Back to Journals » Medical Devices: Evidence and Research » Volume 13
The AngelMed Guardian® System in the Detection of Coronary Artery Occlusion: Current Perspectives
Authors Kazmi SHA, Datta S , Chi G , Nafee T, Yee M, Kalia A , Sharfaei S , Shojaei F, Mirwais S, Gibson CM
Received 18 June 2019
Accepted for publication 13 December 2019
Published 7 January 2020 Volume 2020:13 Pages 1—12
DOI https://doi.org/10.2147/MDER.S219865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Syed Hassan Abbas Kazmi, Sudarshana Datta, Gerald Chi, Tarek Nafee, Megan Yee, Akshun Kalia, Sadaf Sharfaei, Fahimehalsadat Shojaei, Sabawoon Mirwais, C Michael Gibson
Division of Cardiovascular Medicine, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Correspondence: C Michael Gibson
PERFUSE Study Group, Division of Cardiovascular Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, 930 Commonwealth Ave. #3, Boston, MA 02215, USA
Tel +1-617-975-9950
Email [email protected]
Abstract: Total ischemic time, which specifies the time from the onset of chest pain to initiation of reperfusion during percutaneous coronary intervention, consists of two intervals: symptom to door time and door to balloon time. A door to balloon time of 90 mins or less has become a quality-of-care metric in the management of ST elevation myocardial infarction (STEMI). While national efforts made by the American College of Cardiology (ACC) and American Heart Association (AHA) have curtailed in-hospital door to balloon time over the years, a reduction in pre-hospital symptoms to door time presents a challenge in modern interventional Cardiology. Early and complete revascularization has been associated with improved clinical outcomes in MI and strategies that may help reduce symptom to door time, and thus the total ischemic time, are crucial. Rapidly evolving ST-segment changes commonly develop prior to ischemia-related symptom onset, and are detectable even in patients with clinically unrecognized silent MIs. Therefore, a highly intelligent ischemia detection system that alerts patients of ST segment deviation may allow for rapid identification of acute coronary occlusion. The AngelMed Guardian® System is a cardiac activity monitoring and alerting system designed for rapid identification of intracardiac ST-segment changes among patients at a high risk for recurrent ACS events. This article reviews the clinical studies evaluating the design, safety and efficacy of the AngelMed Guardian System and discusses the clinical implications of the device.
Keywords: acute coronary syndrome, myocardial infarction, ST elevation myocardial infarction, ischemia monitoring, electrocardiography
Introduction
Atherosclerotic cardiovascular disease (ASCVD) represents a common yet preventable condition and affects 18.2 millions of individuals aged ≥20 years in the United States.1 Approximately 70% of these people have never had a previous episode of myocardial infarction (MI), whereas about 30% are those who suffer from a recurrent MI. In addition, silent MI (i.e., MI that escapes clinical recognition) is more common than previously thought and accounts for ≥20% of MI cases.2,3 Such high numbers place MI among one of the leading causes of death in Western countries.
Over the past two decades, advances in coronary reperfusion strategy using angioplasty or thrombolytic therapy have dramatically transformed the management of acute MI. However, the rate of rapid reperfusion may affect both the short-term and long-term prognosis of patients with ST-elevation myocardial infarction (STEMI).4–6 Therefore, given the prognostic implications of the total ischemic time (i.e., a composite of symptom to door [S2D] and door to balloon [D2B] time), a delay of diagnosis tends to prevent the optimal derivation of benefit from these treatment modalities.
Studies have shown that the total ischemic time is an independent predictor for infarct size among patients with STEMI.7,8 The duration of myocardial ischemia secondary to coronary occlusion has a direct relation to irreversible cardiomyocyte injury.9 Specifically, an ischemic time of greater than 20 mins has been associated with cardiomyocyte death.10 Further, the majority of irreversible damage to the myocardium and lethal arrhythmia develop within the initial 60 mins following a coronary occlusion.4,5,11–16 These findings indicate the pivotal role of early detection and intervention in minimizing myocardial necrosis and preventing associated complications.
A key quality metric in the management of STEMI is to reduce ischemic time by reestablishing blood flow to jeopardized myocardium as early as possible. Based on the current practice guidelines, an occluded artery should be reperfused with fibrinolytic agent in 0.5 hr or revascularized with percutaneous coronary intervention (PCI) in 1.5 hr from patient’s arrival in the emergency department. Prior studies have shown that only 20% of patients arrive at the hospital in 60 mins after the onset of symptoms, which represents the timeframe that reperfusion therapy may derive the greatest benefit.17–19 Despite shortened door to needle and D2B time that have been translated into decreased mortality in STEMI,20–24 the S2D time remains at 2.7 hrs on average and can be much longer due to a number of sociodemographic, cognitive, behavioral, technological and illness factors.25 It has been shown that the relative risk of death at 1 year is increased by 7.5% with each 30 mins delay in reperfusion therapy. Consequently, early hospital arrival may be beneficial.26
Current Barriers to Reducing Symptom-to-Door Time
The current methodology employed for the diagnosis of ACS events relies on symptoms, electrocardiogram, and sensitive biochemical markers, with patients relying on symptoms alone for prompt presentation to a medical facility. Lack of improvement in symptom-to-door time may be attributed to patient misconceptions of heart attack symptoms,5 patient denial or anxiety, clinically unrecognized or misdiagnosed MI, and failure to detect electrocardiography (EKG) findings indicative of MI.27,28 It should be noted that unrecognized or misdiagnosed MI and failure to detect EKG findings indicative of MI would have to be in the outpatient setting to affect symptom-to-door time.
Of note, patient education to improve symptom recognition as well as to prevent delayed presentation to medical care did not significantly improve outcomes in acute myocardial infarction (AMI).29 Furthermore, a substantial proportion of individuals with MI do not have typical chest discomfort and therefore may not seek medical attention promptly.27,28,30 Silent MI is defined as myocardial ischemia (evidenced by ischemic ST-segment changes, reversible regional wall motion abnormality, or reversible myocardial perfusion defect) in the absence of chest pain or other symptoms associated with ischemia (termed “anginal equivalents”).31 It has also been shown that around 2–4% of the population has myocardial ischemia that is clinically silent but detectable with ambulatory monitoring or exercise treadmill.32 Silent MI is commonly noted in subjects who have ASCVD risk factors such as hypertension (prevalence 1.3–2.4% for men and 1.5–3.3% for women),33,34 a prior history of CVD, female gender, diabetes (prevalence 4–37%), and the elderly (prevalence 0.3–5.4%).28,35–39 These patients represent a major proportion of undertreated high-risk ACS population and have substantially greater all-cause death and cardiovascular death compared to symptomatic MI patients.40 Additionally, patients with chronic angina may not react to the onset of MI when they find it difficult to discern any change in symptoms from MI onset compared to their ongoing background level of chest discomfort and pain.
Another impediment to the implementation of timely treatment is mis- or under-diagnosis of acute thrombotic occlusion. Traditional 12-lead surface EKG has demonstrated limited sensitivity and specificity for detecting posterior or lateral MI. Thus, posterior MI causing isolated ST depression in anterior leads is often misinterpreted as anterior wall ischemia.41 The diagnosis of posterior wall MI is challenging due to inconsistent presentation on EKG and the relatively minor contribution to QRS complex in the anterior leads from the posterior myocardium.42 Therefore, physicians recognized a very small fraction of posterior infractions with an anterior segment depression due to a prolonged delay from the performance of EKG to PCI. Furthermore, a reduction in life expectancy and quality, higher hospitalization rates, and increased risk of sudden death were a result of the morbidity associated with delays in seeking treatment.43
The AngelMed Guardian® System: Device Design and Components
State-of-the-Art Alarm Design
The application of multimodal alarm systems, including vibrotactile, auditory, and visual alarm systems can be seen in daily practice within the healthcare system.44–50 The effectiveness and reliability of these clinical alarm systems are influenced by various human factors, such as sensory perception, cognitive capacity and behavioral processes.51,52 For example, the optimal use of auditory or visual alarms in patients can be limited by auditory or visual impairment.53 Similarly, alerting via vibration alone also presents problems in patients with decreased vibration sensitivity.45 It has been shown that vibrotactile alarms, in combination with visual and auditory alarms, are far more superior in alerting compared to monomodal alerting systems (vibrotactile, visual or auditory alarms alone).46 Thus, an intelligent clinical alarm system is one that is designed in a patient-centered manner, takes physiological variance into account and reflects trigger severity for adequate triage.54–56
The utilization of audio-visual alarms in patients suffering from cardiovascular diseases, such as those used in bedside monitors and implantable cardioverter-defibrillators, is widespread.57–60 Given the clinical significance of urgency associated with treatment of myocardial ischemia, and the unchanged symptom-to-door time over the years, the AngelMed Guardian System has been designed as a patient-focused, multimodal, highly sensitive alarm system that detects EKG changes in real-time for prompt presentation to a medical facility.47 Further, a more definitive pre-hospital diagnosis of an ischemic event through the Guardian System may result in the reduction of D2B times through effective triage decisions when the patient presents at the hospital door.61
Components of AngelMed Guardian System
The AngelMed Guardian System consists of an implantable medical device (IMD) and an external device (EXD). The size of the IMD is similar to that of a single-chamber pacemaker. The miniature IMD is subcutaneously placed in the upper-left region of the anterior chest, and senses myocardial electrical changes from a standard steroid-eluting lead that attaches to the right ventricular apex.62,63 The Guardian programmer is configured to communicate with the IMD and retrieve EKG data for analysis. The IMD continuously checks for ST-segment deviation and other electrocardiographic alterations, such as irregular heartbeats or rhythms. In case of an event, the IMD notifies the patient by sending vibrating alarms, whereas the EXD beeps and flashes red or yellow indicators.48 The device design and components of the AngelMed Guardian System can be seen in Figures 1 and 2.
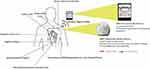 |
Figure 1 AngelMed Guardian® system device design and components. |
 |
Figure 2 Implantable medical device (IMD) and external medical device (EXD). |
Alarm Types That Reflect Event Mapping
The ST-segment denotes the horizontal, isoelectric section of the electrocardiogram between the S wave and the T wave that represents the interval between depolarization and repolarization of the ventricle. Every 90 s, the Guardian analyzes a 10-s intracardiac electrogram. The 24 hr average ST-segment level, average PQ segment level, R-wave height, and the RR interval (i.e. instantaneous heart rate) are calculated for each electrocardiogram sample. For every heartbeat waveform in the electrocardiogram sample, the ST segment deviation relative to the preceding PQ segment level is calculated and this difference is compared to corresponding difference in the patient’s 24 hr composite baseline to derive a “ST shift”. The percentage of ST shift (“ST-Shift%”) is normalized in reference to the amplitude of the R wave, and is then compared against the patient’s ST-shift detection threshold (i.e., 3 standard deviations from the patient’s baseline range, as determined by the Guardian programmer’s Autopick function and as calculated upon a prior 10–14-day sample of data). The Guardian System captures both positive and negative ischemia detection thresholds as ST-shift%. The intracardiac electrogram (ICEG) denoting the ST-shift% is obtained in a patient-specific manner based on comparison with a 24 hr baseline ST-shift at various heart rate ranges. To characterize whether a particular ST-segment shift is indicative of acute ischemia, the IMD utilizes these heart rate-dependent thresholds, with ST-segment depressions registered at higher heart rates. The IMD allows the detection thresholds for a positive ST shift% to be set at different levels than the thresholds for a negative ST shift%. This not only allows for lower false-positives but also differentiates ST-segment elevations from depressions. If abnormal rhythm or ST Shift% is noted, the interval between the sampling of electrograms shorten to once every 30 s. Events recorded by the Guardian System are mapped to different alarm types outlined in Table 1.
 |
Table 1 AngelMed Guardian® System Alarm Types |
Proof-of-Concept Study, CARDIOSAVER, DETECT and ALERTS Trials
Alterations in the ST-segments and T waves are the earliest electrocardiographic findings of myocardial ischemia. In STEMI patients, acute coronary artery occlusion is indicated by rapid and progressive alterations in the ST-segments.64 Human studies have shown that ST-segment deviation may develop within 15 s after obstructing a coronary vessel.15 This finding justifies the importance of real-time monitoring of ST-segment for detecting myocardial ischemia without relying solely on non-specific symptoms. Various clinical studies have attempted to devise patient alerting systems via continuous surface-based EKG monitoring for this purpose. The timeline for the clinical studies of the AngelMed Guardian System is outlined in Figure 3. These evidence generating studies for the AngelMed Guardian System are summarized in Table 2.
 |
Table 2 Evidence Generating Studies for the AngelMed Guardian® System |
 |
Figure 3 Timeline for the clinical studies of the AngelMed Guardian® system. |
Proof-of-Concept Study
During the early 2000’s, several human studies began to assess the ability of a temporary pacemaker lead to measure right ventricular (RV) apical voltage during coronary artery occlusion among individuals undergoing percutaneous transluminal coronary angioplasty. The investigators concluded that ST-segment changes arising as a result of coronary occlusion were magnified when recorded from an intracardiac RV apical electrogram as compared to when recorded from the skin surface.15
CARDIOSAVER (2005) and DETECT (2006) Studies
CARDIOSAVER was a Phase I clinical feasibility study conducted in Brazil. The primary objective of the CARDIOSAVER study was to test the performance of the Guardian system in detecting intracardiac ST shifts resulting from subendocardial and transmural ischemia. The study enrolled 20 subjects with an increased risk of a recurrent coronary occlusion and at least one of the following: (1) ischemia on an exercise stress test; (2) stenosis of a coronary artery on angiogram; or (3) clinical indication for stenting and/or angioplasty. Following the implantation of the device, subjects underwent a 3 mins long balloon occlusion of the target artery and intracardiac ST-segment changes were found to be significant in the case of vessel stenosis with no collateral flow. This finding provided evidence that the AngelMed Guardian System may potentially allow for early detection of myocardial ischemia and initiation of life-saving interventions.47,49
DETECT was another phase I study aimed to assess the safety profile of the Guardian system. The study also explored ST-segment shift thresholds for detecting myocardial ischemia. Overall, the CARDIOSAVER and DETECT studies enrolled 37 patients at risk of recurrent thrombosis who received Guardian System implantation. The median follow-up duration was 1.52 years. The results indicated that the Guardian System is a safe and feasible device for detecting ischemia and notifying patients. It is concluded that intracardiac ST shifts lasting more than 2 mins and exceeding three standard deviations from normal daily may be a threshold with acceptable sensitivity and specificity for identifying coronary thrombosis and occlusion secondary to ruptured atherosclerotic plaques. In contrast to the median delay of 2–3 hrs after the onset of symptoms, the time from alert by the Guardian System to hospital arrival was 19.5 mins.47,49
ALERTS Trial
The ALERTS trial (NCT: 00781118) was a Phase III prospective, randomized, multicenter trial that enrolled 1020 subjects at high-risk of MI due to acute coronary syndrome or bypass surgery. A total of 907 subjects received implantation of the Guardian System, and were randomized in a 1:1 ratio into Treatment (Alarms activated) and Control (Alarms deactivated-for first 6 months) groups. After the six-month randomization period, alarms were activated in the control arm and all subjects were followed until study termination (mean duration of 3.05 years). The primary safety endpoint of the ALERTS trial was to determine if the fraction of subjects free from system or device-related complications was greater than 90%. A total of 30 subjects developed 31 complications, resulting in a 96.7% complication-free rate, meeting the primary safety endpoint. A total of 20 participants had the device removed with eleven of those participants experiencing infection. Device-related complications of the AngelMed Guardian System in the ALERTS trial are outlined in Table 3.
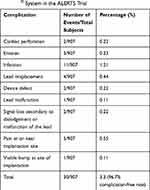 |
Table 3 Device-Related Complications of the AngelMed Guardian® System in the ALERTS Trial |
The primary efficacy endpoint was the composite of cardiac or unexplained death, new Q-wave MI, and time-to-door for a confirmed occlusive event at a medical facility >2 hrs. “Look-back” intervals among the control arm were used to determine subject arrival for an event that exceeded the 2 hr window. The AngelMed Guardian System did not significantly reduce the occurrence of the primary composite endpoint using a 7-day look-back window with 16 (3.8%) events occurring in the treatment group (pt) versus 21 (4.9%) events occurring in the control group (pc) (Posterior probability [Pr] = 0.786). A trend towards significant difference was seen using a 90-day look-back window (3.8% vs. 6.8%; Pr [pt < pc] = 0.974), which was the furthest look-back window used, since it was the longest period between scheduled check-up visits where new Q-waves could be detected. Using a pre-specified 7-day look back window, the alarms significantly reduced detection to arrival time at a medical facility (51 mins versus 30.6 hrs; Pr [pt < pc] >0.999). When a 90-day look back window was used, the control group median time-to-door arrival further increased to 22 days. In addition to this, use of a “dual baseline” (pre-implant EKG and EKG at randomization) for the Q-wave analysis demonstrated statistical significance for the reduction in primary composite endpoint.
Secondary endpoints comprises the individual components of the primary composite endpoint as well as the median time to ED arrival. Authors did not complete statistical analyses for cardiovascular death as only 4 occurred in the trial. Similar to the primary endpoint, new Q-wave MI was lower in the treatment group (10 [2.4%] vs. 14 [3.3%], which increased to 7 [1.7%] vs. 13 [3.0%] in an additional dual-baseline analysis), but this difference was not statistically significant. A statistically significant reduction in number of subjects with late arrival when using the 90-day look-back window was seen, with 4 (0.9%) in the treatment arm and 17 (3.8%) in the control arm. Additionally, while the endpoint was defined for late arrivals, rather than those <2 hrs, it should be noted that the arrival pattern for all confirmed events that occurred for the control and treatment groups were different with arrival ≤2 hrs for 85% of the events in the treatment arm compared to only 5% of the events in the control arm.
An expanded analysis that included all emergency department (ED) visits during the randomized period of the trial, and also those which occurred after the randomized portion of the trial, when all subjects had their alarms turned on, was also completed. The results of the expanded analyses revealed an 18.2% positive predictive value (PPV) for the ALARMS OFF (symptoms only) group versus a positive predictive value (PPV) of 25.8% for the ALARMS ON (alarm with or without symptoms) group, though this difference was not statistically significant. Interestingly, the ALARMS ON group had a false-positive rate of 0.164 per person-year which was statistically lower than the ALARMS OFF group, which had a false-positive rate of 0.678 per person-year. Further, the Guardian System detected 42 acute coronary events in asymptomatic subjects.
Another important aspect of care was the evaluation of the subject’s own perception of the disease in relation to mental and physical health status. To throw light on this, the ALERTS Quality of Life (AQOL) study, with the use of two established quality of life (QOL) instruments (EuroQOL EQ-5D and MacNew) and a custom-designed QOL survey, was designed to assess certain aspects of ALERTS subjects lives, such as anxiety and productivity. All the surveys demonstrated a significant improvement in subjects’ quality of life. After Guardian alerting was enabled, 70% of the subjects reported an improvement in the quality of life at 6 months. This included success in resuming work, normal day-to-day recreational activities and improvement in health issues.
Data collected via the ALERTS trial have provided evidence that the Guardian System has a superior accuracy in alerting for coronary occlusion and subsequent ischemia when compared to patient-perceived symptoms alone. An expanded statistical analysis performed on follow-up data of the post-randomization period demonstrated a reduction in false-positive rate (patient presentations without an occlusive event) when compared to patient-perceived symptoms alone. Moreover, it was also shown that the AngelMed Guardian System was able to identify asymptomatic coronary occlusion (silent MIs) and prompt the patient to seek medical attention.65
Future Perspectives
The AngelMed Guardian System has regulatory approval in Europe and Brazil and received approval by Food and Drug Administration for use in the United States. A post-approval study design has been accepted by the FDA which will investigate the diagnostic accuracy of the AngelMed Guardian System in a commercial environment, and to assess the patient and physician training programs. A minimum of 500 patients with a history of ACS who remain at high risk for recurrent occlusive events are to be included in a prospective, non-randomized, single-arm, event-based, multi-center trial, for the purpose of accruing 314 ACS events adjudicated as true positive or false positive. The co-primary endpoint will assess the non-inferiority of positive predictive value (PPV) and false-positive rate (FPR) of the AngelMed Guardian System relative to what was found in the ALERTS trial. Secondary endpoints consist of the frequency of occlusive coronary events detected only by the Guardian System (i.e., silent ACS events or silent MIs) and symptom-to-door times.
There remains an unmet clinical need to develop strategies that allow for swift and accurate detection of thrombotic coronary occlusion for optimal secondary prevention in a high-risk population. Ischemia-detecting algorithms have been incorporated and tested in newer generations of implantable cardioverter-defibrillators (ICDs) that are originally designed to prevent sudden cardiac death from ventricular arrhythmia. For instance, the prospective ESTIMATION trial demonstrated that the use of ICD with a continuous ST‐monitoring via intracardiac EKG was safe and effective for detecting asymptomatic myocardial ischemia, with a sensitivity, specificity, and negative predictive value of 75.0%, 72.5%, and 93.5% among participants who underwent myocardial perfusion imaging.66 The Guardian System is the first implanted device in ambulatory subjects with advanced multi-vessel cardiac disease to provide ST-segment shift alerting, thereby helping in the detection of a disease process with high morbidity and mortality, where earlier intervention can result in clinical benefit. The Guardian System alert has the capability to accurately recognize coronary occlusion as compared to patient-perceived symptoms alone. Results from randomized clinical trials have shown that even in the absence of symptoms, the Guardian System can precisely detect asymptomatic ACS events (silent MIs), thereby prompting the patients to seek medical attention. The ALERTS clinical trial has provided evidence for both safety and efficacy of this novel myocardial ischemia detection system. Moreover, there is an extensive list of benefits to patients receiving implantation of the Guardian System. While smartwatches and smartphone algorithms are showing great promise to identify cardiac arrhythmias, the reliable detection of coronary ischemia necessitates a highly intelligent alarm system superior in detecting silent MIs compared to patient recognition and extracardiac EKG detection tools.67–70 The AngelMed Guardian System, therefore, should serve as a valuable option in the cardiologists' arsenal for many years to come.
Abbreviations
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; EKG, electrocardiography; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; S2D, symptom to door time; D2B, door to balloon time.
Disclosure
Dr C. Michael Gibson reports grants, personal fees from Angel Medical, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Writing GM, Mozaffarian D, EJ B, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi:10.1161/CIR.0000000000000366
2. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603.
3. Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135:801–811. doi:10.7326/0003-4819-135-9-200111060-00010
4. Faxon D, Lenfant C. Timing is everything: motivating patients to call 9-1-1 at onset of acute myocardial infarction. Circulation. 2001;104:1210–1211. doi:10.1161/circ.104.11.1210
5. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225. doi:10.1161/01.CIR.0000121424.76486.20
6. De Luca G, Suryapranata H, Zijlstra F, et al. Symptom-onset-to-balloon time and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol. 2003;42:991–997. doi:10.1016/S0735-1097(03)00919-7
7. Hasche ET, Fernandes C, Freedman SB, Jeremy RW. Relation between ischemia time, infarct size, and left ventricular function in humans. Circulation. 1995;92:710–719. doi:10.1161/01.CIR.92.4.710
8. Liem AL, van ‘t Hof AW, Hoorntje JC, de Boer MJ, Suryapranata H, Zijlstra F. Influence of treatment delay on infarct size and clinical outcome in patients with acute myocardial infarction treated with primary angioplasty. J Am Coll Cardiol. 1998;32:629–633. doi:10.1016/S0735-1097(98)00280-0
9. Gersh BJ, Stone GW, White HD, Holmes DR
10. Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi:10.1161/01.CIR.56.5.786
11. Brown AL, Mann NC, Daya M, et al. Demographic, belief, and situational factors influencing the decision to utilize emergency medical services among chest pain patients. Rapid Early Action for Coronary Treatment (REACT) study. Circulation. 2000;102:173–178. doi:10.1161/01.CIR.102.2.173
12. Meischke H, Ho MT, Eisenberg MS, Schaeffer SM, Larsen MP. Reasons patients with chest pain delay or do not call 911. Ann Emerg Med. 1995;25:193–197. doi:10.1016/S0196-0644(95)70323-3
13. Chew DP, Moliterno DJ, Herrmann HC. Present and potential future paradigms for the treatment of ST-segment elevation acute myocardial infarction. J Invasive Cardiol. 2002;14(Suppl A):3A–20A.
14. Sekulic M, Hassunizadeh B, McGraw S, David S. Feasibility of early emergency room notification to improve door-to-balloon times for patients with acute ST segment elevation myocardial infarction. Catheter Cardiovasc Interv. 2005;66:316–319. doi:10.1002/(ISSN)1522-726X
15. Fischell TA, Fischell DR, Fischell RE, et al. Potential of an intracardiac electrogram for the rapid detection of coronary artery occlusion. Cardiovasc Revasc Med. 2005;6:14–20. doi:10.1016/j.carrev.2005.05.002
16. Varriale P, Niznik J. Unipolar ventricular electrogram in the diagnosis of right ventricular ischemic injury. Pacing Clin Electrophysiol. 1978;1:335–341. doi:10.1111/pace.1978.1.issue-3
17. Taylor DM, Garewal D, Carter M, Bailey M, Aggarwal A. Factors that impact upon the time to hospital presentation following the onset of chest pain. Emerg Med Australas. 2005;17:204–211. doi:10.1111/emm.2005.17.issue-3
18. Goldberg RJ, Steg PG, Sadiq I, et al. Extent of, and factors associated with, delay to hospital presentation in patients with acute coronary disease (the GRACE registry). Am J Cardiol. 2002;89:791–796. doi:10.1016/S0002-9149(02)02186-0
19. Crumlish CM, Bracken J, Hand MM, Keenan K, Ruggiero H, Simmons D. When time is muscle. Am J Nurs. 2000;100:26–33. doi:10.1097/00000446-200001000-00032.
20. McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–2186. doi:10.1016/j.jacc.2005.12.072
21. Wilson JH, Rath R, Glaser R, Panke T. Severe hemolysis after incomplete mitral valve repair. Ann Thorac Surg. 1990;50:136–137. doi:10.1016/0003-4975(90)90108-I
22. Gibson CM, Pride YB, Frederick PD, et al. Trends in reperfusion strategies, door-to-needle and door-to-balloon times, and in-hospital mortality among patients with ST-segment elevation myocardial infarction enrolled in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1035–1044. doi:10.1016/j.ahj.2008.07.029
23. Furman MI, Dauerman HL, Goldberg RJ, Yarzebski J, Lessard D, Gore JM. Twenty-two year (1975 to 1997) trends in the incidence, in-hospital and long-term case fatality rates from initial Q-wave and non-Q-wave myocardial infarction: a multi-hospital, community-wide perspective. J Am Coll Cardiol. 2001;37:1571–1580. doi:10.1016/S0735-1097(01)01203-7
24. Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–1900. doi:10.1001/jama.297.17.1892
25. DeVon HA, Hogan N, Ochs AL, Shapiro M. Time to treatment for acute coronary syndromes: the cost of indecision. J Cardiovasc Nurs. 2010;25:106–114. doi:10.1097/JCN.0b013e3181bb14a0
26. Gibson CM. Time is myocardium and time is outcomes. Circulation. 2001;104:2632–2634. doi:10.1161/circ.104.22.2632
27. Kannel WB. Silent myocardial ischemia and infarction: insights from the Framingham Study. Cardiol Clin. 1986;4:583–591. doi:10.1016/S0733-8651(18)30577-0
28. Davis TME, Fortun P, Mulder J, Davis WA, Bruce DG. Silent myocardial infarction and its prognosis in a community-based cohort of Type 2 diabetic patients: the Fremantle Diabetes Study. Diabetologia. 2004;47:395–399. doi:10.1007/s00125-004-1344-4
29. Luepker RV, Raczynski JM, Osganian S, et al. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: the Rapid Early Action for Coronary Treatment (REACT) Trial. JAMA. 2000;284:60–67. doi:10.1001/jama.284.1.60
30. Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283:3223–3229. doi:10.1001/jama.283.24.3223
31. Hollenberg NK. Controversies in cardiovascular care: silent myocardial ischemia. Complicat Card Patient. 1987;1:24–30.
32. Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi:10.21037/atm
33. Kannel WB, Dannenberg AL, Abbott RD. Unrecognized myocardial infarction and hypertension: the Framingham Study. Am Heart J. 1985;109:581–585. doi:10.1016/0002-8703(85)90566-6
34. Brennan P. Coronary heart disease in the medical research council trial of treatment of mild hypertension. Medical Research Council Working Party on Mild Hypertension. Br Heart J. 1988;59:364–378. doi:10.1136/hrt.59.3.364.
35. DeLuca AJ, Kaplan S, Aronow WS, et al. Comparison of prevalence of unrecognized myocardial infarction and of silent myocardial ischemia detected by a treadmill exercise sestamibi stress test in patients with versus without diabetes mellitus. Am J Cardiol. 2006;98:1045–1046. doi:10.1016/j.amjcard.2006.05.026
36. Niakan E, Harati Y, Rolak LA, Comstock JP, Rokey R. Silent myocardial infarction and diabetic cardiovascular autonomic neuropathy. Arch Intern Med. 1986;146:2229–2230. doi:10.1001/archinte.1986.00360230169023
37. Nesto RW, Watson FS, Kowalchuk GJ, et al. Silent myocardial ischemia and infarction in diabetics with peripheral vascular disease: assessment by dipyridamole thallium-201 scintigraphy. Am Heart J. 1990;120:1073–1077. doi:10.1016/0002-8703(90)90118-H
38. Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–1020. doi:10.1161/CIRCULATIONAHA.107.727826
39. Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Milan Study on Atherosclerosis and Diabetes (MiSAD) Group. Am J Cardiol. 1997;79:134–139. doi:10.1016/S0002-9149(96)00699-6.
40. Burgess DC, Hunt D, Li L, et al. Incidence and predictors of silent myocardial infarction in type 2 diabetes and the effect of fenofibrate: an analysis from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Eur Heart J. 2010;31:92–99. doi:10.1093/eurheartj/ehp377
41. Pride YB, Tung P, Mohanavelu S, et al. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression: a TRITON-TIMI 38 (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38) substudy. JACC Cardiovasc Interv. 2010;3:806–811. doi:10.1016/j.jcin.2010.05.012
42. Boden WE, Kleiger RE, Gibson RS, et al. Electrocardiographic evolution of posterior acute myocardial infarction: importance of early precordial ST-segment depression. Am J Cardiol. 1987;59:782–787. doi:10.1016/0002-9149(87)91091-5
43. McAlister FA, Quan H, Fong A, Jin Y, Cujec B, Johnson D. Effect of invasive coronary revascularization in acute myocardial infarction on subsequent death rate and frequency of chronic heart failure. Am J Cardiol. 2008;102:1–5. doi:10.1016/j.amjcard.2008.02.089
44. Kerr JH. Symposium on anaesthetic equipment. Warning devices. Br J Anaesth. 1985;57:696–708. doi:10.1093/bja/57.7.696
45. Block FE
46. Hedley-Whyte J Standardization of interface design for medical devices: international electrotechnical commission and international organization for standardization medical alarm systems.
47. Kerr JH, Hayes B. An “alarming” situation in the intensive therapy unit. Intensive Care Med. 1983;9:103–104. doi:10.1007/BF01772574
48. Morris RW, Montano SR. Response times to visual and auditory alarms during anaesthesia. Anaesth Intensive Care. 1996;24:682–684. doi:10.1177/0310057X9602400609
49. Edworthy J, Hellier E. Fewer but better auditory alarms will improve patient safety. BMJ Qual Saf. 2005;14:212–215. doi:10.1136/qshc.2004.013052
50. Edworthy J, Hellier E. Alarms and human behaviour: implications for medical alarms. Br J Anaesth. 2006;97:12–17. doi:10.1093/bja/ael114
51. Wogalter MS, Young SL. Behavioural compliance to voice and print warnings. Ergonomics. 1991;34:79–89. doi:10.1080/00140139108967290
52. Wogalter MS, Conzola VC, Smith-Jackson TL. Research-based guidelines for warning design and evaluation. Appl Ergon. 2002;33:219–230. doi:10.1016/S0003-6870(02)00009-1
53. Simons EC, Feigenblum DY, Nemirovsky D, Simons GR. Alert tones are frequently inaudible among patients with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol. 2009;32:1272–1275. doi:10.1111/pace.2009.32.issue-10
54. Zhang Y, Szolovits P. Patient-specific learning in real time for adaptive monitoring in critical care. J Biomed Inform. 2008;41:452–460. doi:10.1016/j.jbi.2008.03.011
55. Hay WW
56. Zhang Y. Real-time development of patient-specific alarm algorithms for critical care. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4351–4354. doi:10.1109/IEMBS.2007.4353300
57. Epstein AE, Abraham WT, Bianco NR, et al. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J Am Coll Cardiol. 2013;62:2000–2007. doi:10.1016/j.jacc.2013.05.086
58. Olgin JE, Pletcher MJ, Vittinghoff E, et al. Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med. 2018;379:1205–1215. doi:10.1056/NEJMoa1800781
59. Tachinardi U, de Sa Rebelo M, de Magalhaes Oliveira PP
60. DiMarco JP. Implantable cardioverter-defibrillators. N Engl J Med. 2003;349:1836–1847. doi:10.1056/NEJMra035432
61. Brodie BR, Gersh BJ, Stuckey T, et al. When is door-to-balloon time critical? Analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trials. J Am Coll Cardiol. 2010;56:407–413. doi:10.1016/j.jacc.2010.04.020
62. Crossley GH, Brinker JA, Reynolds D, et al. Steroid elution improves the stimulation threshold in an active-fixation atrial permanent pacing lead. A randomized, controlled study. Model 4068 Investigators. Circulation. 1995;92:2935–2939. doi:10.1161/01.CIR.92.10.2935
63. Mond HG, Stokes KB. The steroid-eluting electrode: a 10-year experience. Pacing Clin Electrophysiol. 1996;19:1016–1020. doi:10.1111/pace.1996.19.issue-7
64. Vogel B, Claessen BE, Arnold SV, et al. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5:39. doi:10.1038/s41572-019-0090-3
65. Gibson CM, Holmes D, Mikdadi G, et al. Implantable cardiac alert system for early recognition of ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2019;73:1919–1927. doi:10.1016/j.jacc.2019.01.014
66. Watanabe T, Hirooka K, Furukawa Y, et al. Continuous ST-monitoring function of implantable cardioverter defibrillator detects silent ischemia in patients with coronary artery disease. J Am Heart Assoc. 2018;7:e009332.
67. Tajrishi FZ, Chitsazan M, Chitsazan M, Shojaei F, Gunnam V, Chi G. Smartwatch for the detection of atrial fibrillation. Crit Pathw Cardiol. 2019;18:176–184. doi:10.1097/HPC.0000000000000192
68. Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136:1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
69. Chan PH, Wong CK, Pun L, et al. Head-to-head comparison of the AliveCor heart monitor and microlife WatchBP office AFIB for atrial fibrillation screening in a primary care setting. Circulation. 2017;135:110–112. doi:10.1161/CIRCULATIONAHA.116.024439
70. Reed MJ, Grubb NR, Lang CC, et al. Multi-centre randomised controlled trial of a smart phone-based event recorder alongside standard care versus standard care for patients presenting to the emergency department with palpitations and pre-syncope - the IPED (Investigation of Palpitations in the ED) study: study protocol for a randomised controlled trial. Trials. 2018;19:711.
71. Gibson CM, Guimarães V, John MS, et al. Magnitude and duration of intracardiac ST-segment changes for confirmed clinical events: first in-man experience. J Am Coll Cardiol. 2012;59:E414. doi:10.1016/S0735-1097(12)60415-X
72. Hopenfeld B, John MS, Fischell DR, Medeiros P, Guimarães HP, Piegas LS. The Guardian: an implantable system for chronic ambulatory monitoring of acute myocardial infarction. J Electrocardiol. 2009;42:481–486. doi:10.1016/j.jelectrocard.2009.06.017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
