Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 12
The AKR1D1*36 (rs1872930) Allelic Variant Is Independently Associated With Clopidogrel Treatment Outcome
Authors Kapedanovska-Nestorovska A , Dimovski AJ , Sterjev Z, Matevska Geskovska N , Suturkova L, Ugurov P, Mitrev Z, Rosalia R
Received 12 July 2019
Accepted for publication 23 September 2019
Published 21 October 2019 Volume 2019:12 Pages 287—295
DOI https://doi.org/10.2147/PGPM.S222212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Aleksandra Kapedanovska-Nestorovska,1 Aleksandar J Dimovski,1,2 Zoran Sterjev,1 Nadica Matevska Geskovska,1 Ljubica Suturkova,1 Petar Ugurov,3 Zan Mitrev,4 Rodney Rosalia5
1Center for Biomolecular and Pharmaceutical Analysis, Faculty of Pharmacy, University Ss Cyril and Methodius, Skopje, Republic of North Macedonia; 2Research Center for Genetic Engineering and Biotechnology “Georgi D.Efremov”, Macedonian Academy of Sciences and Arts, Skopje, Republic of North Macedonia; 3Semi Intensive Care Unit, Zan Mitrev Clinic, Skopje, Republic of North Macedonia; 4Department of Cardiovascular Surgery, Zan Mitrev Clinic, Skopje, Republic of North Macedonia; 5Department of Clinical Research, Zan Mitrev Clinic, Skopje, Republic of North Macedonia
Correspondence: Rodney Rosalia
Zan Mitrev Clinic, Bledski Dogovor #8, Skopje 1000, Republic of Macedonia
Tel +389 71305957
Email [email protected]
Aleksandra Kapedanovska-Nestorovska
Faculty of Pharmacy, University Ss Cyril and Methodius, Mother Theresa 47, Skopje 1000, Republic of Macedonia
Tel +389 72228998
Email [email protected]
Aims: The present observational cohort study evaluated the association between the AKR1D1*36 (rs1872930) allele and the risk of major adverse cardiovascular and cerebrovascular events (MACCE) in clopidogrel treated patients.
Methods: We screened 198 consecutive cardiovascular patients on clopidogrel therapy admitted in October to November 2010 with cardiovascular or cerebrovascular symptoms; of these 118 met the study protocol entry criteria; the median age of the cohort was 62.5 years (IQR 57–66 years), and 55% were females.
Results: The median follow up time was 38.5 (IQR 24–48) months; Kaplan-Meier/Log-rank analysis showed that patients carrying the AKR1D1*36 allelic variant have a shorter event-free-survival compared to wild type patients, hazard ratio = 2.193 (95% CI, 1.091 to 4.406); p = 0.0155. Multivariable Cox regression analysis confirmed the AKR1D1*36 allele as an independent risk factor (HR = 2.36; 95% CI, 1.34 to 4.18) and identified 3 other risk factors for MACCE; previous percutaneous interventions (PCI), HR = 2.78; (95% CI, 1.34 to 5.78), and a history of myocardial infarction, HR = 2.62; (95% CI, 1.48 to 4.64) at baseline and the previously reported CYP2C19*2 polymorphism (HR = 2.33; 95% CI, 1.33 to 4.06).
Conclusion: The AKR1D1*36 (rs1872930) variant is independently associated with a higher risk for MACCE and shorter event-free survival time.
Keywords: AKR1D1, CYP2C19, MACCE, DAPT, clopidogrel, North Macedonia
Introduction
Single antiplatelet therapy (SAPT) or Dual antiplatelet therapy (DAPT) comprising of a P2Y12 inhibitor and aspirin is commonly prescribed during the treatment of Coronary Artery Disease (CAD), Peripheral Artery Disease (PAD) and vertebrobasilar disease.
However, the relatively high incidence of Major Adverse Cardiovascular and Cerebrovascular Events (MACCE) observed in cardiovascular patients despite ongoing antiplatelet therapy has prompted several studies evaluating risk factors associated with DAPT/SAPT treatment failure.
MACCE is associated with a high morbidity rate and significant mortality; demographic, clinical, biochemical and genetic risk factors for MACCE have been previously reported. Risk factors such as old age, diabetes, reduced left ventricular ejection fraction and CYP2C19 gene polymorphisms are well-described.1–5
In particular, interindividual response variability to clopidogrel therapy6,7 and MACCE have been linked to the CYP2C19*2 and CYP2C19*3 loss-of-function (LOF) and the CYP2C19*17 gain-of-function (GOF) allelic variants. These polymorphisms lead to aberrant metabolism of clopidogrel, ineffective antiplatelet therapy and consequently an increased risk of adverse events8,9
Nevertheless, the known CYP2C19 polymorphisms fail to account for all observed interindividual differences and phenotypes related to clinical clopidogrel response; we hypothesise that other (genetic) risk factors are involved.
Recent studies point to a putative role of the AKR1D1 gene10–12 as a trans-genetic regulator of the Cytochrome P450 (CYP450) network. The AKR1D1*36 (rs1872930) allelic variation augmented hepatic CYP450 mRNA expression and increased CYP3A4, CYP2C8, CYP2C9, CYP2C19, and CYP2B6 enzyme activity. Hence, it is plausible that the AKR1D1*36 polymorphism contributes to observed MACCE during Clopidogrel therapy.
Several published reports describe risk factors for MACCE in cardiovascular patients of diverse demographics, racial and ethnic background; however, those studies rarely evaluated ethnicities indigenous to the South Eastern European Region.
To this end, this study evaluated candidate risk factors for MACCE in a cohort of patients on clopidogrel therapy from the Republic of North Macedonia. We performed an ambidirectional cohort study assessing clinical, demographic and genetic candidate risk factors, particularly CYP2C19*2, CYP2C19*17 and AKR1D1*36 polymorphisms.
Materials And Methods
Study Subjects And Design
In this single-centre study; we screened 198 consecutive urgent or emergent cardiovascular patients admitted at our clinic in the period between October and December 2010.
All patients enrolled had previously established cardiovascular, peripheral vascular or cerebrovascular disease that required antiplatelet therapy.
At the time of the patient enrollment and follow-up; Ticagrelor and Prasugrel were not routinely available in the country; hence, all patients indicated for antiplatelet therapy received clopidogrel.
The study complied with the 1964 Declaration of Helsinki and the protocol, #02-789/3, was approved by the local Ethical Committee of the University Ss Cyril and Methodius, Faculty of Pharmacy, Skopje, the Republic of North Macedonia. Individual Informed Consent was waived on the condition of full anonymity.
We retrospectively collected data from the medical histories going back five years (2005–2010). Patients were subsequently followed prospectively until October 2015. The primary composite endpoint was the first clinical sign of bleeding or MACCE: death, myocardial infarction, stent thrombosis, stroke, hospitalisation due to unstable angina, transient ischemic attack, cerebrovascular insult (CVI), and target vessel revascularisation. The primary endpoint of the trial is the time of the first occurrence of any element of the composite of CV death, nonfatal MI, or nonfatal stroke.
The Event-Free-Survival (EFS) was determined from the starting date of the clopidogrel therapy. Exclusion criteria were 1) incomplete medical history, 2) follow up information in a period shorter than 12 months after the initiation of Clopidogrel therapy, 3) prior history of bleeding (e.g. peptic ulcer, intracranial haemorrhage, 4) clinically significant platelet abnormality or Factor V Leiden thrombophilia. Moreover, we also excluded patients who reported 5) concomitant use of other drugs known to modify hepatic drug metabolism, 6) drug addiction and alcohol abuse. Patients whom 7) donated blood within the last two months before initialisation of clopidogrel-based antiplatelet therapy were also excluded. Finally, we omitted those patients who were on 8) hormone replacement therapy or using an intrauterine contraception device; consequently, we excluded 80 patients from the study. Thus, we performed the subgroup analysis according to the occurrence of MACCE during the follow-up period based on 118 out of 198 patients (60.6%) screened. The raw data set describing the demographic, clinical and genetic information is freely available via the Figshare repository.13
Genotyping
The genetic screening for AKR1D1 and CYP2C19 polymorphisms was performed at the Center for Biomolecular and Pharmaceutical Analysis, Faculty of Pharmacy, University Ss Cyril and Methodius, Skopje, the Republic of Macedonia.
EDTA-blood aliquots obtained from routine pre-procedural blood samples were obtained and stored at 4°C. The DNA extraction was performed within 24 h. The Qiamp DNA Blood kit (Qiagen GmbH, Hilden, Germany) was used according to the manufacturer’s protocol.
All variants are available on the NCBI dbSNP Entrez Repository. The genotyping of the CYP2C19 variant alleles *2 (rs424485, https://goo.gl/KxhjFh), *3 (rs4986893, https://goo.gl/guVt4c) and *17 (rs12248560, https://goo.gl/ZYMJrR) and AKR1D1*36 (rs1872930, https://goo.gl/1Y2TuA), was performed using TaqMan allelic discrimination assay (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions on a Real-Time PCR machine (Stratagene MxPro 3005P, Agilent Technologies, Edinburgh, UK).
Statistical Analysis
Statistical reporting was performed in compliance with the SAMPL guidelines.14
The Chi-square test was used to compare the observed vs expected genotype frequencies according to the Hardy-Weinberg equilibrium (HWE).
Continuous variables were evaluated using the D’Agostino–Pearson normality test – Parametric data were analysed using the Student`s t-test; the Mann–Whitney test was used for non-parametric data.
The Fisher exact test was applied to evaluate the association between categorical variables with the outcome: ethnicity, age, gender, Diabetes mellitus, prior Myocardial Infarction, previous Percutaneous Intervention (PCI) with or without stenting and previous other (non-) cardiac surgical interventions. The Chi-square test used to test for the distribution of categorical variables; A chi-square for trend analysis was done to evaluate whether carriers of both genetic variants are exposed to higher risks for MACCE compared to single carriers.
Time-to-event (MACCE) are expressed as Kaplan-Meier curves and compared using the log rank test between carriers and non-carriers of evaluated CYP2C19*2 and AKR1D1*36 allelic variants. All statistical analyses were two-tailed. Variables with significant Odds or Hazard ratio (p <0.05) for MACCE were subsequently evaluated in a Cox Proportional Hazards Model with MACCE as a stratification factor. The likelihood chi-square statistic and deviance are reported as a measure of model performance.
Statistical analyses were performed using Graphpad Prism, version 7.03 and Statsdirect, version 3.1.20.
Results
Demographic, Clinical And Genetic Characteristics Of The Study Cohort
We screened 198 patients; of these, 118 met the study inclusion criteria (Figure 1). The patient characteristics and diagnosis at admission are described in Table 1 and Supplementary Figure 1.
 |
Table 1 Demographic And Clinical Characteristics Of 118 Patients Monitored For The Long-Term Clinical Outcome During Continuous Clopidogrel Therapy |
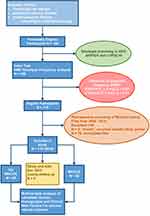 |
Figure 1 STROBE study flow diagram. Patient eligibility evaluation; genetic screening and comparison to Hardy-Weinberg Equilibrium and subsequent exclusion and inclusion based on medical history. |
We tested the outcome of the performed genotyping against the Hardy-Weinberg principle;15 the observed allele and genotype frequencies of AKR1D1*36 (Supplementary Table 1) and CYP2C19 polymorphisms, *2 and *17 (Supplementary Table 2) were in agreement with the expected frequencies according to the Hardy-Weinberg equilibrium. Of note, the prevalence of the AKR1D1*36 allelic variant (25.4%) in the study group was higher than the CYP2C19*2 allelic variant (19.1%), the CYP2C19*3 variant was not detected in the present cohort.
Finally, the AKR1D1*36 and CYP2C19*2 and *17 genotype distribution and allelic frequencies in our patient were comparable to previous observations in the healthy population (Supplementary Table 1 and 2).16,17
Carriers Of AKR1D1*36 And CYP2C19*2 Alleles Are Exposed To Higher Risks Of MACCE Despite Continuous Clopidogrel Therapy
The median follow-up was 38.5 (range 12–85) months with a total of 4608 patient-months.
The cohort was stratified in two sub-groups; patients, N = 60 (50.85%) who had an uneventful follow-up period and those who experienced a MACCE, N = 58 (49.15%) during their postprocedural course The distribution of the gender, ethnicity and the mean age for each sub-group were comparable (Table 1). There were no major bleeding events, but we observed a single mortality case (1/118, 0.85%) during the follow-up period.
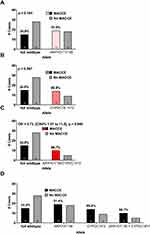
We next determined the incidence of MACCE in full wildtype (AKR1D1*1/*1 + CYP2C19*1/*1) patients in comparison to those who carry AKR1D1*36 and/or CYP2C19*2 polymorphisms (Figure 2). The two polymorphisms were associated for 43 out of 58 (74%) cases of MACCE. The incidence of MACCE in full wildtype patients was 34.88%, (15 out of 43).
Compared to full wildtype patients, we observed a non-significant trend for increased adverse events in patients who were single carriers of the AKR1D1*36 allele (19 out of 37, 51.4%) (Figure 2A) and in patients carrying only the CYP2C19*2 allele, 14 out of 23, 60.9% (Figure 2B).
MACCE was observed in 10 out of 15 (66.67%) for patients stratified as dual carriers of AKR1D1*36 and CYP2C19*2 alleles, Odds Ratio = 3.733 [CI95% 1.007 to 11.89], p = 0.0398 (Figure 2C). We performed a chi-square-for-trend analysis by ordering the variants according to their population frequency,18 suggests an additive effect of both variants on the risk of MACCE, X2 = 6.342, p =0.0118 (Figure 2D).
The study was underpowered to assess an association between clinical event and homozygosity; nonetheless, in homozygous carriers of CYP2C19*2 or AKR1D1*36 variants, the MACCE rate was 57.1% (4/7) and 37.5% (3/8), respectively. Furthermore, there were no cases of dual mutant homozygosity for both genetic polymorphisms (AKR1D1*36/*36 + CYP2C19*2/*2).
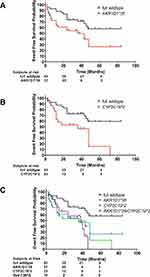
Kaplan-Meier analysis of MACCE suggests that the rs1872930 minor allelic variant is associated with a significantly shorter time-to-event period compared to full wildtype counterparts. For instance, the AKR1D1*36 allele carriers were exposed to an unadjusted Hazard Ratio of 2.193 [CI95% 1.091 to 4.406], p = 0.0155. (Figure 3A)
Nevertheless, the risks for MACCE is higher for CYP2C19*2 carriers, unadjusted Hazard ratio, = 2.628 [CI95% 1.147 to 6.02], p = 0.0064 (Figure 3B).
We next questioned whether dual carriers of the AKR1D1*36 and CYP2C19*2 polymorphisms experienced increased risks for MACCE compared to single carriers. However, our results do not support a combined worse clinical outcome for the presence of both polymorphisms; dual carriers were not exposed to increased risks for MACCE; AKR1D1*36/CYP2C19*2 vs AKR1D1*36, p=0.7047 and AKR1D1*36/CYP2C19*2 vs CYP2C19*2, p = 0.5924 (Figure 3C)
In contrary to full wildtype patients (AKR1D1*1/CYP2C19*1), dual carriers (AKR1D1*36/CYP2C19*2) are exposed to significantly higher risks for MACCE, unadjusted Hazard Ratio = 2.909 [CI95% 1.063 to 7.96], p = 0.0048. (Figure 3C).
We compared the EFS for carriers of the CYP2C19*17 allele with wildtype patients and observed a comparable postprocedural follow-up course in the absence of any adverse bleeding events between the two groups, unadjusted Hazard Ratio = 1.137 [CI95% 0.3789 to 3.411], p = 0.8099.
The Combined Impact Of Various Clinical Risk Factors On The Clopidogrel Therapy Treatment Outcome
We screened for additional candidate clinical and demographic predictors for MACCE (Table 1) and observed a significant increase in risk for patients with a previous history of Myocardial Infarction, those that had undergone PCI or invasive Cardiac surgery in the past with Odds Ratios of 2.629 [1.138 to 5.759], p = 0.026; 6.042 [1.504 to 28.28], p = 0.015 and 5.108 [1.0873 to 13.62], p = 0.0005, respectively.
The risk factors, detected with univariate analysis, were combined with the genetic factors in a Cox Hazards Model of Proportional risks.
The Cox Hazards Model of Proportional risks identified 4 independent risk factors (Table 2) showing a significant influence on the event-free-survival, MACCE: Previous PCI, adjusted Hazard Ratios (aHR) = 2.78; [95% CI, 1.34 to 5.78], a history of Myocardial Infection, aHR = 2.62; [95% CI, 1.48 to 4.64] at baseline followed by the genetic factors AKR1D1*36, aHR = 2.36; [95% CI, 1.34 to 4.18] and CYP2C19*2 (aHR = 2.33; 95% CI, 1.33 to 4.06).
 |
Table 2 Cox Proportional Hazards Model |
In summary, the incidence of MACCE in clopidogrel-treated patients is associated with the presence of the AKR1D1*36 and CYP2C19*2 polymorphisms, a history of Myocardial Infarction and whether the patients previously underwent percutaneous interventions.
Discussion
The present study evaluated the association between AKR1D1*36 and CYP2C19*2 polymorphisms with adverse clinical outcome during continuous Clopidogrel DAPT in a cohort of patients. Therefore, we assessed the incidence of MACCE accounting for demographic, clinical and genetic candidate risk factors.
Individual drug response variability is well-recognised; its causes are likely to be multifactorial, the patient’s age, sex, weight, health status genetics, nutrition, infections, concomitant medications and clinical history, all play an essential role.19
DAPT based on aspirin and Clopidogrel, a P2Y12 receptor antagonist, forms the cornerstone of the pharmacologic treatment of patients undergoing PCI or Coronary Artery Bypass Grafting (CABG).7
However, the negative consequences of the CYP2C19 polymorphisms in post-procedural outcome are well-acknowledged; it is considered the primary genetic risk factor for ineffective DAPT for patients receiving Clopidogrel.20–24
The CYP2C19*2 LOF polymorphism is the most common reported variant among others LOF alleles; *3, *4, *5, *6, *7, and *8 CYP2C19 variants. Nevertheless, according to the literature data, the CYP2C19*2 genetic variant explains 12% of the variability in the therapeutic effect of Clopidogrel.25 Given the significantly lower prevalence of the other alleles, the near-complete absence of the CYP2C19*3 allele in the population form Republic of North Macedonia,16 it is unlikely that the residual LOF variants could explain the remaining 78% of the variability in Clopidogrel response.
Another possible candidate is the CYP2C19*17 gain-of-function (GOF) allele that has been linked to adverse events, notably, excessive bleeding.26–28 However, our results do not point to an association between the CYP2C19*17 variant and adverse (bleeding) events.
Collectively, other demographic, clinical and perhaps novel genetic risk factors might be implicated in the occurrence of adverse cardiac events in our patient cohort despite continuous DAPT. Indeed, several other risk factors for MACCE have been described.1–5
In light of the recent work by Chaundry et al, and Chen et al,10–12 our results point to a novel role for the AKR1D1*36 (rs1872930) GOF allelic variant in the metabolism of Clopidogrel. The CYP2C19 wildtype patients who carry the AKR1D1*36 GOF allelic variant are exposed to an increased risk for MACCE.
Thus, our data suggest that the effectiveness of Clopidogrel-based DAPT is substantially affected by the transcriptional control CYP2C19 by the AKR1D1 gene.
Alternatively, the polymorphic AKR1D1 enzyme might modulate Clopidogrel metabolic biotransformation. The AKR1D1 enzyme is involved in the metabolic reduction of various drugs;10–12 however, we are unaware of any published data showing a direct enzymatic activity of AKR1D1 in Clopidogrel metabolism.
Consequently, we speculate that the adverse clinical outcomes associated with the AKR1D1*36 genetic variant relate to AKR1D1’s function as a trans-regulator of the co-expression network and the activity of the CYP450 genes; among others, the CYP2C19 gene.10
Interestingly, the AKR1D1*36 variant has a higher prevalence compared to the CYP2C19*2 variant. Similar frequencies were previously observed in a healthy cohort.17 Those results are not unique to ethnicities in the South-Eastern European region; the AKR1D1*36 variant is more frequently detected than the CYP2C19*2 variant, 28% vs 22%, within the global population.18
A secondary finding of this study was the affirmation of two clinical variables, a history of MI and previous PCI, as independent predictors of MACCE. Past MI is a known risk factor for future adverse cardiac events, especially in the first 12 months.29 In the patient sub-group with a previous history of MI; several cases of MACCE occurred after the first year on DAPT. Therefore, we support the proposals by Tangri et al, to improve monitoring and to consider prolonging DAPT beyond the first year after MI.29
The association of MACCE and PCI observed here aligns with the results by Mahmoud et al,.30 Considering the reported increased incidence of MACCE after non-cardiac surgery, PCI may be a general risk factor for future adverse cardiac events.
In regards to the research methodology, our study has a complete follow-up rate; the analyses are based on a mixed ethnic population, characteristic of the Southern Balkan peninsula, with genotype distributions that follow the Hardy-Weinberg Equilibrium. Collectively, these observations strengthen our study results.
Nevertheless, our study has several weaknesses - it is a single centre study; furthermore, the cohort size was underpowered to perform sub-group analysis based on homozygosity vs heterozygosity for the respective AKR1D1 and CYP2C19 polymorphisms. Also, the study results might have benefitted from the collection and inclusion of additional clinical, demographic and genetic factors in the Cox Proportional Hazards Model.
However, model analyses suggest that our current methodology is reliable; we present for the first time an overview of possible risk factors for MACCE in an ethnically unique cohort of patients receiving antiplatelet therapy.
In conclusion, the AKR1D1*36 (rs1872930) polymorphism is independently associated with MACCE despite continuous clopidogrel-based dual antiplatelet therapy.
Acknowledgments
The authors are grateful to Dr T Van Der Straaten for critically reviewing earlier versions of the manuscript. An abstract of this paper with interim findings was accepted as a poster presentation at the ESC Conference, 2019; FP Number: P6412.
Disclosure
Dr Zan Mitrev is the hospital director at the Zan Mitrev Clinic. The authors report no other conflicts of interest in this work.
References
1. Lenzi J, Fantini MP, Avaldi VM, et al. [Characteristics and outcomes of acute coronary syndrome in Italian-born patients and immigrants: a population-based observational study using health administrative data of the Emilia-Romagna Region]. G Ital Cardiol (Rome). 2017;18(9):650–659. doi:10.1714/2741.27948
2. Maupas E, Lipiecki J, Levy R, et al. Safety and efficacy outcomes of 3rd generation DES in an all-comer population of patients undergoing PCI: 12-month and 24-month results of the e-Biomatrix French registry. Catheter Cardiovasc Interv. 2017;90(6):890–897. doi:10.1002/ccd.27081
3. Obeid S, Yousif N, Schelldorfer A, et al. Short-term outcome after left main interventions in patients presenting with acute coronary syndrome. J Invasive Cardiol. 2018;30(3):98–104.
4. Stahli BE, Wischnewsky MB, Jakob P, et al. Predictive value of the age, creatinine, and ejection fraction (ACEF) score in patients with acute coronary syndromes. Int J Cardiol. 2018;270:7–13. doi:10.1016/j.ijcard.2018.05.134
5. Xia J, Xu J, Li B, et al. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta. 2017;466:162–166. doi:10.1016/j.cca.2017.01.022
6. Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019. doi:10.1056/NEJMoa1907096
7. Rosano GMC, Seferovic P. 2017 ESC guidelines focus on dual antiplatelet therapy. Eur Heart J Cardiovasc Pharmacother. 2018;4(3):131–132. doi:10.1093/ehjcvp/pvy007
8. Erlinge D, James S, Duvvuru S, et al. Clopidogrel metaboliser status based on point-of-care CYP2C19 genetic testing in patients with coronary artery disease. Thromb Haemost. 2014;111(5):943–950. doi:10.1160/TH13-09-0767
9. Cresci S, Depta JP, Lenzini PA, et al. Cytochrome p450 gene variants, race, and mortality among clopidogrel-treated patients after acute myocardial infarction. Circ Cardiovasc Gene. 2014;7(3):277–286. doi:10.1161/CIRCGENETICS.113.000303
10. Chaudhry AS, Thirumaran RK, Yasuda K, et al. Genetic variation in aldo-keto reductase 1D1 (AKR1D1) affects the expression and activity of multiple cytochrome P450s. Drug Metab Dispos. 2013;41(8):1538–1547. doi:10.1124/dmd.113.051672
11. Tracy TS, Chaudhry AS, Prasad B, et al. Interindividual variability in cytochrome P450-mediated drug metabolism. Drug Metab Dispos. 2016;44(3):343–351. doi:10.1124/dmd.115.067900
12. Chen M, Penning TM. 5beta-Reduced steroids and human Delta(4)-3-ketosteroid 5beta-reductase (AKR1D1). Steroids. 2014;83:17–26. doi:10.1016/j.steroids.2014.01.013
13. The AKR1D1*36 (rs1872930) allelic variant is independently associated with Clopidogrel treatment outcome. DOI:10.6084/m9.figshare.7232978.v2. 2019. https://figshare.com/articles/The_AKR1D1_36_rs1872930_allelic_variant_is_independently_associated_with_Clopidogrel_treatment_outcome_in_Acute_Coronary_Syndrome_patients/7232978.
14. Lang TA, Altman DG. Basic statistical reporting for articles published in biomedical journals: the “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int J Nurs Stud. 2015;52(1):5–9. doi:10.1016/j.ijnurstu.2014.09.006
15. Stark AE. Estimation of divergence from Hardy-Weinberg form. Twin Res Hum Genet. 2015;18(4):399–405. doi:10.1017/thg.2015.41
16. Jakovski K, Nestorovska AK, Labacevski N, Dimovski AJ. Characterization of the most common CYP2C9 and CYP2C19 allelic variants in the population from the Republic of Macedonia. Pharmazie. 2013;68(11):893–898.
17. Kapedanovska Nestorovska A, Jakovski K, Naumovska Z, et al. Distribution of the most common genetic variants associated with a variable drug response in the population of the Republic of Macedonia. Balkan J Med Genet. 2014;17(2):5–14. doi:10.2478/bjmg-2014-0069
18. Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi:10.1093/nar/29.1.308
19. Mathur S, Sutton J. Personalized medicine could transform healthcare. Biomed Rep. 2017;7(1):3–5. doi:10.3892/br.2017.922
20. Gladding P, Webster M, Zeng I, et al. The pharmacogenetics and pharmacodynamics of clopidogrel response: an analysis from the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc Interv. 2008;1(6):620–627. doi:10.1016/j.jcin.2008.09.008
21. Siller-Matula JM, Trenk D, Schror K, et al. Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6(11):1111–1128. doi:10.1016/j.jcin.2013.06.011
22. Yang Y, Lewis JP, Hulot JS, Scott SA. The pharmacogenetic control of antiplatelet response: candidate genes and CYP2C19. Expert Opin Drug Metab Toxicol. 2015;11(10):1599–1617. doi:10.1517/17425255.2015.1068757
23. Cavallari LH, Lee CR, Beitelshees AL, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(2):181–191. doi:10.1016/j.jcin.2017.07.022
24. Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360(4):363–375. doi:10.1056/NEJMoa0808227
25. Anderson CD, Biffi A, Greenberg SM, Rosand J. Personalized approaches to clopidogrel therapy: are we there yet? Stroke. 2010;41(12):2997–3002. doi:10.1161/STROKEAHA.110.594069
26. Harmsze AM, van Werkum JW, Hackeng CM, et al. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet Genomics. 2012;22(3):169–175. doi:10.1097/FPC.0b013e32834ff6e3
27. Huang B, Cui DJ, Ren Y, Han B, Yang DP, Zhao X. Effect of cytochrome P450 2C19*17 allelic variant on cardiovascular and cerebrovascular outcomes in clopidogrel-treated patients: A systematic review and meta-analysis. J Res Med Sci. 2017;22:109. doi:10.4103/jrms.JRMS_976_16
28. Li Y, Tang HL, Hu YF, Xie HG. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10(2):199–206. doi:10.1111/j.1538-7836.2011.04570.x
29. Tangri N, Ferguson TW, Whitlock RH, et al. Long term health outcomes in patients with a history of myocardial infarction: A population based cohort study. PLoS One. 2017;12(7):e0180010. doi:10.1371/journal.pone.0180010
30. Mahmoud KD, Sanon S, Habermann EB, et al. Perioperative cardiovascular risk of prior coronary stent implantation among patients undergoing noncardiac surgery. J Am Coll Cardiol. 2016;67(9):1038–1049. doi:10.1016/j.jacc.2015.11.063
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.