence of genetic and environmental factors, the dysfunction of innate immunity and adaptive immunity leads to the destruction of autoimmune tolerance, which is the most basic factor leading to pathogenesis. The mechanism by which the immune system prevents pathogens from invading the body is very complex. The immune system can eliminate senescent cells and immune complexes in the body through various immune cells (macrophages, dendritic cells (DCs), T lymphocytes, B lymphocytes, etc.) and can recognize the body’s own tissues and cells as “self” to enable immune tolerance.2 If autoimmune tolerance is destroyed, the immune system will produce a strong and sustained immune response to self-molecules, cells or tissues, which induces cell and tissue damage and even triggers clinical symptoms, leading to autoimmune diseases.3
Central tolerance in the thymus and bone marrow plays an important role in maintaining immune system homeostasis. In the thymus, developing lymphocytes undergo positive selection in the cortex before maturing and entering the circulation. In a healthy host, lymphocytes that are potentially responsive to self-peptides are negatively selected and deleted in the thymic medulla. Upon leaving the thymus, mature T cells are selected through peripheral tolerance, resulting in the deletion or anergy of most self-reactive T cells. However, even under strict selection for central and peripheral tolerance, a small number of potentially self-reacting lymphocytes can still “leak out” into the periphery. The presence of these potentially autoreactive T and B lymphocytes and the ability of these cells to produce autoantibodies do not necessarily lead to pathology.4 Therefore, autoimmunity can be divided into “physiological” and “pathological” autoimmunity.5,6 Physiological autoimmunity is usually transient without evidence of clinical disease. For example, the production of natural autoantibodies helps to maintain homeostasis by eliminating degraded autoantigens and foreign antigens. The other type of autoimmunity is “pathological” autoimmunity. That is, when immune tolerance is destroyed, autoantibodies and autoreactive lymphocytes cause inflammatory responses, which lead to pathological autoimmunity, eventually leading to tissue damage and ultimately to autoimmune diseases.1 Therefore, the optimal strategy for autoimmune diseases is to modify the host immune system to restore tolerance. The ideal effect of therapeutic autoimmune diseases is to eliminate the autoantigen-specific spontaneous immune response without interfering with the immune response against other antigens. Therapeutic vaccines that produce immune tolerance conform to this principle.
In recent years, nanocarrier technology has developed rapidly and has been effective in improving the pharmacokinetics and bioavailability of drugs.7,8 Nanocarrier technology can realize the effective encapsulation and delivery of adjuvants and antigens, improve antigen stability and increase antigen loading, and provide the possibility for multiple routes of vaccination (such as oral administration, inhalation delivery, etc.).
Moreover, nanotechnology can lead to the creation of nanovaccines as targets for the immune response. Once nanovaccines are internalized by antigen-presenting cells (APCs), nanoparticles can induce the formation of inflammatory complexes, promote the secretion of inflammatory cytokines, and promote the recruitment of immune cells. In addition, adjusting the shape, particle size and charge of nanovaccines or performing surface modification can enhance the tissue targeting of vaccines, improve uptake efficiency and reduce cytotoxicity.9,10 Therefore, nanovaccines are a very promising vaccine platform.
Here, we review the latest research progress of nanovaccines for autoimmune diseases and the design strategies of nanovaccines to promote the development of more effective nanovaccines for autoimmune diseases in the future.
Etiology of Autoimmune Diseases
The breakdown of immune tolerance results in the immune system attacking host tissues, leading to the development of autoimmune diseases. Autoimmune diseases occur when the immune system attacks host tissues or initiates abnormal, excessive immune reactions; these disordered reactions cause autoimmune diseases, hypersensitivity reactions, and immune deficiency disorders.
Breakdown of Immune Tolerance Leads to Autoimmune Diseases
The main mechanism underlying the onset of autoimmune diseases is changes in immune tolerance, which lead to the generation of self-reactive T cells, B cells, and autoantibodies. The binding of autoantibodies to self-antigens can drive target cell destruction, mediate cell activation, and lead to the formation of antigen-antibody complexes that are deposited and induce inflammation; these phenomena ultimately damage tissues and organs.1 The breakdown of B-cell and T-cell peripheral immune tolerance is regulated by three main signaling pathways: the antibody secretion pathway, the Toll-like receptor (TLR) signaling pathway, and the complement pathway. In the antibody secretion pathway, helper T cells (Th) play a crucial role in breaking peripheral immune tolerance in incompetent B cells. Th can mediate the recognition of double-stranded DNA by certain incompetent B cells and promote their differentiation into plasma cells, thus breaking B-cell peripheral immune tolerance and promoting the production of antibodies. Regulation of this pathway can disrupt peripheral immune tolerance to self-antigens in autoimmune diseases. The TLR signaling pathway is regulated by TLRs, which are transmembrane proteins that are expressed mainly by immune cells. TLRs specifically recognize ligands, transduce signals, and induce the production of corresponding cytokines (such as IL-1). In the absence of helper T cells, the TLR pathway induces antibody production and activates the costimulatory signals B7.1 and B7.2 in B cells. Costimulatory signals further activate more self-reactive T cells. In the complement pathway, a lack of complement components leads to the breakdown of peripheral immune tolerance. A deficiency of early components in the classical complement pathway triggers the activation of incompetent B cells and the secretion of various lymphokines that regulate immune responses, resulting in autoimmune diseases.
Inducing Immune Tolerance is an Important Strategy for Treating Autoimmune Diseases
The pathogenesis of autoimmune diseases is very complex, involving innate immunity and adaptive immunity. The most basic mechanism leading to pathogenesis is the destruction of autoimmune tolerance. Under normal physiological conditions, autoreactive T and B cells with high affinity are eliminated during central (thymus) selection. Autoreactive T and B cells with low affinity that have not been eliminated in central selection are released into the peripheral blood and then encounter immature dendritic cells (iDCs) lacking cell surface costimulatory receptors in the peripheral circulation, resulting in the dysfunction or suppression of autoreactive T cells to produce peripheral immune tolerance through apoptosis or regulatory T-cell (Treg) mechanisms.
DCs are the most important APCs in vivo and are important regulators of innate and acquired immunity. The T-cell immune response is a key part of the organism’s immune response to antigens, and DCs are the only APCs that can activate naive T cells; therefore, DCs play a very important role in inducing T-cell activation or tolerance. DCs have two different states, immature and mature, which are different stages of their development. Mature DCs express CD80, CD86, CD40 and other costimulatory receptors on the cell surface and secrete interleukin-6 (IL-6), transforming growth factor-beta (TGF-β), IL-12 and interferon-γ (IFN-γ), triggering adaptive immunity and inducing initial T cells to differentiate into CD4+ helper T (CD4+ Th) cells, including Th1, Th2, Th17 and CD4+CD25+ Foxp3 Tregs. Normally, most DCs in organisms are immature and have strong phagocytosis and swallowing functions. In addition, DCs also express chemokine receptors (CCR1, CCR5, and CCR6) and therefore have a strong migratory ability, as well as the ability to take up and process antigens. However, low-level expression of histocompatibility complex II (MHC-II) and costimulatory receptors on its surface cannot activate T cells but do induce antigen-specific T-cell dysfunction or apoptosis, thus inducing antigen-specific tolerance11 (Figure 1).
 |
Figure 1 Functions of DCs in autoimmunity and tolerance. DCs interact with T cells through MHC-I and -II. Activated pro-inflammatory DCs presenting autoantigens that trigger autoreactive killing CD8+ and CD4+ helper T cells. Tolerant DCs (Tol DCs) express different co-stimulatory receptors and cytokines that induce Tregs and deactivate autoreactive CD4+ and CD8+ T cells. Data from Cifuentes-Rius et al.11 |
DCs induce peripheral immune tolerance by inducing antigen-specific T-cell apoptosis or by inducing immune tolerance through Tregs. In the immune response, the activation of antigen-specific T cells can only be achieved through contact with APCs. APCs with T-cell apoptosis factors (such as nitric oxide (NO), FasL, etc.) can induce antigen-specific T-cell apoptosis, thus inducing immune tolerance. In addition, DCs can induce immune tolerance by activating Tregs. Tregs are a specific subset of T cells that express FOXP3, and they perform immunoregulatory functions. Tregs are divided into different subgroups, such as CD4+ Tregs, CD8+ Tregs, and NK Tregs, and current research has focused primarily on the therapeutic potential of CD4+CD25+FOXP3+ Tregs. Tregs can exert immunoregulatory effects through various mechanisms. Under steady-state conditions, Tregs express the IL-2 receptor CD25, which binds to IL-2 with high affinity; this interaction maintains low levels of IL-2 and prevents excessive immune responses. Tregs can also produce immunosuppressive molecules, such as IL-10, IL-35, and TGF-β; express the extracellular enzymes CD39 and CD73 to degrade extracellular ATP; and secrete granzyme and perforin to kill APCs. Additionally, Tregs can bind to the costimulatory ligands CD80 and CD86 on the surfaces of APCs via cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), preventing these costimulatory ligands from binding to CD28, increasing T-cell activation thresholds, and limiting T-cell activation.12 Tregs can also inhibit the proliferation of CD8+ cytotoxic T lymphocytes via the secretion of exosomes.13 The main mechanism by which Tregs induce immune tolerance is as follows: Tregs inhibit the proliferation and activation of effector cells by secreting anti-inflammatory cytokines. The release of granzyme B from Tregs induced apoptosis of effector cells and played a cytotoxic role. Tregs interfered with effector cell metabolism and inhibited effector cell proliferation and killing. Tregs inhibit the maturation and activation of DCs to block antigen presentation and regulate autoimmunity. Current studies suggest that when there is no inflammatory stimulus signal in the organism, immature peripheral DCs can take up the antigenic components of apoptotic cells, enter secondary lymph nodes, and stimulate the precursor of T cells to differentiate into Tregs instead of effector T cells. When inflammation occurs, sensitized Tregs return to the peripheral tissue, regulate the maturation of DCs and inhibit the proliferation of antigen-specific T cells by feedback, inducing peripheral immune tolerance to antigens. Tregs downregulate the levels of MHC and costimulatory receptors on the DC surface by negative feedback, thus inhibiting the antigen presentation function of DCs via cellular contact. However, DCs in contact with Tregs could no longer stimulate T-cell proliferation but could induce the generation of more Tregs, thus inducing immune tolerance. Therefore, the induction of immune tolerance may be the best strategy for the treatment of several autoimmune diseases.
Nanomaterials Used as Nanovaccines
Nanomaterials have emerged as a novel tool for improving the effectiveness of subunit vaccines or other types of vaccines. Nanomaterials provide a platform for antigen loading and modification due to their unique physical (size, morphology, large surface area) and chemical (surface characteristics, high reactivity) properties. Nanovaccines based on nanomaterial technology can simultaneously enable antigens and adjuvants to be absorbed by immune cells or tissues and induce rapid and durable cellular immunity and humoral immunity. In addition, nanovaccines have a variety of drug delivery routes and good stability, presenting many advantages compared with traditional subunit vaccines. In addition, nanovaccines have the advantages of being able to be designed and loaded and of better protecting antigens from premature degradation. Nanovaccines also have the ability to target specific tissues or cells through optimized design.
Therefore, in the process of nanovaccine preparation, the selection of nanomaterials is highly critical, and different nanomaterials provide disparate functions in nanovaccines. Nanomaterials can not only transport antigens but also carry therapeutic cargo through biological barriers such as mucous membranes to protect antigens from being degraded in advance. In addition, some nanomaterials can target lymphoid tissue to effectively present antigens and adjuvants to immune cells.
Inorganic Nanoparticles
Inorganic nanoparticles are nanocarriers composed of a solid core to which antigen(s) of interest are conjugated. Such nanoparticles are applied in vaccinology as antigenic adjuvants and antigenic carriers to enhance the immune response. The advantages of inorganic nanoparticles for nanovaccines are hard structure, stable performance and controllable synthesis, but they have the disadvantage that they cannot be made biodegradable. As nanovaccine carriers, inorganic nanomaterials need to be modified to change their physical and chemical properties to improve their biocompatibility. Currently, a variety of inorganic nanoparticles have been used as vaccine carriers, particularly carbon, gold, silica and calcium nanoparticles (Figure 2).
Gold Nanoparticles
Gold nanoparticles (GNPs) can interact with different functional groups or high-affinity ligands, making their surfaces easy to modify and therefore making them suitable candidates for nanovaccine manufacturing processes.14 GNP has a high affinity for sulfhydryl groups, which can be used to modify the surface of GNP to improve biosafety and pharmacokinetics. In addition, GNPs possess good biocompatibility, low immunogenicity and high antigen loading capacity, as well as strong immune stimulation, which can activate immune cells and induce the production of inflammatory cytokines. Therefore, GNP can be applied not only as an antigen carrier but also as an adjuvant to improve the efficacy of vaccines.15 Studies have shown that GNPs can effectively deliver antigens to major APCs such as DCs, promoting the downstream immune response, cross-presentation, and CD8+ cytotoxic T-cell response.16 In addition to passive targeting, which changes the size and shape of GNPs to make them more easily internalized by individual cell types, active targeting can be achieved through surface modification and functionalization. For example, GNPs modified with DEC205, CD40, CD11c, or mannose antibodies can be selectively absorbed by DCs through receptor-mediated endocytosis. Almeida et al reported that GNP-coupled OVA (AuNP-OVA) prevented the degradation of antigenic peptides and targeted antigen delivery to APCs. After subcutaneous administration of AuNP-OVA, DCs released a large amount of cytokines and induced a strong antigen-specific response in mice.17 Yeste et al constructed gold (Au) nanoparticles loaded with the AhR ligand ITE (2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester) and MOG35-55 antigen to promote FoxP3+ Treg differentiation by activating the AhR signaling pathway, as well as promote the generation of CNS-specific Tregs by DCs through carrying the MOG35-55 antigen. The results showed that the NPITE+MOG treated DCs exhibited a tolerogenic phenotype and promoted differentiation of Tregs in vitro. In addition, NPs carrying ITE and MOG35–55 expanded the FoxP3+ Treg compartment and inhibited the development of MS in experimental autoimmune encephalomyelitis (EAE) mouse model18 (Table 1). They also constructed Au nanoparticles loaded with ITE and β cell antigen proinsulin for the treatment of Type 1 diabetes (T1D). The NPITE+Ins can also induce the tolerogenic phenotype and promote the differentiation of FoxP3+ Treg cells, thus reestablishing the tolerance of T1D (Table 1).19
 |
Table 1 Application of Nanovaccines in the Treatment of Autoimmune Diseases |
The advantages of gold nanoparticle carriers include their hard structure, stable properties and controllable synthesis, but their disadvantages include the fact that they are not biodegradable.26
Carbon Nanoparticles
The surface of carbon nanoparticles is easily functionalized and modified to enable use as adjuvants or carriers of different kinds of vaccines. The ability of carbon nanoparticles to carry antigens and stimulate immune responses is usually influenced by structural and physical characteristics. Carbon nanoparticles with immunomodulatory properties have been reported thus far, including fullerenes, carbon nanotubes, graphene/graphene oxide, etc.
Carbon nanotubes (CNTs) are seamless nanotube-like crystals curled up by graphite lamels containing hexagonal meshes. CNTs can be divided into single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs). Compared with SWCNTs, MWCNTs are more easily functionalized. SWCNTs may bind to proteins, enter endosomes by endocytosis and transport to lysosomes, or form phagosomes by phagocytosis.27 MWCNTs are lamellar carbon nanotubes formed from multiple concentric graphene sheets that spontaneously form coiled or bunched structures, typically entering cells by endocytosis.28 CNTs need to be functionalized to function as drug carriers. The modification can effectively improve the drug-carrying capacity, dispersion and biocompatibility of CNTs, which can not only be used to carry chemotherapy drugs but also gene drugs, antigens and antibodies. Hassan et al demonstrated that the surface properties of carbon nanotubes affect their efficacy as vaccine nanocarriers in vitro and in vivo and that MWCNTs stimulate immune responses by enhancing antigen uptake by cells.29 Moraes et al reported that stimulation of APCs with MWCNTs prior to neuroantigen presentation affected the development of the Th17 subset of encephalitogenic CD4+ T lymphocytes, resulting in less severe experimental autoimmune encephalomyelitis (EAE) that could be applied to treat multiple sclerosis (MS). MWCNTs are internalized by APCs and stimulate the production of IL-27 in these cells, which affects the development of encephalitogenic Th17 lymphocytes. The adoptive transfer of encephalitogenic T cells devoid of Th17 cells resulted in less severe EAE.30
Graphene quantum dots (GQDs) are 1–10-layer-thick sheets of graphene, a carbon allotrope consisting of a single layer of carbon atoms in a honeycomb structure. Previous studies have shown that GQD can suppress proinflammatory human T-cell responses via the induction of tolerogenic DCs (Tol DCs).31 Tosic et al reported the therapeutic capability of GQDs in an EAE model. GQD treatment reduced the number of interferon-γ-expressing T-helper (Th)1 cells and the expression of Th1 transcription factor T-bet and pro-inflammatory cytokine tumor necrosis factor, interleukin-1, and granulocyte-macrophage colony-stimulating factor in the lymph nodes and central nervous system (CNS) immune infiltrates, as well as reduced immune infiltration, demyelination, axonal damage and apoptotic death in the CNS of EAE animals.32
With further research, the toxicity of carbon nanoparticles has also attracted increasing amounts of attention from researchers. The application of carbon nanoparticles in the development of drugs, vaccines and gene therapies still needs to be improved, and efforts should be made to develop carbon nanoparticles that are safe, efficient and functional.
Silicon Nanoparticles
Silicon nanoparticles (SiNPs) can be used as carriers for anticancer drugs, biomacromolecules and polypeptides because of their simple preparation process. The size of SiNPs can be adjusted from 3 nanometers to several hundred nanometers, their surface can be functionalized by a large number of different organic, biological, or other functional groups, and the size of the (reservoir) pore in the core can be adjusted. In addition, SiNPs also have good biocompatibility and biodegradability. Chemical modification of SiNPs can enrich the silanol groups on their surface to improve cell recognition, cell interaction, cell uptake and absorption of specific biomolecules. Compared with other porous silica nanoparticles, mesoporous silica nanoparticles (MSNs) with pore sizes of 2 to 50 nm serve as nanopores and adjuvants to effectively deliver antigens. MSNs are widely used in the field of biomedicine because of their large specific area, controllable pore size, easy surface modification and good biocompatibility. In addition, MSNs are able to control the release of drugs or proteins based on their size, shape and surface modification. These properties of MSNs make them an attractive vehicle for the targeted delivery and release of biomolecules such as nucleic acids, enzymes, proteins and peptides. Nguyen et al prepared MSNs loaded with myelin oligodendrocyte glycoprotein (MOG) peptide for the treatment of multiple sclerosis (MS). The high antigen-loading capacity of MSNs enables them to deliver sufficient amounts of their own antigens to spleen APCs, even at small doses. Immunization with MSNs loaded with autoantigens produced Foxp3+ regulatory T cells in the spleen and produced systemic immune tolerance in EAE mice, reducing central nervous system infiltration of APCs and autoreactive CD4+ T cells. Antigen-specific inhibition of EAE in the advanced chronic stage of the disease20 (Table 1).
Iron Oxide Nanoparticles
Iron oxide nanoparticles, which have unique magnetic properties, exhibit outstanding performance and significant potential for use in biomedical applications, such as magnetic resonance imaging and targeted drug delivery.33 As a delivery system, iron oxide nanoparticles can transport antigens to the immune system or serve as immune adjuvants to enhance antigen processing. To prevent the aggregation and oxidation of magnetic nanoparticles after synthesis, these nanoparticles can be coated with single-layer ligands, polymers, combinations of polymers and biomolecules (phospholipids and carbohydrates), or inorganic materials (silica and gold). These coatings not only stabilize nanoparticles in solution but also facilitate the binding of various biological ligands (antibodies, proteins, transferrin, folate) that are needed for medical applications to the surface of the nanoparticles. These ligands can be chemically coupled to polymer-coated magnetic nanoparticles, endowing them with targeting capabilities. Therefore, iron oxide nanoparticles can be applied in nanovaccines. Pusic et al reported that iron oxide nanovaccines containing malaria parasite schizont surface protein 1 (recombinant merozoite surface protein 1, rMSP1) can elicit a robust antigen-specific antibody response in the absence of other adjuvants.34 Rezaei et al loaded hepatitis B surface antigen and mannose onto the surface of iron oxide nanoparticles to enhance the efficacy of the hepatitis B surface antigen vaccine. The chemical adsorption of mannose and HBsAg, which target immune cells, on the surface of iron oxide nanoparticles led to enhanced vaccine efficacy, indicating that mannose-mediated immune targeting has a positive impact on improving immune responses in various ways.35
However, the morphology and size of iron oxide nanoparticles are contributing to particle cytotoxicity. Studies suggest that rod-shaped iron oxide nanoparticles are more toxic than spherical iron oxide nanoparticles.36 Additionally, iron oxide nanoparticles induce IL-1β release by macrophage in a size- and dose-dependent manner. Moreover, PEG-coated 10-nm iron oxide nanoparticles were taken up by cells at a higher than 30-nm particles. Research also indicates that slightly larger iron oxide nanoparticles are more effective at inhibiting the release of inflammatory factors by macrophages. The modulatory effects of iron oxide nanoparticles on proinflammatory macrophages are inconsistent, which can be attributed to differences in coating modifications and sizes. Hence, considering the size and coating performance of these materials is crucial for their application.37,38
In addition, inorganic nanoparticles mainly carry antigens through electrostatic adsorption and other mechanisms, which easily leads to antigen instability and makes it difficult to preserve vaccines for a long time. Therefore, there is an urgent need to develop new strategies for stably carrying antigens to promote the further clinical application of inorganic nanoparticle vaccines.
Polymer Nanoparticles
Polymer NPs are solid structures with particle sizes ranging from 10 nm to 500 nm. They have high biodegradability, good biocompatibility and safety. Some polymer NPs can also be used as adjuvants to enhance the immunoactivity of vaccines. In addition, polymer NPs have low mucosal adhesion toxicity, a stable structure and highly controllable drug release, which can enhance drug stability and utilization. Polymer NPs can not only protect antigens from enzymatic hydrolysis but also have the effects of antigen storage, APC targeting and immune regulation. They also have the advantages of easy surface modification, good stability and high safety, so they are increasingly widely used in the field of biological vaccine adjuvants. Polymers commonly used in nanovaccine carriers can be divided into synthetic polymer nanocarriers and natural polymer nanocarriers. Synthetic polymers mainly include copolymers, such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone, polyanhydride, dendritic macromolecules, and poly(lactic acid). Natural polymers include chitosan, alginate, hyaluronic acid, amylopectin, inulin, and glucan.
PLGA is a commonly used drug delivery system.39 PLGA is a copolymer formed by random polymerization of polylactic acid (PLA) and poly(glycolic acid) (PGA). PLGA supports DC targeting and can be widely used as a vaccine delivery platform for many diseases. PLGA/PLA-based NPs can deliver encapsulated antigens to APCs in a controlled and sustained manner. Controlling the proportion of monomers and chemical modification is expected to enhance the hydrophobicity and drug release characteristics of PLGA and produce a durable immune response and immune memory. PLGA NPs can encapsulate drugs or antigens, which can not only protect drugs or antigens from enzymatic hydrolysis but also release drugs or antigens slowly for a long time to achieve long-term drug action or durable immune protection for antigens. For example, PLGA-based vaccine adjuvants can release antigens for a long time due to their good slow release effect, providing a more effective immune protection response for the organism, reducing the number of immune times, and achieving economical and effective protection for the body. Polymer nanovaccines provide safer and more effective allergen-specific immunotherapy (AIT) because the polymer material protects the allergen from phagocytosis by active mast cells while promoting the production of protective IgG antibodies by B cells.40 In addition, the use of a PLGA-encapsulated antigen can effectively save the amount of antigen used. Due to its good safety and adjuvant effect, the use of PLGA as a vaccine adjuvant has attracted extensive attention. Recently, nanovaccines that deliver autoantigens based on PLGA NPs without immunosuppressive drugs have been widely studied for the treatment of multiple sclerosis (MS). For example, Cappellano et al used PLGA NPs to envelope MOG35-55 autoantigen and recombinant (r) IL-10 for the treatment of MS. PLGA NPs located with MOG35-55 and rIL-10 reduced histopathological damage of central nervous tissue, as well as decreased secretion of IL-17 and IFN-γ in spleen T cells induced by MOG35-55, significantly ameliorating the course of EAE21 (Table 1). Hunter et al prepared a nanovaccine (PLP139-151-PLG-PEMA) against EAE mice using PLGA nanoparticles modified with poly (ethylene-co-maleic acid) (PEMA) as a surfactant and coupled myelin peptide to PLG-PEMA. PLP139-151-PLG-PEMA can significantly reduce the CNS infiltration of encephalitogenic Th1 (IFN-γ) and Th17 (IL-17a) cells as well as inflammatory monocytes/macrophages, and the use of these nanovaccines at the onset of MS can significantly improve persistent disease and prevent recurrence at the peak of acute disease22 (Table 1). Similarly, Al-Ghobashy et al loaded recombinant human myelin basic protein (rhMBP) onto poly (ε-caprolactone) (PCL) NPs to construct nanovaccine rhMBP PCL NPs. The PCL NPs are hydrophobic and provide an improved drug delivery system for rhMBP, allowing rhMBP to cross the BBB to the mouse brain. The nanovaccine composed of rhMBP and PCL NPs significantly enhanced the efficacy of rhMBP as a therapeutic vaccine and ameliorated EAE symptoms in an EAE animal model23 (Table 1).
The mechanism by which nanovaccines induce immune tolerance involves several aspects, including increased expression of programmed cell death ligands, downregulation of APC-positive costimulatory molecules, inhibition of T-cell activity, and immune regulation of hepatic sinusoidal endothelial cells (LSECs). McCarthy et al constructed a nanovaccine composed of Ag-encapsulated NPs composed of poly(lactide-co-glycolide) [PLG(Ag)] to treat autoimmune model mice with Th1/17 dysfunction-relapse-remitting experimental EAE (R-EAE). The results showed that PLG(Ag) increased programmed death ligand 1 (PD-L1) expression on APCs, while programmed cell death receptor 1 (PD-1) blocking reduced tolerance induction. Therefore, PD-1/PD-L1 was confirmed to be involved in restoring immune tolerance.41 Another study also demonstrated that PLGA(Ag) downregulates the positive costimulatory receptors CD86, CD80, and CD40 on the surface of APCs and inhibits the proliferation of T cells.42 In addition, Kishimoto et al found that codelivery of antigen and rapamycin using PLGA NPs led to durable antigen-specific immune tolerance in mice with EAE.43 They also studied PLGA tolerance nanovaccines containing RPM, chicken OVA, and MHC-II-restricted OVA323-339 peptide, which inhibited T-cell activation and induced antigen-specific Tregs and regulatory B cells (Breg cells) after subcutaneous or intravenous administration24 (Table 1). They were surprised to find that the PLGA tolerance nanovaccines induced durable antigen-specific immune tolerance in EAE model mice for at least 200 days, even after repeated antigen attacks. Moreover, neither free RPM nor nanoparticle-encapsulated antigens nor nanoparticles containing only RPM had this effect.24 This may be because PLGA nanoparticles can encapsulate drugs or antigens in nanoparticles, which can not only protect drugs or antigens from enzymatic hydrolysis but also enable long-term slow release of drugs or antigens to achieve the purpose of long-term drug action or durable immune protection of antigens.
The size of PLGA NPs can affect the pathway into cells and the type and strength of immune response mediated by PLGA NPs. Therefore, the size of the PLGA nanovaccine can be adjusted to effectively promote DC-targeting phagocytosis. For example, An et al prepared three NPs with particle sizes of 20 nm, 40 nm and 100 nm based on PLGA NPs and compared the lymph node transport efficiency and effective targeting of DCs in LNs with different particle sizes.44,45 Among the three kinds of NPs, 20 nm NPs entered the LNs the fastest and were efficiently taken up by DCs, thus rapidly inducing an effective immune response. NPs with a size of 40 nm can also enter LNs quickly, but the uptake efficiency of DCs is lower than that of 20 nm NPs, so the induced immune response is weaker than that of the former. However, although 100 nm NPs took longer to enter LNs, their immune duration was stronger than that of the previous two NPs. The time and way of the three NPs entering LNs were different, so the strength and duration of the mediated immune response were different. NPs with a diameter of 20–100 nm can enter LNs directly through the paracellular pathway, deliver vaccines to LNs, and generate a strong immune response. However, NPs larger than 100 nm were recruited at the injection site through the cell carrier pathway, and after the uptake of a large number of APCs, the vaccine migrated to the KNs and produced a durable immune response.46 In addition, cationic modification of PLGA NPs enhanced the antigen loading rate of the nanoparticles.47 Commonly used cationic modifiers are chitosan, polylysine, polyvinylimine, polydopamine and liposomes containing cationic dimethyl dioctadecyl ammonium bromide.48 These cations can be directly modified by electrostatic adsorption to improve the antigen loading rate of PLGA nanoparticles. After cationic modification, the surface of nanoparticles can not only load more antigens through electrostatic adsorption but also induce the body to produce strong cellular immune activity.48 The surface charge of NPs plays an important role in cell uptake. Positively charged NPs are more susceptible to cell internalization because positively charged NPs are more readily taken up by cells due to electrostatic interaction with negative charges in the plasma membrane. Moreover, after the positively charged NPs enter the lysosome, they can rapidly scavenge a large amount of H+ in the lysosome, thus causing the influx of a large number of salt ions in the cytoplasm, leading to the breakdown of the lysosome and the escape of the effective lysosome. The escaped NPs are then presented by MHC-I molecules, which can effectively induce the activation of Th1 cells and produce a strong cellular immune response.
Chitosan is a kind of natural polysaccharide cationic polymer material that has the advantages of good biocompatibility and biodegradability, as well as low toxicity. Chitosan is also widely studied and applied in the field of nanomedicine.49 Chitosan NPs as immune adjuvants can significantly enhance the local response and the antibody response of the system. As adjuvants, chitosan nanoparticles have the properties of mucosal absorption, immune stimulation, osmotic promotion, slow release and control, and specific targeting. Many studies have shown that chitosan is an effective mucosal vaccine adjuvant for a variety of antigens, such as ovalbumin protein (OVA).50 In addition, chitosan can temporarily open intercellular tight junctions, facilitating the passage of drugs through the intercellular space, thereby improving tissue penetration and enhancing humoral and cellular immunity.51 Chitosan-loaded antigen NPs can be absorbed by APCs, epithelial cells and M-cells and transported by these cells to lymphoid tissues to stimulate the immune response.52,53
Nanomicelles are amphiphilic molecules with a hydrophobic core and a hydrophilic shell that can self-assemble to form spherical particles similar to a core-shell structure. Nanomicelles can carry lipophilic drugs within their core. As a drug delivery carrier, nanomicelles have the advantages of good stability, long retention time in vivo, high drug loading capacity and wide drug loading range.54 In recent years, nanomicelles have attracted much attention as vaccine delivery adjuvants. Depending on the chemical properties of the nanomicelles and the size and properties of the antigens, the nanomicelles can deliver vaccine candidates in two different ways. One method is to covalently link the antigen to the hydrophilic group of micelles. The combined product can induce APCs to generate a strong immune response to antigen stimulation. Another approach is to produce amphiphilic polypeptides consisting of peptides attached to the tails of hydrophobic alkyl groups that self-assemble into micellar structures in aqueous medium. For example, Li et al designed an amphiphilic diblock copolymer that can penetrate into lymphatic vessels and reach draining lymph nodes quickly after subcutaneous injection and then deliver antigen OVA and immune-stimulating adjuvant (CL264) to DCs. This amphiphilic diblock copolymer, to a certain extent, enhances antigen uptake by DCs, cytokine production and cross-presentation of antigen to T cells and promotes strong cellular immunity in animals.55
Liposome-Based Nanoparticles
Liposome carrier systems are considered to be one of the most flexible and successful drug delivery systems due to their advantages of biocompatibility, strong encapsulation capacity, high loading capacity and variable surface modification methods. Liposomes consist of a biocompatible and biodegradable bilayer of phospholipids, which are amphiphilic and characterized by a lipophilic tail and a hydrophilic head on the same molecule. Antigens or adjuvants can be inserted into the hydrophilic core of the liposome or into the amphiphilic lipid bilayer to protect the antigen from degradation during transport and thus efficiently deliver the antigen to immune cells. The size, lamellar property, surface charge and bilayer fluidity of liposomes influence the efficacy of liposome-based nanovaccines in vivo. Smaller liposomes with particle sizes <100 nm were more likely to activate Th2 responses, while larger liposomes with particle sizes >400 nm were more likely to activate Th1 responses.56 Nanovaccines based on cationic liposomes can better encapsulate negatively charged antigenic substances and significantly enhance humoral and cellular immune responses. In addition, monolayer lipid nanomaterials have limited stability in serum, while the stability of multilamellar vesicle (MLV) NPs has improved, as well as enhanced stable antigen embedding in the hydrophilic core or lipid bilayer.57
However, to improve the effects of lipid nanoparticles within the body, a series of challenges, such as the easy degradation of antigens or mRNAs, the renal retention of nanoparticles, difficulty in specifically targeting tissues, challenges in achieving nuclear escape, and issues related to adjuvant-induced inflammation or toxicity, still needs to be addressed.58 Research on approaches to increase the stability and blood retention of nanoparticles has suggested that modifying lipid nanoparticles with polyethylene glycol (PEG) can enhance their stability by reducing particle aggregation and increasing particle hydrophilicity, thereby decreasing their clearance by the mononuclear phagocyte system (MPS) and extending their circulation time.59
Biomembrane-Based Nanoparticles
Biomimetic membrane-cloaked nanoplatforms can deliver antigens to the target site efficiently due to their diverse functions and have the advantages of high biocompatibility, long-term circulation in vivo, unique antigen characteristics, good flexibility and so on. At present, many studies have been conducted on preparing vaccines using biomimetic membrane nanomaterials.
Biomembranes exhibit a bilayer structure, mainly composed of lipids, proteins and a small amount of sugars, which undertake the important tasks of material exchange and information transmission between cells.60 In addition, the surface of the biomembrane retains the important molecular structure and physical and chemical properties of the surface of living cells. For example, the erythrocyte cell membrane expresses the CD47 molecule and signal-regulatory protein α (SIRPα) receptors to send the “don’t eat me” signal to the body, thus easily escaping phagocytic immune cell-controlled clearance and degradation.61 Nanodrug delivery systems based on erythrocyte membranes have been widely studied and applied [9]. Therefore, the encapsulation and assembly of biomembranes and drugs into biomimetic drug-carrying nanoparticles can not only increase the biocompatibility of nanoparticles but also greatly reduce the probability that nanoparticles will be recognized and cleared by reticuloendothelial tissue or mononuclear phagocytes in the human immune system after entering the blood and effectively inhibit the formation of protein coronas on the surface of nanoparticles.62 Natural biomimetic nanocarriers have the following characteristics: natural biomimetic nanoparticles retain the basic physical and chemical properties and biological activity of the cell membrane surface, have good biocompatibility, are safe and degradable, and have low immunogenicity. Natural biomimetic nanocarriers can evade blood clearance and realize long-term circulation and slow drug release. The surface of natural biomimetic nanoparticles can be genetically modified to facilitate the display of targeted peptides, functional proteins and antibodies. In addition, the preparation cost of natural biomimetic nanocarriers is low, and cell engineering preparation can be realized.63
Biomembrane-based nanovaccines shown promise as the mainstay of the next generation of nanovaccines. However, their widespread clinical application is limited by challenges, such as complex preparation processes, difficulties in large-scale production, complex product compositions, and high production costs.
Strategies of Nanovaccines for the Treatment of Autoimmune Diseases
Loading Self-Antigens to Generate Tolerogenic Dendritic Cells
The tolerogenic DCs for therapeutic use can be generated using methods adapted from DC vaccination. In general, when the vaccine enters the body from the injection site, the foreign antigens it carries are recognized by pattern recognition receptors (PRRs) on the surface of APCs, such as peripheral macrophages or DCs, and transported to immune organs, such as draining lymph nodes (LNs) or the spleen. Depending on the antigens contained in vaccines, they can be recognized directly by B cells to activate humoral responses or presented to T cells in immune organs by antigenic macrophages or DCs.64 Induced tolerogenic DCs can provide non-specific immunosuppression, so loading disease-specific autoantigens on DCs can produce tolerogenic DCs that are specific to specific autoimmune diseases.
For example, Nguyen et al constructed a therapeutic vaccine based on immunosuppressive biomaterials that carry autologous antigens and tolerance-inducing inorganic nanoparticles (NPs) for the treatment of multiple sclerosis (MS). MS is an autoimmune disease characterized by white matter demyelination of the central nervous system, which is mainly caused by the targeted attack of autoreactive CD4+ T cells on the myelin sheath. They took advantage of the high antigen-loading capacity of mesoporous silica NPs (MSNs) and loaded myelin oligodendrocyte glycoprotein antigen (MOG35-55 peptide) onto MSNs to generate antigen-specific immune tolerance. Injecting MOG35-55 peptide-loaded MSNs maintained the expression of MHC-II but did not upregulate the expression of CD86 on APCs in the spleen, thus inducing tolerance in APCs. In addition, immunization with mesoporous NPs loaded with autoantigens produced Tregs in the spleen and generated systemic immune tolerance in the experimental autoimmune encephalomyelitis (EAE) mouse (a mouse model mimicking human MS), resulting in a reduction in central nervous system infiltrated APCs and autoreactive CD4+ T cells. Furthermore, they introduced reactive oxygen species (ROS)-scavenging cerium oxide NPs (CeNPs) into specific antigen-supported nanovaccines to inhibit APC activation in the inflammatory setting of chronic MS through ROS clearance, increasing Treg cell frequency, and inhibiting self-reactive CD4+ T cells in the CNS, thus improving the therapeutic effect of restoring motor function. Therefore, nanovaccines carrying autoantigens show great potential in the treatment of MS and potentially other autoimmune diseases through immune tolerance20. Park et al also reported a tolerogenic nanovaccine for the treatment of autoimmune encephalomyelitis. The nanovaccine (AbaLDPN-MOG) consists of a phospholipid layer (LDPN) containing dexamethasone wrapped around the core of polydopamine NPs (PN), and the surface was modified with abatacept and myelin oligodendrocyte glycoprotein (MOG) autoantigen peptides. AbaLDPN-MOG can be targeted and endocytosed by DCs and then transported to lysosomes due to the modification of abatacept. AbaLDPN-MOG can block the interaction between CD80/CD86 and CD28 in APCs and T cells, resulting in decreased IFN-γ secretion. In addition, AbaLDPN-MOG treatment reduced the expression levels of CD86, CD80, and MHC-II in DCs, suggesting that these particles were effective in inducing DC tolerance. At the same time, AbaLDPN-MOG treatment significantly decreased the production of the proinflammatory factors tumor necrosis factor (TNF)-α, IL-1β, IL-6 and IL-12 and significantly increased the production of IL-10 and TGF-β. In experimental EAE mice, subcutaneous administration of AbaLDPN-MOG induced a high proportion of Tregs and reduced the proportion of IFN-γ-secreting CD4+ T cells and IL-17A-expressing CD4+ T cells, thereby reducing immune cell invasion. Furthermore, AbaLDPN-MOG increased the integrity of the myelin sheath, significantly alleviating symptoms in the EAE mouse model. Therefore, AbaLDPN can be used to deliver MOG peptides in EAE models and is expected to be widely used to prevent and alleviate other autoimmune diseases25 (Figure 3, Table 1).
 |
Figure 3 Loaded with autoantigens to generate tolerant dendritic cells. AbaLDPN-MOG nanovaccine induced tolerogenic DCs. (A) The composition of AbaLDPN-MOG tolerogenic nanovaccine, and the mechanism of antigen-specific tolerance induction in lymph nodes. (B) The nanoparticles induced tolerogenic DC and detected the expression of related CD molecules and inflammatory factors. All statistical data are presented as mean ± SD (n = 5; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Adapted with permission from Park J, Le QV, Wu Y, Lee J, Oh YK. Tolerogenic nanovaccine for prevention and treatment of autoimmune encephalomyelitis. Adv Mater. 2023;35(1):e2202670;25 © 2022 Wiley-VCH GmbH. |
Enhancing DC Targeting
Adjusting the Size of the Nanovaccine
Prolonging antigen persistence or reducing unnecessary antigen degradation will enhance the immune response. The persistence of antigens at injection sites, lymphatic tissues, and even APCs is enhanced by binding or loading antigens in nanomaterials. Currently, only a small amount of antigen from the injection site can be delivered to the lymph nodes (LNs) via APCs. To improve the efficiency of delivery to LNs, a variety of physical properties of NPs can be adjusted, such as charge, shape, and size; moreover, different nanomaterials may have different optimal sizes for LN delivery. Thus, the physical properties of NPs (such as charge, shape, and size) can be modulated to facilitate the absorption of antigens by APCs, resulting in a strong immune response.
The size of the nanovaccine is also a key parameter in determining whether the NPs present in the tissue fluid enter the lymphatic capillaries and remain in the LNs. For example, NPs with sizes of 20–200 nm are free to enter the lymphatic system without being taken up by APCs, resulting in direct contact between NPs and T or B cells in LNs. NPs smaller than 50 nm can be transported more efficiently than those larger than 100 nm. However, NPs with sizes of 200–500 nm could not enter lymphatic capillaries effectively and needed active transport of DCs to the LNs. Meanwhile, NPs larger than 500 nm were mainly absorbed by macrophages rather than DCs. Therefore, the optimal particle diameter effectively captured by DCs is 200–500 nm.65,66
Surface Modification to Enhance DC Targeting
The surface of the nanovaccine was modified to enhance DC-specific delivery. Studies have shown that the formation of the protein corona is inhibited by surface modification of hydrophilic poly-(ethylene glycol) (PEG), thereby reducing nonspecific uptake by other phagocytes.67
The protein corona is a structure composed of one or more layers of protein adsorbed on the surface of nanomaterials after they enter the biological environment, such as serum-containing medium or body fluids such as blood. The formation of a protein corona can mediate the absorption and rapid clearance of nanomaterials by macrophages.68,69 Therefore, the surface modification of nanomaterials with PEG can effectively reduce the adsorption of proteins on the surface of nanomaterials to enhance nanovaccine targeting.
In addition, different PEG linker length modifications also show different DC-targeting capabilities. For example, Cruz et al compared the PEG linker length of antigen-loaded and antibody-coated PLGA NPs, showing that shorter PEG constructors (2–3 kDa) interact more strongly with DCs and induce higher T-cell proliferation than longer PEG constructors (6–20 kDa).70 Brückner et al studied the effect of surface modification of NPs with PEG linker lengths of different molecular weights (0.65, 2 and 5 kDa) on DC targeting. They found that antibody-functionalized NPs with a shorter PEG linker length of 0.65 kDa showed the best DC targeting in DC2.4 cell lines, while NPs with a longer PEG linker length of 5 kDa specifically accumulated in bone marrow-derived dendritic cells (BMDCs) and conventional type 1 DCs (cDC1) from the spleen.71 Therefore, these key aspects need to be considered when targeting the subpopulation of DCs, which has important implications in the field of autoimmune disease treatment and vaccine development.
The targeted delivery of NPs to DCs can be achieved by modifying the DC-specific surface receptor ligand onto the surface of the nanovaccine. These ligands include Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), such as DEC-205, Clec9A, the mannose receptor, and dendritic cell inhibitory receptor 2 (DCIR2), which present antigens directly to DCs.72 Studies have shown that anti-Clec9A and anti-DEC-205 monoclonal antibodies enhance DC internalization and prime Treg activation over cytotoxic T cells.73,74 In addition, White et al demonstrated that targeting DCs with mannosylated liposomes increased nanoparticle uptake without increasing T-cell activation.75
However, nanovaccines can be directed to internalization by different DCs by modification with the ligands of different surface receptors. Studies have shown that the expression of CLRS in different DC subtypes leads to different targeting and antigen-processing abilities of DC subtypes. For example, CD8+ DCs express DEC-205, leading to cross-presentation of antigens to CD8+ T cells and initiating cellular immune responses. DCIR2 is expressed on CD8− DCs, and the antigens targeting DCIR2 are preferentially presented to CD4+ T cells via MHC-II.72,76,77 Compared with other C-type lectins and most DC marker molecules, the expression of Clec9A appears to be more tightly restricted to DCs. For example, in mice, Clec9A expression is restricted by the subtype of DCs and is limited to the CD8+ CD24+ Sirpα− cDC subtype. CD8+ DCs are effective activators of CD4 T cells, thus enhancing the antibody response. In humans, the expression of CLEC9A is also restricted to the DC subtype. For example, CLEC9A is expressed in a small subset of DC blood and is consistent with the expression of BDCA-3. However, it does not seem to be expressed in plasmacytoid DCs (pDCs).73
The treatment of autoimmune diseases requires inducing immune tolerance rather than initiating a cellular immune response. Therefore, it is necessary to be selective about the DC subtypes that present the antigen. For example, Price et al used chimeric antibodies specific for DCIR2 or DEC-205 to target self-antigens to CD11b+ (cDC2) DCs and CD8+ (cDC1) DCs in autoimmune-prone nonobese diabetic (NOD) mice to determine which subsets of DCs can induce tolerance in the context of chronic autoimmunity. They found that antigen presentation by DCIR2+ DCs, but not DEC-205+ DCs, elicited a tolerogenic CD4+ T-cell response. Furthermore, delivery of beta-cell antigen to DCIR2+ DCs delayed diabetes induction and induced increased apoptosis of T cells, as well as in the absence of IFN-γ or sustained expansion of autoreactive CD4+ T cells. DCIR2+ DCs inhibited T-cell differentiation by stimulating T cells to not produce IFN-γ but increased zinc finger and BTB domain 32 (Zbtb32) expression. Thus, they demonstrated that DCIR2+ DCs can induce antigen-specific immune tolerance in the face of persistent autoimmunity and identified Zbtb32 as an inhibitory transcription factor controlling T-cell-mediated autoimmunity.78 Tabansky et al fused αDCIR2 antibodies with the highly encephalitogenic peptide 139–151 of myelin-derived proteolipid protein (PLP139-151) to target CD11c+ CD8− DCs with a DEC-205-DCIR2+ phenotype in vivo. The αDCIR2-PLP139-151 fusion monoclonal antibody immunization can significantly improve the clinical symptoms of EAE mice induced by PLP139-151, while free PLP139-151 fusion monoclonal antibody immunization failed to produce a protective effect, suggesting that the protective effect of fusion monoclonal antibody may be mediated by DC targeting. In addition, αDCIR2-PLP139-151 fusion monoclonal antibody-mediated EAE protection relies on targeting the immature state of DCIR2+ DCs, eliminating IL-17- and IFN-γ-producing pathogenic T cells, and enhancing Foxp3+ Treg activity. These results indicate that different DC subsets and targeted receptors can mediate different immune responses. Therefore, the selection of certain ligand modifications, such as DCIR2, can allow the targeting of immune tolerogenic DCs, thereby inducing anergic effector T-cell or Treg activation to produce immune tolerance for the treatment of autoimmune diseases (Figure 4).79
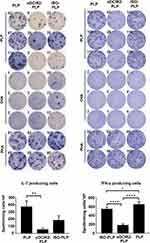 |
Figure 4 Pretreatment with αDCIR2-PLP139–151 mAb reduces activity of pathogenic T helper cells. The numbers of IL-17 producing cells (pathogenic Th17) and IFN-γ producing cells are significantly reduced in mice treated with αDCIR2-PLP139–151 mAb. All statistical data are presented as mean ± SD (n =2; *p < 0.05, **p < 0.01, ****p = 0.0001). Adapted with permission from Tabansky I, Keskin DB, Watts D et al. Targeting DEC-205(-)DCIR2(+) dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Molecular medicine. 2018;24(1):17.79 Creative Commons. |
In addition, several studies have shown that NPs with a positive surface charge are generally more easily absorbed by DCs than NPs with a neutral or negative surface charge.80 However, positively charged NPs are not effective vaccine vectors, and their delivery efficiency depends on the model system used. For example, the lipid composition of liposomes not only affects the surface charge and uptake efficiency of APCs but also determines whether liposomal content is released in early or late endosomal compartments, which has an important effect on the presentation of different peptide epitopes. Positive surface charges may immobilize the vaccine carrier through electrostatic interactions with negatively charged components present in the extracellular matrix, thus impeding the efficacy of the vaccine by reducing tissue penetration. When charged liposomes come into contact with plasma, they interact with complementary proteins and are rapidly phagocytosed and cleared by macrophages of the reticuloendothelial system. For polymer NPs, hydrophobicity appears to be a key factor in regulating interactions. Although the rapid clearance of this process is generally considered a negative feature of drug delivery systems, complement activation as a signal to activate DCs may benefit vaccination strategies.
Nanovaccines Modified with Cell-Penetrating Peptides Enhance Antigen Delivery Efficiency
Cell-penetrating peptides (CPPs) are a large class of short peptides composed of 10–30 amino acids and have the ability to penetrate cell membranes. CPPs, also known as protein translocation domains (PTDs), are carriers that can carry proteins, small molecules, siRNA, and other peptides into cells. The CPP-mediated transmembrane mechanism mainly includes clathrin-, caveolae-, and jutunyin-mediated endocytic pathways, and CPPs direct intracellular transport of substances.
CPPs are mainly divided into cationic peptides, hydrophobic peptides and amphoteric peptides. Cationic peptides are generally rich in arginine and need to contain at least eight positively charged residues to be effectively taken up by cells, mainly R9, TAT and penetration.81 Hydrophobic CPPs contain KGF and FGF sequences, such as the signal sequences in integrin β3 and carbozi fibroblast growth factor, but their applications are rarely studied.82,83 Amphiphilic CPPs consist of a hydrophilic domain and a hydrophobic domain, usually covalently linked to nuclear localization sequences (NLS) by hydrophobic domains, including MPG, VP22, and Pep-1, which have also been successfully used for vaccine delivery.84,85 Many studies have shown that CPP-mediated nanovaccines can penetrate the plasma membrane of cells and release the vaccine encapsulated in the endosome, mediating immune responses by enhancing antigen processing and immunogenicity. Nanotechnology-based CPP-mediated vaccines can actively or passively target APCs and stimulate APCs such as DCs through CPP-mediated delivery systems to improve vaccine efficiency and extend vaccine circulation time in the body. CPP-modified nanovaccines easily interact with biomolecules on the cell surface and penetrate cells due to their small size and large specific surface area. After CPP modification, the drug delivery system changes, which is conducive to the induction of the immune response. With the assistance of CPPs, NPs are used to deliver encapsulated vaccines to DCs, where soluble antigens are taken up by cells more efficiently.
For example, Liu et al linked antigens (ovalbumin, OVA) to MPGΔNLS, which were subsequently encapsulated in poly (lactide-co-glycolide) acid (PLGA) NPs to produce nanovaccines. MPGΔNLS promotes antigen escape from the lysosome into the cytoplasm, increases the amount of processed antigens in the cytoplasm, enhances antigen cross-presentation via MHC-I molecules, and induces cytotoxic CD8+ T-cell responses. The results showed that MPGΔNLS-OVA-loaded PLGA NPs improved the release of OVA into the cytoplasm of BMDCs and promoted the maturation and activation of BMDCs. In vivo, MPGΔNLS modification has also been shown to stimulate OVA-specific T-cell expansion, OVA-specific IgG antibody production, and OVA-specific memory T-cell proliferation. CPPs can affect the intracellular localization of antigens encapsulated in nanovaccines, promote antigen cross-presentation and stimulate antigen-specific immune responses. Therefore, CPP modification of antigens is an innovative way to improve the immunotherapeutic effect of nanovaccines.86
CPPs may be an attractive strategy for delivering macromolecules to immune cells, but most CPPs, including TAT, are less efficient for primary lymphocyte delivery. Therefore, some newly discovered CPPs can replace previously discovered CPPs, such as TAT, which can directly target T cells to induce an immune response and be applied in the treatment of autoimmune diseases.87 For example, Lim et al identified a novel CPP dNP2 from the human novel LZAP-binding protein (NLBP), which is a lysine-rich tandem repeat sequence. The cell-penetrating ability of dNP2 was at least twofold greater than that of TAT or R9 and was able to deliver antigen molecules to primary T cells in mice and humans with very high efficiency, inhibiting the activity of T effector cells. dNP2 has the ability to cross the blood‒brain barrier (BBB) and localize in the central nervous system (CNS), which could be effective in the treatment of CNS autoimmune diseases. Treatment of EAE mice with recombinant DNP2-ctCTLA-4 protein produced by dNP2 fusion with cytotoxic T-lymphocyte antigen 4 (ctCTLA-4) significantly decreased the expression levels of IFN-γ and IL-17A in activated splenocytes, decreased demyelination and CNS-infiltrating T helper 1 and T helper 17 cells, and consistently improved the symptoms of EAE mice.88 In another study, Koo et al constructed the recombinant protein dNP2-LRR produced by fusion of dNP2 with the leucine-rich repeat domain (LRR) of the NOD-like receptor family member X1 (NLRX1) protein, which exhibited high T-cell delivery efficiency. dNP2-LRR treatment of EAE mice can alleviate tissue inflammation and disease severity, reduce the number of infiltrating T cells producing inflammatory cytokines such as IFN-γ, and significantly inhibit T-cell activation, cytokine production, and Th1 differentiation (Figure 5).89 Lee et al also demonstrated the high T-cell delivery efficiency of dNP2. They used dNP2-conjugated VIVIT to synthesize a peptide (dNP2-VIVIT) that can selectively inhibit the interaction between NFAT and calcineurin. dNP2-VIVIT treatment inhibited the differentiation of Th1 and Th17 cells and significantly reduced disease severity in EAE model mice.90 Therefore, dNP2 has been shown to be an effective CPP that can be used for T-cell regulation and potential applications in autoimmune diseases by fusing dNP2 to enhance its effectiveness in delivering antigens directly to the cytoplasm of T cells.
 |
Figure 5 Cell-penetrating peptide-modified nanovaccines can improve antigen delivery efficiency. (A) The intracellular delivery efficiency of dNP2-LRR is increased compared with LRR alone. Reproduced with permission from Koo JH, Kim DH, Cha D, Kang MJ, Choi JM. LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis. Theranostics. 2020;10(7):3138–3150.89 Creative commons. (B) dNP2-LRR inhibits T cell activation and specifically inhibits Th1 differentiation. n=3 per group and error bars indicate S.D. Reproduced with permission from Koo JH, Kim DH, Cha D, Kang MJ, Choi JM. LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis. Theranostics. 2020;10(7):3138–3150.89 Creative commons. **P<0.01 and ***P<0.001. N.S., not significant. |
Combination of Nanovaccines and Immune Checkpoint Agonists
Immune checkpoints, such as the CTLA4-B7 and PD-1/PD-L1 pathways, are negative regulatory molecules that prevent excessive T-cell activation to maintain immune tolerance in the body. PD-1/PD-L1 plays a crucial role in inhibiting T-cell signal transduction, thus mediating immune tolerance and maintaining immune homeostasis. Dysregulation of PD-1/PD-L1 can occur during the development of various autoimmune diseases. The PD-1/PD-L1 pathway is involved in T-cell activation, proliferation, and apoptosis, and it inhibits T-cell-mediated cellular immune responses. This pathway regulates the induction and maintenance of peripheral tolerance and, under physiological conditions, protects tissues from autoimmune attack. Tregs that are activated by the PD-1 pathway may contribute to the maintenance of immune homeostasis by maintaining sufficiently high T-cell activation thresholds that prevent autoimmunity.91 For example, in a mouse model of EAE, increased expression of PD-1 and PD-L1 in the central nervous system blocked PD-1, leading to the activation of antigen-specific T cells, the production of proinflammatory cytokines, and further worsening of EAE symptoms.92 Therefore, enhancing PD-1/PD-L1 signaling is a promising strategy for treating autoimmune diseases; such approaches include the indirect activation of PD-1 signaling, the administration of extracellular agonist antibodies, the mimicking of PD-L1 function with small molecules/proteins, the activation of intracellular signals downstream of PD-1 with small-molecule compounds, and the depletion of PD-1+ cells.
Encapsulating immune checkpoint agonists in nanoparticles not only enhances the immune therapeutic response but also reduces off-target effects.93 Additionally, nanoparticles are recognized as versatile delivery platforms that are capable of encapsulating vaccines or drugs for chemotherapy, photothermal therapy, etc. Combining immune checkpoint agonists with nanoparticles carrying different types of drugs can enhance immune tolerance and be used to treat autoimmune diseases. For instance, Hou et al constructed nanovaccines (PRM NDs) by encapsulating PLGA nanoparticles within a macrophage membrane (PRM) with high PD-L1 expression. These nanoparticles were designed to treat autoimmune diseases by targeting multiple factors. IFN-γ treatment induces the overexpression of PD-L1 on macrophage membranes and increases the expression of various proinflammatory cytokine receptors and adhesion molecule receptors. Thus, PRM NDs exhibit strong inflammatory factor-targeting and proinflammatory cytokine-clearing capabilities, as well as immune inhibitory properties. These cells mimic the PD-1/PD-L1 inhibitory axis and suppress CD4+ T-cell activation, ultimately restoring immune tolerance. Consequently, in mouse models of Zymosan A-induced arthritis and dextran sulfate sodium-induced ulcerative colitis, PRM NDs exerted strong synergistic anti-inflammatory and immune inhibitory effects, alleviating autoimmune damage.94
Conclusion and Prospects
The optimal strategy for treating autoimmune diseases is to modify the host immune system to restore tolerance. The ideal effect of treating autoimmune diseases is to eliminate the autoantigen-specific spontaneous immune response without interfering with the immune response to other antigens. Therapeutic vaccines that generate immune tolerance conform to this principle. Nanomaterials are emerging as novel tools for improving the effectiveness of subunit vaccines or other types of vaccines. Nanovaccines are a new generation of vaccines based on nanotechnology that can induce long-term and effective immune responses through vector design, surface ligand modification, etc., which provide promising clinical application prospects. We have reviewed the advances in the development of nanovaccines for the treatment of autoimmune diseases. These nanovaccines, based on the design rules that promote the induction of tolerant DCs, promote DC-targeting absorption and internalization, and promote antigen delivery efficiency, can lead to better treatment outcomes.
However, there are still many difficulties and challenges in realizing the wide and safe clinical application of nanovaccines. For example, how to achieve specific targeting of target cells such as APCs without triggering negative regulation within cells or suppressing immune signals is a major challenge for nanovaccine research. Second, some nanomaterials will accumulate in cells, leading to long-term retention of nanomaterials in the body, which may cause the formation of blood clots. In addition, the differences between animal models and the human immune system, combined with the complexity of clinical conditions, mean that the results of animal experiments and clinical effects may be far from each other. For example, some therapies may cause the same effects in mice as in humans but may be accompanied by important side effects that cannot be predicted by animal models in humans. At the same time, there are also high production and preservation costs.
Although the construction of nanovaccines is in its infancy, with only a handful in early clinical stages, this new generation of vaccines has great potential to prevent and treat a wide range of diseases. In the future, the design of nanovaccines will focus on improving biosafety and biocompatibility. We further explored the mechanism of interaction between nanoparticles and organisms and constructed more in vivo models to further improve the performance of nano adjuvants. Nanovaccines are expected to achieve the organic unity of safety and efficacy.
Funding
This work was supported by funding from National Natural Science Foundation of China (No. 81960562) and Guangxi Natural Science Foundation (No. 2019GXNSFBA185018).
Disclosure
The authors declare that they have no competing financial interests.
References
1. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Internal Med. 2015;278(4):369–395. doi:10.1111/joim.12395
2. Juarranz Y. Molecular and cellular basis of autoimmune diseases. Cells. 2021;10:2.
3. Moorman CD, Sohn SJ, Phee H. Emerging therapeutics for immune tolerance: tolerogenic vaccines, T cell therapy, and IL-2 therapy. Front Immunol. 2021;12:657768. doi:10.3389/fimmu.2021.657768
4. Salinas GF, Braza F, Brouard S, Tak PP, Baeten D. The role of B lymphocytes in the progression from autoimmunity to autoimmune disease. Clin Immunol. 2013;146(1):34–45. doi:10.1016/j.clim.2012.10.005
5. Hang LM, Nakamura RM. Current concepts and advances in clinical laboratory testing for autoimmune diseases. Crit Rev Clin Lab Sci. 1997;34(3):275–311. doi:10.3109/10408369708998095
6. Avrameas S, Selmi C. Natural autoantibodies in the physiology and pathophysiology of the immune system. J Autoimmun. 2013;41:46–49. doi:10.1016/j.jaut.2013.01.006
7. Zhang Y, Zhang L, Wang M, Li P. The applications of nanozymes in neurological diseases: from mechanism to design. Theranostics. 2023;13(8):2492–2514. doi:10.7150/thno.83370
8. Zhang Y, Yu W, Chen M, Zhang B, Zhang L, Li P. The applications of nanozymes in cancer therapy: based on regulating pyroptosis, ferroptosis and autophagy of tumor cells. Nanoscale. 2023;15(29):12137–12156. doi:10.1039/d3nr01722b
9. Clemente-Casares X, Blanco J, Ambalavanan P, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:7591):434–40. doi:10.1038/nature16962
10. Tsai S, Shameli A, Yamanouchi J, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32(4):568–580. doi:10.1016/j.immuni.2010.03.015
11. Cifuentes-Rius A, Desai A, Yuen D, Johnston APR, Voelcker NH. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nature Nanotechnol. 2021;16(1):37–46. doi:10.1038/s41565-020-00810-2
12. Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J Clin Invest. 2017;127(7):2505–2512. doi:10.1172/jci90598
13. Chen L, Huang H, Zhang W, Ding F, Fan Z, Zeng Z. Exosomes derived from T regulatory cells suppress CD8+ cytotoxic T lymphocyte proliferation and prolong liver allograft survival. Med Sci Monit. 2019;25:4877–4884. doi:10.12659/msm.917058
14. Sengupta A, Azharuddin M, Al-Otaibi N, Hinkula J. Efficacy and immune response elicited by gold nanoparticle- based nanovaccines against infectious diseases. Vaccines. 2022;10(4):505. doi:10.3390/vaccines10040505
15. Mateu Ferrando R, Lay L, Polito L. Gold nanoparticle-based platforms for vaccine development. Drug Discov Today. 2020;38:57–67. doi:10.1016/j.ddtec.2021.02.001
16. Ahmad S, Zamry AA, Tan HT, Wong KK, Lim J, Mohamud R. Targeting dendritic cells through gold nanoparticles: a review on the cellular uptake and subsequent immunological properties. Mol Immunol. 2017;91:123–133. doi:10.1016/j.molimm.2017.09.001
17. Almeida JPM, Lin AY, Figueroa ER, Foster AE, Drezek RA. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;11(12):1453–1459. doi:10.1002/smll.201402179
18. Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109(28):11270–11275. doi:10.1073/pnas.1120611109
19. Yeste A, Takenaka MC, Mascanfroni ID, et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signaling. 2016;9(433):ra61. doi:10.1126/scisignal.aad0612
20. Nguyen TL, Choi Y, Im J, et al. Immunosuppressive biomaterial-based therapeutic vaccine to treat multiple sclerosis via re-establishing immune tolerance. Nat Commun. 2022;13(1):7449. doi:10.1038/s41467-022-35263-9
21. Cappellano G, Woldetsadik AD, Orilieri E, et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine. 2014;32(43):5681–5689. doi:10.1016/j.vaccine.2014.08.016
22. Hunter Z, McCarthy DP, Yap WT, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS nano. 2014;8(3):2148–2160. doi:10.1021/nn405033r
23. Al-Ghobashy MA, ElMeshad AN, Abdelsalam RM, Nooh MM, Al-Shorbagy M, Laible G. Development and pre-clinical evaluation of recombinant human myelin basic protein nano therapeutic vaccine in experimental autoimmune encephalomyelitis mice animal model. Sci Rep. 2017;7:46468. doi:10.1038/srep46468
24. Maldonado RA, LaMothe RA, Ferrari JD, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112(2):E156–65. doi:10.1073/pnas.1408686111
25. Park J, Le QV, Wu Y, Lee J, Oh YK. Tolerogenic nanovaccine for prevention and treatment of autoimmune encephalomyelitis. Adv Mater. 2023;35(1):e2202670. doi:10.1002/adma.202202670
26. Feng Q, Yang X, Hao Y, et al. Cancer cell membrane-biomimetic nanoplatform for enhanced sonodynamic therapy on breast cancer via autophagy regulation strategy. ACS Appl Mater Inter. 2019;11(36):32729–32738. doi:10.1021/acsami.9b10948
27. Yaron PN, Holt BD, Short PA, Losche M, Islam MF, Dahl KN. Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. J Nanobiotechnol. 2011;9:45. doi:10.1186/1477-3155-9-45
28. Maruyama K, Haniu H, Saito N, et al. Endocytosis of multiwalled carbon nanotubes in bronchial epithelial and mesothelial cells. Biomed Res Int. 2015;2015:793186. doi:10.1155/2015/793186
29. Hassan HA, Smyth L, Rubio N, et al. Carbon nanotubes’ surface chemistry determines their potency as vaccine nanocarriers in vitro and in vivo. J Control Release. 2016;225:205–216. doi:10.1016/j.jconrel.2016.01.030
30. Chemmannur SV, Bhagat P, Mirlekar B, Paknikar KM, Chattopadhyay S. Carbon nanospheres mediated delivery of nuclear matrix protein SMAR1 to direct experimental autoimmune encephalomyelitis in mice. Int J Nanomed. 2016;11:2039–2051. doi:10.2147/IJN.S93571
31. Tomic S, Janjetovic K, Mihajlovic D, et al. Graphene quantum dots suppress proinflammatory T cell responses via autophagy-dependent induction of tolerogenic dendritic cells. Biomaterials. 2017;146:13–28. doi:10.1016/j.biomaterials.2017.08.040
32. Tosic J, Stanojevic Z, Vidicevic S, et al. Graphene quantum dots inhibit T cell-mediated neuroinflammation in rats. Neuropharmacology. 2019;146:95–108. doi:10.1016/j.neuropharm.2018.11.030
33. Nigam S, Mohapatra J, Makela AV, et al. Shape anisotropy-governed high-performance nanomagnetosol for in vivo magnetic particle imaging of lungs. Small. 2023:e2305300. doi:10.1002/smll.202305300
34. Pusic K, Aguilar Z, McLoughlin J, et al. Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. FASEB J. 2013;27(3):1153–1166. doi:10.1096/fj.12-218362
35. Rezaei M, Hosseini SN, Khavari-Nejad RA, Najafi F, Mahdavi M. HBs antigen and mannose loading on the surface of iron oxide nanoparticles in order to immuno-targeting: fabrication, characterization, cellular and humoral immunoassay. Artif Cells Nanomed Biotechnol. 2019;47(1):1543–1558. doi:10.1080/21691401.2019.1577888
36. Lee JH, Ju JE, Kim BI, et al. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ Toxicol Chem. 2014;33(12):2759–2766. doi:10.1002/etc.2735
37. Chen S, Chen S, Zeng Y, et al. Size-dependent superparamagnetic iron oxide nanoparticles dictate interleukin-1β release from mouse bone marrow-derived macrophages. J Appl Toxicol. 2018;38(7):978–986. doi:10.1002/jat.3606
38. Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8(1):2082. doi:10.1038/s41598-018-19628-z
39. Su Y, Zhang B, Sun R, et al. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Delivery. 2021;28(1):1397–1418. doi:10.1080/10717544.2021.1938756
40. Pohlit H, Bellinghausen I, Frey H, Saloga J. Recent advances in the use of nanoparticles for allergen-specific immunotherapy. Allergy. 2017;72(10):1461–1474. doi:10.1111/all.13199
41. McCarthy DP, Yap JW, Harp CT, et al. An antigen-encapsulating nanoparticle platform for T(H)1/17 immune tolerance therapy. Nanomedicine. 2017;13(1):191–200. doi:10.1016/j.nano.2016.09.007
42. Kuo R, Saito E, Miller SD, Shea LD. Peptide-conjugated nanoparticles reduce positive co-stimulatory expression and T cell activity to induce tolerance. Mol Ther. 2017;25(7):1676–1685. doi:10.1016/j.ymthe.2017.03.032
43. LaMothe RA, Kolte PN, Vo T, et al. Tolerogenic nanoparticles induce antigen-specific regulatory T cells and provide therapeutic efficacy and transferrable tolerance against experimental autoimmune encephalomyelitis. Front Immunol. 2018;9:281. doi:10.3389/fimmu.2018.00281
44. An M, Li M, Xi J, Liu H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Inter. 2017;9(28):23466–23475. doi:10.1021/acsami.7b06024
45. Howard GP, Verma G, Ke X, et al. Critical size limit of biodegradable nanoparticles for enhanced lymph node trafficking and paracortex penetration. Nano Res. 2019;12(4):837–844. doi:10.1007/s12274-019-2301-3
46. Gu P, Xu S, Zhou S, et al. Optimization of angelica sinensis polysaccharide-loaded Poly (lactic-co-glycolicacid) nanoparticles by RSM and its immunological activity in vitro. Int J Biol Macromol. 2018;107(Pt A):222–229. doi:10.1016/j.ijbiomac.2017.08.176
47. Lim HJ, Kim JK, Park JS. Complexation of Apoptotic Genes with Polyethyleneimine (PEI)-Coated Poly-(DL)-Lactic-Co-Glycolic Acid Nanoparticles for Cancer Cell Apoptosis. J Biomed Nanotechnol. 2015;11(2):211–225. doi:10.1166/jbn.2015.1880
48. Kang BS, Choi JS, Lee SE, et al. Enhancing the in vitro anticancer activity of albendazole incorporated into chitosan-coated PLGA nanoparticles. Carbohydr Polym. 2017;159:39–47. doi:10.1016/j.carbpol.2016.12.009
49. Gao X, Liu N, Wang Z, et al. Development and optimization of chitosan nanoparticle-based intranasal vaccine carrier. Molecules. 2021;27:1.
50. Bedford JG, Caminschi I, Wakim LM. Intranasal delivery of a chitosan-hydrogel vaccine generates nasal tissue resident memory CD8(+) T cells that are protective against influenza virus infection. Vaccines. 2020;8:4.
51. Zhuo SH, Wu JJ, Zhao L, Li WH, Zhao YF, Li YM. A chitosan-mediated inhalable nanovaccine against SARS-CoV-2. Nano Res. 2022;15(5):4191–4200. doi:10.1007/s12274-021-4012-9
52. Duran V, Yasar H, Becker J, et al. Preferential uptake of chitosan-coated PLGA nanoparticles by primary human antigen presenting cells. Nanomedicine. 2019;21:102073. doi:10.1016/j.nano.2019.102073
53. Kadiyala I, Loo Y, Roy K, Rice J, Leong KW. Transport of chitosan-DNA nanoparticles in human intestinal M-cell model versus normal intestinal enterocytes. Eur J Pharm Sci. 2010;39(1–3):103–109. doi:10.1016/j.ejps.2009.11.002
54. Bose A, Roy Burman D, Sikdar B, Patra P. Nanomicelles: types, properties and applications in drug delivery. IET Nanobiotechnol. 2021;15(1):19–27. doi:10.1049/nbt2.12018
55. Li C, Zhang X, Chen Q, et al. Synthetic polymeric mixed micelles targeting lymph nodes trigger enhanced cellular and humoral immune responses. ACS Appl Mater Inter. 2018;10(3):2874–2889. doi:10.1021/acsami.7b14004
56. Singha S, Shao K, Ellestad KK, Yang Y, Santamaria P. Nanoparticles for immune stimulation against infection, cancer, and autoimmunity. ACS nano. 2018;12(11):10621–10635. doi:10.1021/acsnano.8b05950
57. Whitener R, Henchir JJ, Miller TA, et al. Localization of multi-lamellar vesicle nanoparticles to injured brain tissue in a controlled cortical impact injury model of traumatic brain injury in rodents. Neurotr Rep. 2022;3(1):158–167. doi:10.1089/neur.2021.0049
58. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094. doi:10.1038/s41578-021-00358-0
59. Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem. 2010;49(36):6288–6308. doi:10.1002/anie.200902672
60. Cheng X, Smith JC. Biological membrane organization and cellular signaling. Chem Rev. 2019;119(9):5849–5880. doi:10.1021/acs.chemrev.8b00439
61. Ai X, Hu M, Wang Z, et al. Recent advances of membrane-cloaked nanoplatforms for biomedical applications. Bioconjugate Chem. 2018;29(4):838–851. doi:10.1021/acs.bioconjchem.8b00103
62. Liu G, Zhao X, Zhang Y, et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv Mater. 2019;31(32):e1900795. doi:10.1002/adma.201900795
63. Wang L, Wang X, Yang F, et al. Systemic antiviral immunization by virus-mimicking nanoparticles-decorated erythrocytes. Nano Today. 2021;40:101280. doi:10.1016/j.nantod.2021.101280
64. Shi Y, Lu Y, You J. Antigen transfer and its effect on vaccine-induced immune amplification and tolerance. Theranostics. 2022;12(13):5888–5913. doi:10.7150/thno.75904
65. Fan X, Wang F, Zhou X, Chen B, Chen G. Size-dependent antibacterial immunity of staphylococcus aureus protoplast-derived particulate vaccines. Int j Nanomed. 2020;15:10321–10330. doi:10.2147/IJN.S285895
66. Jorquera PA, Tripp RA. Synthetic biodegradable microparticle and nanoparticle vaccines against the respiratory syncytial virus. Vaccines. 2016;4:4.
67. Pelaz B, Del Pino P, Maffre P, et al. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS nano. 2015;9(7):6996–7008. doi:10.1021/acsnano.5b01326
68. Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Delivery Rev. 2009;61(6):428–437. doi:10.1016/j.addr.2009.03.009
69. Breznica P, Koliqi R, Daka A. A review of the current understanding of nanoparticles protein Corona composition. Med Pharm Rep. 2020;93(4):342–350. doi:10.15386/mpr-1756
70. Cruz LJ, Tacken PJ, Fokkink R, Figdor CG. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials. 2011;32(28):6791–6803. doi:10.1016/j.biomaterials.2011.04.082
71. Bruckner M, Fichter M, da Costa Marques R, Landfester K, Mailander V. PEG spacer length substantially affects antibody-based nanocarrier targeting of dendritic cell subsets. Pharmaceutics. 2022;14:8.
72. Cruz LJ, Tacken PJ, Rueda F, Domingo JC, Albericio F, Figdor CG. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol. 2012;509:143–163. doi:10.1016/B978-0-12-391858-1.00008-3
73. Caminschi I, Proietto AI, Ahmet F, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112(8):3264–3273. doi:10.1182/blood-2008-05-155176
74. Petzold C, Schallenberg S, Stern JN, Kretschmer K. Targeted antigen delivery to DEC-205(+) dendritic cells for tolerogenic vaccination. Rev Diabetic Stud. 2012;9(4):305–318. doi:10.1900/RDS.2012.9.305
75. White KL, Rades T, Furneaux RH, Tyler PC, Hook S. Mannosylated liposomes as antigen delivery vehicles for targeting to dendritic cells. J Pharm Pharmacol. 2006;58(6):729–737. doi:10.1211/jpp.58.6.0003
76. Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–11. doi:10.1126/science.1136080
77. den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192(12):1685–1696. doi:10.1084/jem.192.12.1685
78. Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 Restore CD4+ T-Cell Tolerance and Inhibit Diabetes. Diabetes. 2015;64(10):3521–3531. doi:10.2337/db14-1880
79. Tabansky I, Keskin DB, Watts D, et al. Targeting DEC-205(-)DCIR2(+) dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Mol Med. 2018;24(1):17. doi:10.1186/s10020-018-0017-6
80. Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–322. doi:10.1016/j.ijpharm.2005.03.035
81. Koren E, Apte A, Sawant RR, Grunwald J, Torchilin VP. Cell-penetrating TAT peptide in drug delivery systems: proteolytic stability requirements. Drug Delivery. 2011;18(5):377–384. doi:10.3109/10717544.2011.567310
82. Pujals S, Giralt E. Proline-rich, amphipathic cell-penetrating peptides. Adv Drug Delivery Rev. 2008;60(4–5):473–484. doi:10.1016/j.addr.2007.09.012
83. Lindgren M, Rosenthal-Aizman K, Saar K, et al. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem Pharmacol. 2006;71(4):416–425. doi:10.1016/j.bcp.2005.10.048
84. Deshayes S, Plenat T, Aldrian-Herrada G, Divita G, Le Grimellec C, Heitz F. Primary amphipathic cell-penetrating peptides: structural requirements and interactions with model membranes. Biochemistry. 2004;43(24):7698–7706. doi:10.1021/bi049298m
85. Yu X, Wang Y, Xia Y, Zhang L, Yang Q, Lei J. A DNA vaccine encoding VP22 of herpes simplex virus type I (HSV-1) and OprF confers enhanced protection from Pseudomonas aeruginosa in mice. Vaccine. 2016;34(37):4399–4405. doi:10.1016/j.vaccine.2016.07.017
86. Liu X, Liu J, Liu D, et al. A cell-penetrating peptide-assisted nanovaccine promotes antigen cross-presentation and anti-tumor immune response. Biomater Sci. 2019;7(12):5516–5527. doi:10.1039/c9bm01183h
87. Koo JH, Kim WJ, Choi JM. CPP applications in immune modulation and disease therapy. Methods Mol Biol. 2022;2383:347–368. doi:10.1007/978-1-0716-1752-6_23
88. Lim S, Kim WJ, Kim YH, et al. dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat Commun. 2015;6:8244. doi:10.1038/ncomms9244
89. Koo JH, Kim DH, Cha D, Kang MJ, Choi JM. LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis. Theranostics. 2020;10(7):3138–3150. doi:10.7150/thno.43441
90. Lee HG, Kim LK, Choi JM. NFAT-specific inhibition by dNP2-VIVITAmeliorates autoimmune encephalomyelitisby regulation of Th1 and Th17. Mol Ther Meth Clin Develop. 2020;16:32–41. doi:10.1016/j.omtm.2019.10.006
91. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi:10.1111/j.1600-065X.2010.00923.x
92. Li H, Zheng C, Han J, Zhu J, Liu S, Jin T. PD-1/PD-L1 axis as a potential therapeutic target for multiple sclerosis: a T cell perspective. Front Cell Neurosci. 2021;15:716747. doi:10.3389/fncel.2021.716747
93. Toy R, Roy K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng Transl Med. 2016;1(1):47–62. doi:10.1002/btm2.10005
94. Hou M, Wei Y, Zhao Z, et al. Immuno-engineered nanodecoys for the multi-target anti-inflammatory treatment of autoimmune diseases. Adv Mater. 2022;34(12):e2108817. doi:10.1002/adma.202108817
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.