Back to Journals » Infection and Drug Resistance » Volume 15
The Anti-Virulence Effect of Sub-Minimal Inhibitory Concentrations of Levofloxacin on Hypervirulent Klebsiella pneumoniae
Authors Ma X, Zhang L, Yue C, Liu Y , Li J
Received 19 April 2022
Accepted for publication 24 June 2022
Published 4 July 2022 Volume 2022:15 Pages 3513—3522
DOI https://doi.org/10.2147/IDR.S370273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xuejiao Ma,1,* Li Zhang,1,* Chengcheng Yue,1 YanYan Liu,2,3 Jiabin Li1– 4
1Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Institute of Bacterium Resistance, Anhui Medical University, Hefei, People’s Republic of China; 3Anhui Center for Surveillance of Bacterial Resistance, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 4Department of Infectious Disease, Chaohu Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiabin Li, Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Jixi Road no. 218, Hefei, 230022, People’s Republic of China, Tel/Fax +86-551-62922713, Email [email protected] YanYan Liu, Anhui Center for Surveillance of Bacterial Resistance, Jixi Road no. 218, Hefei, 230022, People’s Republic of China, Tel/Fax +86-551-62922713, Email [email protected]
Background: Hypervirulent Klebsiella pneumoniae (hvKp) is best described as a virulent pathogen and generally associated with the hypermucoviscosity phenotype. Increased capsule and aerobactin production are established important hvKp-specific virulence factors. Although hvKp strains have been relatively susceptible to antimicrobials, given the high morbidity and mortality, there is a critical need for alternative strategies for the treatment of hvKp infections. Thus, the anti-virulence therapy has been targeted for the hvKp development of therapeutics.
Materials and Methods: Four hvKp isolates with hypermucoviscous phenotype were used in our experiments. Mucoviscosity of the capsule can be assessed by low-speed centrifugation of cultures. CPS amount was determined by glucuronic acid content. The capsule thickness was measured under microscope after ink staining. The transcriptions of gene were measured by quantitative real-time PCR (qRT-PCR). The effect of levofloxacin on the resistance of K. pneumoniae to phagocytosis by macrophages and mouse lethality assay was observed.
Results: Our data revealed that sub-Minimal Inhibitory Concentrations (sub-MIC) of LVX reduce mucoviscosity and CPS production of hvKp. Microscopic observations demonstrated that the capsule of hvkp bacteria became thinned after treatment with LVX. qRT-PCR showed decreased transcript levels of rmpA, wzi, magA, iroN and icuA genes. Down-regulation of these virulence genes occurred leading to increased susceptibility to phagocytosis by macrophages. Mouse lethality assay revealed that the wild strain had the LD50 of 103 CFU, while the sub-MIC LVX-treated bacteria had the LD50 of 105 CFU.
Conclusion: Our data suggested that LVX may serve as a potential anti-virulence agent for refractory infection by hvKp.
Keywords: hypervirulent Klebsiella pneumoniae, hypermucoviscous, capsular polysaccharide, levofloxacin, anti-mucoviscous, anti-virulence agent
Introduction
Klebsiella pneumoniae (kp) is an increasingly important opportunistic pathogen capable of causing pneumonia, urinary tract infections, and bacteremia in immunocompromised or frequently healthcare-exposed individuals.1 Recently, a new and hypervirulent clinical variant of K. pneumoniae has an ability to cause community-acquired and life-threatening infections among young and healthy individuals. Epidemics of hypervirulent K. pneumoniae (hvKp) have occurred mainly in Asian countries, including China, South Korea and Iran, while only sporadic spread have been reported in European and American countries. The patients infected by hvKp often present with multiple sites of infection and/or develop subsequent metastatic spread, which are less common in other Enterobacteriaceae infections. The prevalence of bacteremia caused by hvKp in China is high, ranging from 24.5% to 36.8%,2,3 which is lower than 41.5% reported in Taiwan,4 but higher than 6% (53/878) reported by a teaching hospital in Spain from 2007 to 2013.5 Despite reasonable antibiotic treatment, patients with the invasive syndrome have a mortality rate ranging from 3% to 31%,6 and patients with hypermucoviscous (HMV) K. pneumoniae bacteremia have a mortality rate close to 35% or higher.3 In one case series of 12 French patients with the HMV phenotype, all 5 patients with bacteremia died.7
The RmpA- and/or RmpA2-mediated overproduction of capsular polysaccharide is the unique structural feature of hvKp strains when grown on agar plates. The capsular polysaccharides (CPS) is one of important virulence factors of hvKp, which can protect bacteria from phagocytosis and opsonization, provide protective shield against antimicrobial peptides, and suppresses the early inflammatory response.8 Also, it is a key virulence factor that aids dissemination to the host bloodstream and systemic infection.9,10 More than 70 capsular serotypes have been reported for K. pneumoniae, and isolates that produce CPS are generally more virulent than non-capsulated strains. Among these, most of hvKp isolates belong to capsular serotypes K1 and K2.11 Most HMV K. pneumoniae isolates from patients with bacteremia also had the K1 or K2 serotype (78%).12 Besides CPS, a number of virulence factors have been suggested to contribute to the pathogenesis of hvKp strains, including rmpA, magA, and several iron acquisition factors.
Antibiotics continue to be the only treatment option for K. pneumoniae infections, but it still fails to prevent the occurrence of invasive syndrome. There have been no trials assessing which antibiotics are best suited for treating hvKp infection and local antimicrobial resistance patterns. Many studies have shown that changes in CPS levels due to gene mutant and deletion from the CPS locus can lead to changes in mucus phenotypes, serum, phagocytosis, and virulence. For example, hvKp with mutations in rmpA, or other genes that affect capsule production, typically lose the HMV phenotype and show a strong reduction in virulence when tested in mice.13,14 However, changes in CPS and other virulence-related gene expression through spontaneous and specific gene mutants or deletion may not be applied in clinical patients with hvKp infection. Ofloxacin has been shown to reduce mucobiscosity of hvKp.15 In our study, we want to discuss levofloxacin (LVX) serves as potential anti-virulent agent for hvKp infection.
Materials and Methods
Bacteria
Four hvKp strains were isolated in blood or pyogenic fluid cultures from with liver abscess in the first affiliated hospital of Anhui Medical University. The strains were grown in Mueller–Hinton broth at 37°C with shaking at 130 rounds per minute (RPM). All the strains identified had positive string test results and carried several virulence-associated genes (rmpA, magA, ironB, and iucA). Molecular analysis revealed that the isolates produced K1 capsular polysaccharides and belong to sequence type (ST) 23. They were all susceptible to almost all commonly used antibiotics, including ceftriaxone, cefotaxime, piperacillin–tazobactam imipenem, meropenem, amikacin, and levofloxacin, except ampicillin. The minimum inhibitory concentration (MIC) values of levofloxacin for the four isolates were 0.03125–0.125µg/mL. K. pneumoniae ATCC 700603 is a non-hvKP control strain, which is a K6 serotype possessing neither magA nor rmpA.
Evaluation of Anti-Mucoviscous Activity of Levofloxacin
The mucoviscosity of K. pneumoniae strains was determined as previously described.16 Briefly, equal numbers of phase-cultured bacteria with or without different concentrations of LVX (1/16 ×, 1/8 ×, 1/4 ×, 1/2 × of the MIC) were centrifuged at 1000 g for 5 min. Then, the supernatant was subjected to measurement of the absorbance at 600 nm. B16 isolate was used for verifying the anti-mucoviscous activity of LVX, and then confirmed the effect with other hvKp isolates.
Quantitative Measurement of Bacterial Capsular Polysaccharide
The bacterial extracellular polysaccharide was extracted and the uronic acid, a main component of K. pneumoniae K1 capsule, was quantified as previously described.17,18 Briefly, 500 μL of overnight broth-cultured bacteria was mixed with 100 μL of 1% Zwittergent 3–14 (Sigma-Aldrich, Milwaukee, WI) in 100 mM citric acid (pH 2.0) and then incubated at 50°C for 20 min. After centrifugation, 250 μL of the supernatant was transferred and added with 1 mL of cold ethanol. The mixture was incubated at 4°C for 20 min for precipitation. After centrifugation, the pellet was dried and dissolved in 200 μL of distilled water, and then 1200 μL of 12.5 mM tetraborate in concentrated H2SO4 was added. After vigorous vortex, the mixture was boiled for 5 min. After cooling, 20 μL of 0.15% 3-hydroxydiphenol (Sigma-Aldrich) was added. Then, the absorbance at 520 nm was measured.
Bacterial Growth Curves
To monitor the effects of levofloxacin on bacterial growth, growth curve was generated as previously described.19 Bacteria cultured overnight were added to a 96-well microtiter plate at a final concentration of 106 CFU/mL, together with different concentrations of LVX for 18h (final volume of 200 μL). The optical density (OD) of the bacterial culture at 600 nm was measured every 2h using a spectrophotometer (U-1500, Hitachi, Tokyo, Japan). Experiments were repeated in triplicate and the average absorbance values were used for analysis.
Microscopic Observation of Capsule
Overnight cultures were mixed with equal volumes of India ink, and 2 μL samples of the mixtures placed on microscope slides and covered with a cover slip. The samples were evaluated, and images captured using an Olympus BX50 microscope (Olympus, Tokyo, Japan) with Confocal Laser Scanning Microscopy (CLSM) a 100 objective lens. To determine the change in capsule thickness over time, overnight cultures with or without 1/4MIC LVX were subcultured in fresh M-H B broth starting at 0.05 OD600 units. The cultures were incubated at 37 °C over a series of time-points. The cultures were diluted to 0.1OD600. Then, four slides were prepared according to the above ink staining method to observe and measure the capsule thickness. The shortest diameter of the clear zone around each bacterial cell was recorded as the capsule thickness. Four fields were randomly selected for each slide, all cells were measured and average capsule thickness was calculated.
Quantitative Real-Time Polymerase Chain Reaction
The expression level of rmpA, magA, galF, wzi, manC, iucA, and iroN genes in hvKp strains after treatment with a sub-MIC concentration of LVX were determined via quantitative real-time PCR (qRT-PCR). The primers used for qRT-PCR were synthesized according to the literature.15 RNAs of the K. pneumoniae strains at the logarithmic growth stage were extracted using an RNA extraction kit (Tiangen) according to the kit’s procedure. The quantity and quality of the RNAs were assessed using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Complementary DNA was synthesized using the cDNA Synthesis Kit (Takara, Japan). The qRT-PCR (three replicates) assay for each gene was performed using Applied Biosystems PowerUpTM SYBR® Green Master Mix (Thermo Fisher Scientific) in an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). The 16S rRNA gene was used as internal control. Finally, the relative expression of the above genes was calculated by the 2−ΔΔCt method.
Determination of the Anti-Phagocytosis
Raw264.7 cells were grown in DMEM (Gibco, Grand Island, NY, United States) containing 10% FBS at 37°C. Bacteria were grown in 5 mL of LB broth with or without LVX (1/4 MIC µg/mL) for 8–10 hours and washed once in cold phosphate-buffered saline (PBS). Raw 264.7 cells were infected with K. pneumoniae at a multiplicity of infection (MOI) of 100 and incubated for 2h at 37°C to permit phagocytosis. After 2h incubation, infected cells were washed three times with PBS, and incubated for another 1h with 1mL of DMEM containing 100 µg/mL of gentamycin and 10 µg/mL lysostaphin (Sigma-Aldrich) to kill the extracellular bacteria. The cells were washed three times with PBS again and lysed with 0.2 mL of sterile 0.25% Triton X-100. After being fully mixed, 10-fold serial dilutions were plated on the MHA culture plates for 16h incubation at 37°C, and the number of bacterial colonies was counted. The percentage of phagocytosis rate was expressed as the number of viable bacteria that infect RAW264.7 cells compared with the number of viable bacteria from the pretreatment and multiplied by 100.
Determination of the Virulence in Mouse Lethality Tests
ICR male mice (28–32g) were injected with graded doses of 102 to 106 CFU in 10-fold serial dilutions in 0.1 mL of normal saline via tail vein. Six mice were used to test the effect of each inoculum (10g/0.1mL). Mice receiving injections were followed for 7 days, with an in extremis state or death being used as the study end point. The 50% lethal dose (LD50) of K. pneumoniae strain was calculated using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) according to the number of mice that died. Animal studies were approved by the Animal Care and Use Committee of Anhui Medical University (No. LLSC20190253). All animal care and use protocols in this study were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
Results
Levofloxacin Reduce Mucoviscosity and CPS Production
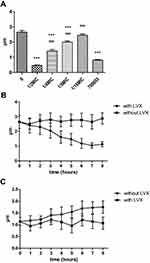
HMV strains do not sediment well during centrifugation, and the supernatant remains turbid. Measurement of the turbidity after centrifugation can therefore serve as a quantitative indicator of HMV. The strains were grown as described above, with or without LVX overnight, and then subjected to low-speed centrifugation. Uronic acid (UA) is a key component of bacterial capsules and has been used as an indicator of capsule production. These results indicate that LVX can reduce the capsule production and mucoviscosity (Figure 1). Obviously, the effect was enhanced with the increase in the concentration of LVX (Figure 1).
Low Concentration of LVX Had No Obvious Effects on the Growth of KP
To monitor different MICs of LVX on the growth of KP, a 18h growth curve was generated. Based on the growth curve, we found that the growth of hvKp was almost unaffected when the concentration of LVX in the medium was less than or equal to 1/4MIC (Figure 2).
 |
Figure 2 Bacterial growth curve at different concentrations of levofloxacin. Low concentration (≤1/4MIC) of LVX does not affect the hvKp growth. Bacterial cultures were grown at 37°C for 18 h. |
Microscopic Observation of Capsule
When hvKp was grown in the presence of sub-inhibitory concentration LVX, the capsule became thinner. When the concentration was reduced to 1/16MIC, the capsule thickness was not significantly different from that of the original strain (Figure 3A). Compared with ATCC700603, when LVX concentration was 1/2MIC, there was no obvious difference in capsule thickness between ATCC700603 and LV. However, at other concentrations of LVX, the capsule was thicker than 700603 in our study. We further evaluate the effect of LVX on bacterial capsule thickness over time following the addition or removal of LVX. After the addition of LVX, the bacterial capsule thickness gradually decreased with time and reached the minimum level after 6 hours (Figure 3B). Conversely, when LVX was removed from an overnight culture of hvKp, the capsule gradually regained its thickness (Figure 3C). But over 8 hours, the bacterial capsule thickness had not returned to the original strain size. Eventually, the bacterial capsule thickness could return to normal after about 12 hours. These results demonstrated that the effect of LVX on the hvKp capsule was reversible.
LVX Represses Capsule Genes and Other Virulence Genes Expression
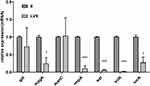
We evaluate the effect of LVX on the expression levels of the capsule-related genes (rmpA, magA, galF, wzi, and manC) and other virulence genes (iucA and iroN) in absence and presence of 1/4 MIC LVX overnight. Not all the gene transcripts were markedly lower when hvKP was grown in the presence of LVX compared to that in the absence of LVX (Figure 4). Transcription of rmpA, wzi, magA, iroN and icuA decreased, especially rmpA, wzi, and iroN. However, expression of galF and manC was not affected.
Phagocytosis of hvKp
A hallmark of the pathogenesis of K. pneumoniae is its resistance to macrophage-mediated phagocytosis, due to the capsule polysaccharide. Given that LVX reduced capsule expression (Figures 3 and 4), bacteria treated with 1/4MIC LVX were more likely to be phagocytosed (Figure 5).
 |
Figure 5 Phagocytosis of K. pneumoniae. The percentage of phagocytosis against hvKp increased significantly after treatment with LVX (***p < 0.001). |
Virulence in Mouse Lethality Tests
Mouse lethality assay revealed that the wild strain had the LD50 of 103 CFU, while the LVX-treated bacteria had the LD50 of 105 CFU. When mice were injected bacteria with doses of 106 CFU, All the mice in group 0 (wild strain) died within 4 days (Figure 6). On day 7, the death rate in group LVX (LVX-treated bacteria) was 50% (Figure 6).
Discussion
Antibiotics have been widely used in the treatment of bacterial infection with high efficiency since their discovery. These drug molecules can kill bacterial pathogens or inhibit their growth if they are used at the right concentration and dosage. However, if the drug concentration is below the minimum inhibitory concentration (MIC), antibiotics can regulate bacterial adaptability and gene expression.20 For example, antibiotics acting on bacterial cell walls can inhibit the synthesis of capsular polysaccharides in Streptococcus pneumoniae.21 In the study by Nomura et al, it was found that after the treatment of K. pneumoniae with different drugs in 1/4MIC, penicillins, cephalosporins and monoamides could significantly reduce the capsular thickness, quinolones had little effect on the capsular thickness, while aminoglycosides, macrolides and carbapenems showed no significant change in the capsular thickness.22 Since the mid-1980s, hvKp, which is usually presented as an HMV phenotype, has become a clinically important pathogen and often causes severe disseminated infections.23,24 The high mucinous phenotype of hvKp is due to the increased production of typical CPS and the presence of specific virulence genes.8,25 Similarly, for hvKp strain, Namekawa H showed that some antibiotics could inhibit the high viscosity of hvKp and some antibiotics could promote the high viscosity of hvKp by testing 18 kinds of drugs at 1/4MIC concentration, among which rifampicin showed a strong inhibitory effect on capsule formation.15 In our study, we found that LVX can inhibit the hvKp capsule formation. At 1/4MIC, LVX had little effect on the growth of the bacteria, but the mucoviscosity and CPS production decreased. Regardless of the effect of drugs on bacterial growth, with the increase in drug concentration, the inhibition effect on bacterial capsule became more obvious. These results can be seen from qualitative measurements of capsule content and direct measurements of capsule thickness in our study. The capsule thickness of hvKp began to thin after the addition of LVX for 2h. When the capsule was reduced to the minimum, the capsule thickness gradually recovered after the removal of the drug. But it did not completely recover until 12h.This indicated that the change of capsule thickness of hvKp caused by LVX is reversible.
Several important genes associated with the capsule production are encoded in the CPS cluster, includes three promoters, which are located upstream of galF, wzi, and manC, respectively.26 The transcriptional regulator RmpA upregulates the activities of all these promoters to activate capsule production, resulting in the HMV phenotype and increase in virulence.27 From the perspective of gene transcription level, LVX has different effects on the expression of these genes. We hypothesized that LVX may reduce the mucoviscosity and CPS production by inhibiting the expression of rmpA. Theoretically, all the CPS genes are expected to be repressed simultaneously. However, in our experiment, only wzi was inhibited significantly, while galF and manC were not affected. The polycistronic mRNA driven by promoter P2 from wzi to gnd (in many cases, corresponds to upstream of manC) consisted of genes of capsule repeat unit synthesis and polymerization, as well as surface assembly.28 It is speculated that down regulation of wzi has an effect on the capsule production in hvKp grown in the presence of LVX and may not be regulated by rmpA. Mucoviscosity-associated gene A (magA) of Klebsiella pneumoniae contributes to K1 CPS biosynthesis. MagA was predicted to be a Wzy-like polymerase based on sequence homology, conserved domain alignment, and an O-antigen synthesis assay.28,29 MagA (K1_wzy) is essential for the synthesis of capsular polysaccharide and plays an important role in hypermucoviscous. So in our experiment, down-regulated magA was also involved in the reduction of the hypermucoviscosity phenotype and CPS production.
In large part, the enhanced virulence of hvKp is due to its ability to synthesize significantly higher levels of iron acquisition molecules.30 These siderophores have a remarkably high affinity for iron and facilitate bacteria acquire iron in iron-depleted environments, such as the human host. HvKp can produce four siderophores: enterobactin, salmochelin, yersiniabactin, and aerobactin, whereas salmochelin (encoded by the iro operon) and aerobactin (iuc) are hvKp specific.31 Recent data have directly demonstrated only aerobactin plays in hvKp growth/survival ex vivo and for the enhanced virulence of the hvKp in vivo.32 As such, it has been proposed as an anti-virulence target.33 There is a strong trend of co-occurence of iro and iuc loci in hvKp strains in a recent analysis of ~2500 Kp genomes, suggesting salmochelin is present in these genomes because it is important to the success of hvKp as well.34 In our experiment, LVX had an inhibitory effect on both iucA and iroN, especially iroN. Iuc and Iro synthesis is encoded by loci (iuc and iro), that are typically co-located on the so-called ‘virulence plasmids’ of K. pneumoniae. These plasmids also carry additional virulence determinants including rmpA genes that upregulate capsule production. QRT-PCR revealed reduced transcription level of these three genes in hvKp grown in the presence of LVX. It can be inferred from the above observations, sub-MIC LVX may not affect bacterial growth but could modulate the overall gene-expression pattern. Specifically, genes located on virulence plasmids.
CPS is probably considered the critical factor in K. pneumoniae pathogenesis to protect K. pneumoniae from killing by phagocytosis or serum factors.35,36 Compared with encapsulated strains, unencapsulated K. pneumoniae strains are more susceptible to killing by serum complement, and they are more readily phagocytosed and killed by phagocytic leukocytes.37,38 In our study, LVX decreases the CPS production that were correlated with the enhancement of phagocytic activity of macrophages. After LVX treatment, the virulence of bacteria in mice decreased with the change of several virulence factors (CPS synthesis-related genes and siderophores expression inhibition).
Conclusion
Taken together, we here demonstrated that LVX repress CPS and siderophores production. This changed bacterial phenotype, including mucoviscosity, impairs the bacterial resistance to phagocytosis and reduce the virulence of aof bloodstream infection in a mouse model . In addition to its bactericidal properties, LVX reduces the virulence of hvKp, making it a perfect or even preferred antibiotic for the treatment or prevention of infections caused by hvKp.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81973983), Collaborative Tackling and Public Health Collaborative Innovation Project in Anhui Province (No. GXXT-2020-018), the joint construction project of clinical medicine university and hospital (No. 2021lcxk006), and Natural Science Research Project of Universities in Anhui Province (No. KJ2020A0176).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi:10.1128/CMR.11.4.589
2. Liu YM, Li BB, Zhang YY, et al. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother. 2014;58(9):5379–5385. doi:10.1128/AAC.02523-14
3. Li J, Ren J, Wang W, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis. 2018;37(4):679–689. doi:10.1007/s10096-017-3160-z
4. Lee HC, Chuang YC, Yu WL, et al. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med. 2006;259(6):606–614. doi:10.1111/j.1365-2796.2006.01641.x
5. Cubero M, Grau I, Tubau F, et al. Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007–2013). Clin Microbiol Infect. 2016;22(2):154–160. doi:10.1016/j.cmi.2015.09.025
6. Choby JE, Howard-Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. J Intern Med. 2020;287(3):283–300. doi:10.1111/joim.13007
7. Decre D, Verdet C, Emirian A, et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol. 2011;49(8):3012–3014. doi:10.1128/JCM.00676-11
8. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. doi:10.1128/MMBR.00078-15
9. Cortes G, Borrell N, de Astorza B, Gomez C, Sauleda J, Alberti S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun. 2002;70(5):2583–2590. doi:10.1128/IAI.70.5.2583-2590.2002
10. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199(5):697–705. doi:10.1084/jem.20030857
11. Jun JB. Klebsiella pneumoniae liver abscess. Infect Chemother. 2018;50(3):210–218. doi:10.3947/ic.2018.50.3.210
12. Jung SW, Chae HJ, Park YJ, et al. Microbiological and clinical characteristics of bacteraemia caused by the hypermucoviscosity phenotype of Klebsiella pneumoniae in Korea. Epidemiol Infect. 2013;141(2):334–340. doi:10.1017/S0950268812000933
13. Wacharotayankun R, Arakawa Y, Ohta M, et al. Enhancement of extracapsular polysaccharide synthesis in Klebsiella pneumoniae by RmpA2, which shows homology to NtrC and FixJ. Infect Immun. 1993;61(8):3164–3174. doi:10.1128/iai.61.8.3164-3174.1993
14. Palacios M, Miner TA, Frederick DR, et al. Identification of two regulators of virulence that are conserved in Klebsiella pneumoniae classical and hypervirulent strains. Mbio. 2018;9(4). doi:10.1128/mBio.01443-18
15. Namikawa H, Oinuma KI, Sakiyama A, et al. Discovery of anti-mucoviscous activity of rifampicin and its potential as a candidate antivirulence agent against hypervirulent Klebsiella pneumoniae. Int J Antimicrob Agents. 2019;54(2):167–175. doi:10.1016/j.ijantimicag.2019.05.018
16. Lin TL, Yang FL, Yang AS, et al. Amino acid substitutions of MagA in Klebsiella pneumoniae affect the biosynthesis of the capsular polysaccharide. PLoS One. 2012;7(10):e46783. doi:10.1371/journal.pone.0046783
17. Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57(12):3778–3782. doi:10.1128/iai.57.12.3778-3782.1989
18. Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54(2):484–489. doi:10.1016/0003-2697(73)90377-1
19. Shang D, Liang H, Wei S, Yan X, Yang Q, Sun Y. Effects of antimicrobial peptide L-K6, a temporin-1CEb analog on oral pathogen growth, Streptococcus mutans biofilm formation, and anti-inflammatory activity. Appl Microbiol Biotechnol. 2014;98(20):8685–8695. doi:10.1007/s00253-014-5927-9
20. Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci U S A. 2006;103(51):19484–19489. doi:10.1073/pnas.0608949103
21. Brook I, Hausfeld JN. In vitro effects of penicillin and telithromycin on the expression of Streptococcus pneumoniae capsule. J Antimicrob Chemother. 2006;58(3):678–679. doi:10.1093/jac/dkl261
22. Nomura S, Murata K, Nagayama A. Effects of sub-minimal inhibitory concentrations of antimicrobial agents on the cell surface of Klebsiella pneumoniae and phagocytic killing activity. J Chemother. 1995;7(5):406–413. doi:10.1179/joc.1995.7.5.406
23. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913–1916. doi:10.1001/archinte.1986.00360220057011
24. Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26(6):1434–1438. doi:10.1086/516369
25. Siu LK, Fung CP, Chang FY, et al. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol. 2011;49(11):3761–3765. doi:10.1128/JCM.00977-11
26. Shu HY, Fung CP, Liu YM, et al. Genetic diversity of capsular polysaccharide biosynthesis in Klebsiella pneumoniae clinical isolates. Microbiology. 2009;155(Pt 12):4170–4183. doi:10.1099/mic.0.029017-0
27. Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol. 2010;192(12):3144–3158. doi:10.1128/JB.00031-10
28. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45(3):284–293. doi:10.1086/519262
29. Yeh KM, Lin JC, Yin FY, et al. Revisiting the importance of virulence determinant magA and its surrounding genes in Klebsiella pneumoniae causing pyogenic liver abscesses: exact role in serotype K1 capsule formation. J Infect Dis. 2010;201(8):1259–1267. doi:10.1086/606010
30. Russo TA, Olson R, Macdonald U, et al. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun. 2014;82(6):2356–2367. doi:10.1128/IAI.01667-13
31. Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56(9). doi:10.1128/JCM.00776-18
32. Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA, Camilli A. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun. 2015;83(8):3325–3333. doi:10.1128/IAI.00430-15
33. Russo TA, Gulick AM. Aerobactin synthesis proteins as antivirulence targets in hypervirulent Klebsiella pneumoniae. Acs Infect Dis. 2019;5(7):1052–1054. doi:10.1021/acsinfecdis.9b00117
34. Lam M, Wyres KL, Judd LM, et al. Tracking key virulence loci encoding aerobactin and salmochelin siderophore synthesis in Klebsiella pneumoniae. Genome Med. 2018;10(1):77. doi:10.1186/s13073-018-0587-5
35. Sahly H, Podschun R, Oelschlaeger TA, et al. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect Immun. 2000;68(12):6744–6749. doi:10.1128/IAI.68.12.6744-6749.2000
36. Lin JC, Chang FY, Fung CP, et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 2004;6(13):1191–1198. doi:10.1016/j.micinf.2004.06.003
37. Domenico P, Salo RJ, Cross AS, Cunha BA. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella pneumoniae. Infect Immun. 1994;62(10):4495–4499. doi:10.1128/iai.62.10.4495-4499.1994
38. Kobayashi SD, Porter AR, Freedman B, et al. Antibody-mediated killing of carbapenem-resistant ST258 Klebsiella pneumoniae by human neutrophils. Mbio. 2018;9(2). doi:10.1128/mBio.00297-18
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.