Back to Journals » International Journal of General Medicine » Volume 16
The Application of Multiple Magnetic Resonance Scanning Techniques in Evaluating the Stability of Intracranial Aneurysms
Authors Ma P, Li Y, Feng Y, Wu G , Li B, Wu H
Received 20 January 2023
Accepted for publication 28 April 2023
Published 24 May 2023 Volume 2023:16 Pages 2003—2011
DOI https://doi.org/10.2147/IJGM.S402255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Pengcheng Ma,1 Yadi Li,2 Yusen Feng,1 Gang Wu,3 Bin Li,4 Haiyan Wu5
1Department of Radiology, Kunming Yan ‘an Hospital, Kunming, 650000, People’s Republic of China; 2Department of Ophthalmology, Affiliated Hospital of Yunnan University, Kunming, 650000, People’s Republic of China; 3Department of Neurology, Kunming Yan ‘an Hospital, Kunming, 650000, People’s Republic of China; 4Department of Neurosurgery, Kunming Yan ‘an Hospital, Kunming, 650000, People’s Republic of China; 5Department of Cardiovascular, Affiliated Hospital of Kunming University of Science and Technology, Kunming, 650000, People’s Republic of China
Correspondence: Gang Wu, Tel/Fax +86 0871 632111408, Email [email protected]
Purpose: To evaluate the stability of unruptured intracranial aneurysm (UIA) with high-resolution magnetic resonance imaging of the vessel wall (HR-VWI).
Materials and Methods: A total of 92 UIA patients were enrolled. After MRA, HR-VWI imaging, the reconstruction of volume rendering (VR) and maximum intensity projection (MIP) were performed to observe the location and size of aneurysms, AR value (ratio of aneurysm height to aneurysmal diameter), SR value (ratio of maximum tumor depth to proximal parent artery diameter), and signal intensity were measured.
Results: There were 7 aneurysms with UIA located in the anterior cerebral artery, 31 aneurysms with UIA in the middle cerebral artery, 1 aneurysm with UIA in the posterior cerebral artery, 18 aneurysms with UIA in the anterior communication, 5 aneurysms with UIA in the posterior communication, 34 aneurysms with UIA in the intracranial segment of the internal carotid artery and 3 aneurysms with UIA in the vertebral artery. Among them, 8 patients had more than two multiple aneurysms. The lesion size was 2– 38mm (6.3 ± 5.09). There are 46 aneurysms with wall enhancement: the maximum SR value was 7.03 and the minimum 1.2, and the maximum AR value was 7.5 and the minimum 1.0. Fifty-five aneurysms showed no enhancement of the tumor wall. The maximum SR value was 4.55 and the minimum 0.58, and the maximum AR value was 4.0 and the minimum 0.6, respectively. Patients were divided into a stable group and an unstable group according to the aneurysm wall. The enhancement rate, SR value, and AR value in the stable aneurysm group were significantly lower than those in the unstable aneurysm group (P < 0.05).
Conclusion: MRA and HR-VWI can objectively reflect the stability of aneurysms by judging the morphology, SR value, and signal enhancement of UIA, and can provide a certain basis for diagnosis and treatment, which has become routine examination.
Keywords: high-resolution magnetic resonance imaging of the vessel wall, unruptured intracranial aneurysm, signal intensity, enhancement, diagnosis
Introduction
Intracranial aneurysm (IA) is a common cerebrovascular disease, which refers to the local pathological dilation of the artery wall and occurs mostly in women aged 40–60 years old. The pathogenesis of IA has not yet been fully determined and may be related to the degeneration of the internal elastic layer.1 According to the survey, the incidence of IA is about 6%, and the incidence of aneurysm rupture is about 0.8–2%,2 with a low incidence. Some patients have no special clinical symptoms, but if an aneurysm rupture leads to subarachnoid hemorrhage, the fatality rate is as high as 32–67%,3 and about 1/3 of patients are accompanied by disability, which brings a great burden to the society and family. Therefore, it is crucial to use accurate and convenient methods for early assessment of aneurysm rupture and provide an objective basis for clinical treatment. Currently, PHASES scoring is used to evaluate the stability of aneurysms,4 but this scoring method is based on the clinical characteristics of patients, which have certain errors and lack certain objectivity. At present, the methods for cerebral vascular imaging examination are magnetic resonance angiography (MRA), CT angiography (CTA), and Digital Subtraction angiography (DSA). MRA has the advantage of a non-injection contrast agent as a routine screening method. Cerebrovascular disease is confirmed by CTA or DSA examination as the standard. The location, size, and shape of aneurysms examined by CTA can only provide the judgment of whether aneurysms tend to rupture. Because of the high rate of misdiagnosis of MRA, approximately 59%,5 CTA remains a key role for the initial diagnosis of intracranial aneurysms because of its advantages of rapid imaging and reproducibility. The sensitivity and specificity of CTA for diagnosing medium and large aneurysms were 96–98% and 100%, respectively.6 In this study, even though the aneurysm was found by MRA, the patient still underwent CTA for the diagnostic accuracy. Although DSA is more sensitive to aneurysms < 5 mm,7 it is usually used as a treatment method and not as a routine examination method. According to literature reports,8 the larger the arterial tumor/aspect ratio is, the more likely it is to make exceptions, the larger the diameter is, the more likely it is to rupture. The aneurysm containing ascus is also likely to rupture. However, the above examination method cannot evaluate the condition of the vascular wall, so it has certain limitations.
With the development of imaging in recent years, MRI advanced scanning sequence has been increasingly applied in clinical practice. The application of high-resolution magnetic resonance vessel wall imaging (HR-VWI) can not only display the morphology of an aneurysm but also evaluate the condition of the aneurysm wall to reflect the stability of the aneurysm. HR-VWI can clearly show the thickness of an aneurysm wall, but Sherif9 measured the thickness of an experimental rabbit aneurysm wall by 3.0T MAGNETIC resonance and found that there was a certain difference between histological measurement and MRI measurement, which may be related to the resolution of the equipment. HR-VWI can measure tumor wall thickness when the lesion exceeds the spatial resolution of the image. Studies have shown that10 an aneurysm wall has an inflammatory reaction due to the effect of inflammatory factors, which will make the wall thicker, and the manifestation of this inflammatory reaction can be demonstrated by HR-VWI technology. HR-VWI technology reflects the inflammatory response of the aneurysm wall by evaluating the enhancement way or whether there is an enhancement, thus reflecting the stability of the aneurysm.11 Therefore, as an emerging imaging technology, HR-VWI imaging can analyze the characteristics of aneurysm walls, make a comprehensive analysis of patients and conduct regular follow-up reviews. In this study, unruptured intracranial aneurysms (UIA) were selected as subjects and aneurysm wall characteristics were analyzed by CTA and HR-VWI imaging to evaluate the stability of the aneurysm, to provide objective diagnosis and treatment basis for clinicians.
Materials and Methods
Research Subjects
A total of 92 patients with UIA diagnosed by CTA in Yan ‘an Hospital affiliated with Kunming Medical University and The Second People’s Hospital of Kunming from January 2018 to May 2021 were collected, including 57 females and 35 males. UIA patients were divided into an unstable group and a stable group on whether there was an enhancement of the vascular wall or not. Patients were admitted to the hospital with headaches and facial muscle convulsion as the chief reason.
Data Collection and Scanning Methods
Siemens Verio 3.0T superconducting MAGNETIC resonance scanner and 8-channel array coil were used.
Firstly, MRA images were collected to search for the lesion, and then the lesion area was scanned with a high-resolution SPACE sequence. An intravenous injection of gadolinium meglumine (Magenweishen) at a dose of 0.2 mL/kg was performed. The scanning parameters were consistent before and after enhancement, and the SPACE scan was performed again. The scanning parameters are as follows: MRA sequence: TR/TE:25/3.45, FOV18x18 cm, layer thickness 0.14 cm, and scan 120 layers. SPACE sequence: TR/TE:800/18, FOV20x18 cm, layer thickness 0.06 cm, scanning 133 layers.
Image Post-Processing
The images were loaded into the Siemens MRI MMWP post-processing station, and the MRA and SPACE images were subjected to multiplanar reformation (MPR) to produce images of different orientations. Image analysis was performed by two senior diagnostic imaging physicians. The measurement results of two physicians in the same method were analyzed, including the measurement of lesion location, size, SR value, and signal intensity. Signal measurements are performed using the Circle tool in the Workstation Tools directory. If there were different opinions, a consensus was reached through further discussion.
Statistical Analysis
SPSS 26.0 was used for data analysis and Origin 2019 for mapping. The measurement data followed a normal distribution and were described as mean ± standard deviation (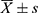 ). A t-test was used for comparison between groups. The count data were described by the number of cases, and the χ2 test was used for comparison between groups. Based on the literature,12–14 the enhancement rate, SR, and AR were transformed into a binary variable. The enhancement rate, SR, AR, and inflow angle were taken as independent variables, respectively, and the stability of the aneurysm was taken as dependent variables for univariate logistic regression analysis. The variables with P < 0.10 in univariate analysis were incorporated into the multivariate logistic regression model through stepwise forward regression screening. In the bilateral test, P < 0.05 was considered statistically significant.
). A t-test was used for comparison between groups. The count data were described by the number of cases, and the χ2 test was used for comparison between groups. Based on the literature,12–14 the enhancement rate, SR, and AR were transformed into a binary variable. The enhancement rate, SR, AR, and inflow angle were taken as independent variables, respectively, and the stability of the aneurysm was taken as dependent variables for univariate logistic regression analysis. The variables with P < 0.10 in univariate analysis were incorporated into the multivariate logistic regression model through stepwise forward regression screening. In the bilateral test, P < 0.05 was considered statistically significant.
Results
Comparison of the Baseline Between the Unstable Group and Stable Group
A total of 92 patients were included in the study. In the unstable group, there were 19 males and 24 females, aged 58.74 ± 11.71 years. In the stable group, there were 16 males and 33 females, aged 56.08 ± 9.37 years. There was no statistical significance in the age and gender of the two groups (Table 1).
 |
Table 1 Comparison of the Baseline Between the Unstable Aneurysm Group and Stable Aneurysm Group |
Analysis of Aneurysm Characteristics
The 92 patients had a total of 101 aneurysms. There were 7 aneurysms with UIA located in the anterior cerebral artery, 31 aneurysms with UIA in the middle cerebral artery, 1 aneurysm with UIA in the posterior cerebral artery, 18 aneurysms with UIA in the anterior communication, 5 aneurysms with UIA in the posterior communication, 34 aneurysms with UIA in the intracranial segment of the internal carotid artery, 2 aneurysms are located in the basilar artery, and 2 aneurysms with UIA in the vertebral artery (Figure 1). The lesion size of the aneurysm was between 2 mm and 38 mm in all collected cases (6.3 ± 5.09). There are 46 aneurysms with wall enhancement: the maximum SR value was 7.03 and the minimum SR value was 1.2; the maximum AR value was 7.5 and the minimum AR value was 1.0. In addition, it was found that there are 55 aneurysms without enhancement walls: the maximum SR value was 4.55 and the minimum SR value was 0.6; the maximum AR value was 4.0 and the minimum AR value was 0.6.
 |
Figure 1 Aneurysm location distribution. |
As shown in Table 1 and Figure 2 the enhancement rate, SR, and AR in the stable group were significantly lower than those in the unstable group (P < 0.05). Furthermore, the differences in enhancement rate, SR, inlet angle, and AR between the unstable group and the stable group were demonstrated by the violin diagram (Figure 2). Figures 3 and 4 show more clearly that the enhancement rate, SR, and AR of the unstable group are higher than those of the stable group.
 |
Figure 2 Violin diagram. (a) Inlet angle, p> 0.05. (b–d) Enhancement rate, AR, and SR, respectively, p< 0.05. |
Discussion
Aneurysm formation includes congenital and acquired factors.15,16 Some scholars believe that aneurysms are familial inheritance, and after exon sequencing, LOXL2c.C133T may be a pathogenic mutation.17,18 However, no evidence of familial heritability was found in this study, which may be due to the small sample size or lack of detailed medical history collection. Follow-up studies can expand the sample size and increase exon sequencing for further research. Epigenetic factors include hemodynamic changes, atherosclerosis, and vascular inflammatory responses. Hypertension may also be a factor. Cerebral et al19 proposed the concept of Wall Shear stress (WSS), which refers to the force generated by the friction between blood and vascular intima. The increase of WSS damages vascular endothelial cells and further leads to the dysfunction of endothelial cells, which is closely related to the occurrence of IA. Rupture of IA is the main cause of subarachnoid hemorrhage (SAH). In the 92 cases, 2 cases were ruptured. In 2012, the American Stroke Association proposed that active blood pressure control and smoking cessation could reasonably reduce the occurrence of SAH. The main causes of IA rupture are 1) inflammatory cells and factors; 2) the location and size of the aneurysm; 3) hemodynamics. Vascular inflammation is an important factor that cannot be ignored in an aneurysm rupture. It has been reported that C3, C9 complement, IgG, IgM immunoglobulin, and macrophages are found in the aneurysm wall of patients with aneurysm rupture, and leukocyte infiltration can be found in patients with aneurysm rupture.20 Autopsy results showed that inflammatory cell infiltration and fibrosis co-existed in 78% of unruptured aneurysms,21 while no fibrosis was found in the tumor wall without inflammatory cell infiltration, indicating that fibrosis may be secondary to inflammatory cell infiltration.22 As previously described, the macrophages have been found in patients with aneurysms and it has been found that they can denature the aneurysm wall, leading to rupture of the aneurysm. The reason may be that macrophages secrete MMP and elastase, which degenerated the elastic layer inside the vessel and thinned the vessel wall, finally leading to aneurysm rupture.23–25 HR-VWI, as a non-invasive and radiation-free examination technology, is widely used in the examination of vascular diseases. During the scanning, the blood within the scanning range cannot produce a magnetic resonance signal, to suppress the blood signal and highlight the surrounding vascular wall, which is called the black-blood technology. The application of this scanning technique was initially evaluated for arterial plaque, but with the continuous improvement of scanning technology, it can also be applied for vasculitis, aneurysm, and dissection.
In this study, aneurysm images of 92 cases were collected and analyzed by HR-VWI. When the inflammatory reaction occurs on the vascular wall, the permeability of the vascular wall increases and the injected contrast agent can penetrate the vascular wall, which can be shown as a uniform enhancement of the aneurysm wall in the image. The middle membrane of the arterial wall maintains the integrity of the vascular wall. The middle membrane is mainly smooth muscle, and the smooth muscle is the main component of the inflammatory reaction.26 Early in the early stage of aneurysm formation, smooth muscle underwent a phenotypic transformation, and smooth muscle cells changed from close arrangement to network arrangement, and the middle membrane could not maintain the integrity of the vascular wall, resulting in aneurysm rupture.27–30
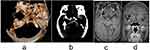
Takashi31 judged the stability of aneurysms according to the ratio of the maximum tumor depth to the diameter of the proximal parent artery (SR). In the analysis of 31 middle cerebral artery aneurysms, it was found that aneurysms located in the middle cerebral artery with SR > 1.7 were more likely to rupture. A study of the anterior cerebral artery showed that aneurysms with SR greater than 0.9 were at risk of rupture.32 In the present study of 31 aneurysms in the middle cerebral artery, SR > 1.7 was found in 4 cases of them (Figure 3). This patient can be judged as an unstable aneurysm from both SR value and enhancement signal intensity, and annular enhancement is more likely to rupture than focal enhancement.33 This patient also presented significant annular enhancement and was in urgent need of treatment, which was consistent with the conclusion of some scholars34–36 that tumor wall enhancement and SR value could be used as independent risk factors for aneurysm rupture. Aneurysm size has also been considered an important factor in determining stability. Some studies suggest that the size and location of aneurysms can determine the stability of IA and aneurysms with a diameter >7 mm located in the anterior communicating artery are more likely to rupture.15,37 The study showed that the enhancement rate increases gradually with the increase of aneurysm volume.38 In this study, 4 aneurysms (4/55) in the stable aneurysm group had lesions larger than 7 mm, and 14 aneurysms (14/55) in the anterior communicating artery. These 18 aneurysms showed no enhancement of the tumor wall, indicating no inflammatory response. One of the reasons for this may be that the number of cases was too small to reach an agreement with the scholar’s findings, and a larger sample size is needed for further validation. The maximum SR in the anterior communicating artery was 0.7, less than 0.9. This was consistent with the findings of Tremmel.32
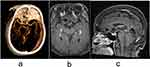
Hemodynamics is related to the formation of IA and also plays an important role in the rupture process.39 There is a positive correlation between WSS and blood flow velocity. The faster the speed is, the more serious the damage to the elastic layer in the blood vessel will be. The smooth muscle and elastic fibroma of the medium membrane would disappear, and the IA were more likely to rupture. This is in contrast to the Tremmel32 study, in which he found that aneurysms are prone to vorticity and thus reduced wall shear stress (WSS). Due to limited conditions, WSS cannot be measured in this study, which can be used as the target of future research. In addition, studies have shown that thrombus formation in aneurysms is an independent factor in aneurysm rupture, aneurysms >8 mm are more likely to form thrombi, the process of thrombosis is due to prolonged blood flow residence time, and both WSS and eddy currents in blood flow are key factors in thrombosis.40 Figure 4c shows the high-resolution vessel wall enhancement imaging. A high signal is visible in the lumen of the aneurysm, indicating that blood flow stays in the lumen for a long time and this patient is more prone to thrombosis. The significant enhancement of the aneurysm wall also indicates that there is a severe inflammatory response in the aneurysm wall, which is also prone to rupture. Therefore, surgery is performed for this patient. In contrast, small aneurysms have a shorter residence time for blood flow; thus, it is less likely to form a thrombus. Figure 3 shows an aneurysm of the cavernous sinus segment of the left internal carotid artery. Although there is an enhancement in the wall, no contrast is seen in the lumen, indicating that the blood residence time for a small aneurysm is short and is less likely to form thrombosis.
For IA, especially unstable IA, HR-VWI is the main noninvasive examination method. Although routine CTA examination is the diagnostic standard of vascular diseases, it is easy to cause misdiagnosis because of its simple judgment method. HR-VWI can be used to identify lesions from various aspects including location, morphology, and tumor wall state, which can significantly improve the diagnostic accuracy of IA and provide a diagnosis and treatment basis.
Conclusion and Limitation
There are many independent risk factors for unruptured intracranial aneurysms, including SR, AR, rate of enhancement, and thrombosis. HR-VWI allows for specific observation of the rate of aneurysm wall enhancement. Although it is limited to some aneurysm walls, it can be supplemented by other parameters to provide a valid assessment of the risk of aneurysm rupture. The shortcomings of this study are the cases included for research are small, especially for patients with a high risk of non-surgical treatment. Future further studies are needed to increase the sample size for more in-depth studies.
Patient Consent for Publication
Informed consent was obtained from all patients.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of Yan ‘an Hospital affiliated with Kunming Medical University and The Second People’s Hospital of Kunming. Informed consent was obtained from all patients. This study complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Joint Project of Kunming Medical University and Department of Science and Technology, Yunnan Province (No.202101AY070001-210); Yunnan High-level Health Technical Talents Reserve Talents Program for Medical Discipline (No. H-2018060); Scientific Research Foundation of Yunnan Education Department (No.2021J0278); Kunming Health Science and Technology Personnel Training Project and “Ten Hundred thousand” Project Training Plan (No. 2020-SW −10); Kunming Municipal Health Commission Health Research Project (No.2020-09-01-111); Hospital Project of Kunming Yan ‘an Hospital (No.YYKY019-021).
Disclosure
The authors declare that they have no competing interests. The institution does not develop products with relevant information, apply for patents, and does not provide experimental funds. The institution does not interfere with the decision to publish and share relevant research results in journals.
References
1. Majewska P, Gulati S, Øie L, et al. Smoking habits and detection rate of unruptured intracranial aneurysms and incidence rate of subarachnoid haemorrhage in Norway between 2008 and 2015. Acta Neurochir. 2020;162(12):3161–3165. doi:10.1007/s00701-020-04541-0
2. Liang ES, Mahady K, Coulthard A, et al. Treatment of a middle cerebral artery aneurysm in the setting of Loeys-Dietz syndrome: case report and review of literature. Radiol Case Rep. 2021;16(1):48–50. doi:10.1016/j.radcr.2020.10.012
3. Darkwah OM, Gembruch O, Pierscianek D, et al. Post-treatment antiplatelet therapy reduces risk for delayed cerebral ischemia due to aneurysmal subarachnoid hemorrhage. Neurosurgery. 2019;85(6):827–833. doi:10.1093/neuros/nyy550
4. Greving JP, Wermer MJH, Brown RD, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66. doi:10.1016/S1474-4422(13)70263-1
5. Schwab KE, Gailloud P, Wyse G, Tamargo RJ. Limitations of magnetic resonance imaging and magnetic resonance angiography in the diagnosis of intracranial aneurysms. Neurosurgery. 2008;63:1.
6. Wang X, Benson JC, Jagadeesan B, et al. Giant cerebral aneurysms: comparing CTA, MRA, and digital subtraction angiography assessments. J Neuroimaging. 2020;30(3):335–341. doi:10.1111/jon.12712
7. You SH, Kim B, Yang K-S, et al. Ultrashort echo time magnetic resonance angiography in follow-up of intracranial aneurysms treated with endovascular coiling: comparison of time-of-flight, pointwise encoding time reduction with radial acquisition, and contrast-enhanced magnetic resonance angiography. Neurosurgery. 2021;88(2):E179–E189. doi:10.1093/neuros/nyaa467
8. Di Bonaventura R, Sturiale CL, Latour K, et al. Comparison between minipterional craniotomy associated with focused sylvian fissure opening and standard pterional approach with extended sylvian fissure dissection for treatment of unruptured middle cerebral artery aneurysms. World Neurosurg. 2021;146:e1293–e1300. doi:10.1016/j.wneu.2020.11.150
9. Sherif C, Krssak M. Evaluation of cerebral aneurysm wall thickness in experimental aneurysms: comparison of 3T-MR imaging with direct microscopic measurements. Acta Neurochir. 2018;160(4):759. doi:10.1007/s00701-018-3476-1
10. Lehman VT, Brinjikji W, Mossa-Basha M, et al. Conventional and high-resolution vessel wall MRI of intracranial aneurysms: current concepts and new horizons. J Neurosurg. 2018;128(4):969–981. doi:10.3171/2016.12.JNS162262
11. Lehman VT, Brinjikji W, Kallmes DF, et al. Clinical interpretation of high-resolution vessel wall MRI of intracranial arterial diseases. Br J Radiol. 2016;89(1067):20160496. doi:10.1259/bjr.20160496
12. Zhai XD, Yu J-X, Li C-J, et al. Morphological characteristics of pericallosal artery aneurysms and their high propensity for rupture. World Neurosurg. 2020;133:e320–e326. doi:10.1016/j.wneu.2019.09.003
13. Duan Z, Li Y, Guan S, et al. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci Rep. 2018;8(1):6440. doi:10.1038/s41598-018-24732-1
14. Gu Y, Zhang Y, Luo M, et al. Risk factors for asymptomatic intracranial small aneurysm rupture determined by electrocardiographic-gated 4D computed tomographic (CT) angiography. Med Sci Monit. 2020;26:e921835. doi:10.12659/MSM.921835
15. Darkwah OM, Jabbarli R, Radbruch A, et al. Blind date with an aneurysm: acute M1 middle cerebral artery thrombus with native computed tomography scan suggesting aneurysm rupture. World Neurosurg. 2019;132:103–105. doi:10.1016/j.wneu.2019.08.206
16. Komunski P, Nowosławska E, Zakrzewski K, et al. Superior hypophyseal artery ruptured aneurysm in a 5-month-old child presenting as an acute subdural hematoma: a case report. Pediatr Neurosurg. 2020;55(6):374–379. doi:10.1159/000511674
17. Wu Y, Li Z, Shi Y, et al. Exome sequencing identifies LOXL2 mutation as a cause of familial intracranial aneurysm. World Neurosurg. 2018;109:e812–e818. doi:10.1016/j.wneu.2017.10.094
18. Chambers WR, Harper BJ, Simpson JR. Familial incidence of congenital aneurysms of cerebral arteries: report of cases of ruptured aneurysms in father and son. J Am Med Assoc. 1954;155(4):358–359. doi:10.1001/jama.1954.73690220001007
19. Cebral JR, Mut F, Weir J, et al. Quantitative characterization of the hemodynamic environment in ruptured and unruptured brain aneurysms. AJNR Am J Neuroradiol. 2011;32(1):145–151. doi:10.3174/ajnr.A2419
20. Hussain S, Barbarite E, Chaudhry NS, et al. Search for biomarkers of intracranial aneurysms: a systematic review. World Neurosurg. 2015;84(5):1473–1483. doi:10.1016/j.wneu.2015.06.034
21. Watanabe T. Neopterin derivatives – a novel therapeutic target rather than biomarker for atherosclerosis and related diseases. Vasa. 2021;50(3):165–173. doi:10.1024/0301-1526/a000903
22. Roa JA, Zanaty M, Ishii D, et al. Decreased contrast enhancement on high-resolution vessel wall imaging of unruptured intracranial aneurysms in patients taking aspirin. J Neurosurg. 2020;134(3):902–908. doi:10.3171/2019.12.JNS193023
23. Rojas HA, Fernandes KSDS, Ottone MR, et al. Levels of MMP-9 in patients with intracranial aneurysm: relation with risk factors, size and clinical presentation. Clin Biochem. 2018;55:63–68. doi:10.1016/j.clinbiochem.2018.03.005
24. Patzig M, Forbrig R, Gruber M, et al. The clinical value of ceMRA versus DSA for follow-up of intracranial aneurysms treated by coil embolization: an assessment of occlusion classifications and impact on treatment decisions. Eur Radiol. 2021;31(6):4104–4113. doi:10.1007/s00330-020-07492-3
25. Zou L, Hou Y, Yu B, et al. The effect of intravascular interventional embolization and craniotomy on MMP-2, MMP-9 and caspase3 in serum of intracranial aneurysm patients. Exp Ther Med. 2018;16(6):4511–4518. doi:10.3892/etm.2018.6740
26. Lai XL, Deng Z-F, Zhu X-G, et al. Apc gene suppresses intracranial aneurysm formation and rupture through inhibiting the NF-κB signaling pathway mediated inflammatory response. Biosci Rep. 2019;39(3). doi:10.1042/BSR20181909
27. Signorelli F, Sela S, Gesualdo L, et al. Hemodynamic stress, inflammation, and intracranial aneurysm development and rupture: a systematic review. World Neurosurg. 2018;115:234–244. doi:10.1016/j.wneu.2018.04.143
28. Xu Z, Rui Y-N, Hagan JP, et al. Intracranial aneurysms: pathology, genetics, and molecular mechanisms. Neuromolecular Med. 2019;21(4):325–343. doi:10.1007/s12017-019-08537-7
29. Gitto L, Richardson TE, Serinelli S, et al. Massive intracranial bleeding due to the rupture of a rare spontaneous pseudoaneurysm of the middle cerebral artery in a pediatric patient: case report with clinical, radiological, and pathologic findings. Forensic Sci Med Pathol. 2019;15(3):474–480. doi:10.1007/s12024-019-00122-5
30. Miyata H, Imai H, Koseki H, et al. Vasa vasorum formation is associated with rupture of intracranial aneurysms. J Neurosurg;2019. 1–11. doi:10.3171/2019.5.JNS19405
31. Sadatomo T, Yuki K, Migita K, et al. Morphological differences between ruptured and unruptured cases in middle cerebral artery aneurysms. Neurosurgery. 2008;62(3):602.
32. Tremmel M, Dhar S, Levy EI, et al. Influence of intracranial aneurysm-to-parent vessel size ratio on hemodynamics and implication for rupture: results from a virtual experimental study. Neurosurgery. 2009;64(4):622–630. doi:10.1227/01.NEU.0000341529.11231.69
33. Khattar NK, White AC, Adams SW, et al. MRI SPACE sequence confirmation of occluded MCA M2 dissection stump masquerading as a ruptured MCA aneurysm. BMJ Case Rep. 2018;2018.
34. Chihi M, Jabbarli R, Gembruch O, et al. A rare case of a completely thrombosed bilobed giant intracranial aneurysm of the anterior cerebral artery with spontaneous parent vessel thrombosis: case report. BMC Neurol. 2019;19(1):297. doi:10.1186/s12883-019-1529-6
35. Rinkel G. Management of patients with unruptured intracranial aneurysms. Curr Opin Neurol. 2019;32(1):49–53. doi:10.1097/WCO.0000000000000642
36. Razaghi R, Biglari H, Karimi A. Risk of rupture of the cerebral aneurysm in relation to traumatic brain injury using a patient-specific fluid-structure interaction model. Comput Methods Programs Biomed. 2019;176:9–16. doi:10.1016/j.cmpb.2019.04.015
37. Gew J, Sokol D, Gallo P, et al. De novo distal middle cerebral artery aneurysm post-excision of intracerebral arteriovenous malformation in an 8-year old. Childs Nerv Syst. 2019;35(11):2211–2218. doi:10.1007/s00381-019-04328-4
38. Zwarzany L, Tyburski E, Poncyljusz W. High-resolution vessel wall magnetic resonance imaging of small unruptured intracranial aneurysms. J Clin Med. 2021;10(2):225. doi:10.3390/jcm10020225
39. Texakalidis P, Sweid A, Mouchtouris N, et al. Aneurysm formation, growth, and rupture: the biology and physics of cerebral aneurysms. World Neurosurg. 2019;130:277–284. doi:10.1016/j.wneu.2019.07.093
40. Gu Y, Miao C, Li A, et al. High-Resolution Magnetic Resonance Imaging (HR-MRI) evaluation of the distribution and characteristics of intra-aneurysm thrombosis to improve clinical diagnosis of thrombotic intracranial aneurysm. Med Sci Monit. 2022;28:e935613. doi:10.12659/MSM.935613
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.