Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
The acceptability of nanocarriers for drug delivery in different contexts of use: perceptions of researchers and research trainees in the field of new technologies
Authors Chenel V, Boissy P, Poirier M, Cloarec J, Patenaude J
Received 6 December 2014
Accepted for publication 22 January 2015
Published 16 March 2015 Volume 2015:10(1) Pages 2125—2139
DOI https://doi.org/10.2147/IJN.S78799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Vanessa Chenel,1–4 Patrick Boissy,1–3 Marie-Sol Poirier,1–3 Jean-Pierre Cloarec,3,4 Johane Patenaude1–3
1Interdisciplinary Institute for Technological Innovation (3IT), 2Department of Surgery, Faculty of Medicine and Health Sciences, Université de Sherbrooke, Sherbrooke, QC, Canada; 3Laboratoire Nanotechnologies et Nanosystèmes (LN2), Centre National de la Recherche Scientifique (CNRS), Université de Sherbrooke, Canada; 4Université de Lyon, Institut des Nanotechnologies de Lyon (INL), site École Centrale de Lyon, France
Background: Despite marked optimism in the field of nanomedicine about the use of drug-delivery nanocarriers, uncertainties exist concerning nanocarriers’ possible unintended impacts and effects. These uncertainties could affect user acceptance and acceptability. “Acceptance” refers to the intention to put a technology or a device to a specified use. “Acceptability” refers to a value judgment that accounts for acceptance. The objectives of this study were to characterize impact perception, acceptance, and acceptability in relation to drug-delivery nanocarriers in different contexts of use, and to explore relationships among these concepts.
Methods: A sample of European and Canadian researchers and graduate research trainees active in the field of new technologies was recruited by targeted email invitation for participation in a web-based questionnaire study. The questionnaire presented scenarios for two contexts of use (lung cancer, seasonal flu) of drug-delivery nanocarriers with two compositions (carbon, synthetic DNA). Respondents’ impact perception, acceptance, and acceptability judgment in relation to each kind of nanocarrier in each context of use were measured with Likert scale questions and scored using categorical values.
Results: Two hundred and fourteen researchers and graduate research trainees completed the questionnaire. The results showed that nanocarrier composition influenced impact perception: as compared with the carbon nanocarrier impact perception, the positive impacts of the synthetic DNA nanocarrier were perceived as more significant and more likely to occur than its negative impacts. Composition did not influence acceptance or acceptability. Context of use significantly influenced acceptance and acceptability of both kinds of nanocarriers: researchers were more likely to accept the use of nanocarriers to treat lung cancer than the seasonal flu. The results also showed a significant relationship between acceptance and the perceived usefulness of the treatments.
Conclusion: Nanocarrier composition does not appear to influence acceptance or acceptability. On the other hand, the nanocarriers’ perceived usefulness and context of use are both major factors in accounting for acceptance and acceptability.
Keywords: acceptance, impact perception, nanomedicine, researchers’ perceptions, ELSI, E3LS
Introduction
Recent advances in nanoscience and nanotechnology offer opportunities for the development of innovative and promising applications in fields ranging from electronics to the food industry and medicine. Expectations for nanomedicine applications are high, and research targeting numerous applications for nanocarriers, such as medical imaging and diagnostics, tissue engineering, and targeted drug delivery, is well under way.1 As of 2006, most research activity and marketing in nanomedicine were related to targeted drug delivery using nanocarriers, with 18 US Food and Drug Administration-approved products and 88 new products at clinical-study stage.2 Although advances in the development of nanocarriers encourage high hopes,3 uncertainty surrounds nanocarriers’ use. Toxicity and long-term adverse effects are causes for concern among scientists, clinicians, and stakeholders.4 Nanocarriers’ potential use also raises ethical, environmental, economic, legal, and social (E3LS) issues that are often overlooked.5
Failure to consider E3LS issues can compromise the acceptance and acceptability encountered by new technologies. For example, in Europe and North America, the rejection of genetically modified (GM) foods and past and current public sentiment against transgenesis clearly indicate the existence of public apprehensions that had been hard to anticipate.6 Rejections like these reflect the inadequacies of traditional methods for risk assessment and for research on the public’s acceptance and perceptions in relation to emerging technologies. Anchored in a behaviorist paradigm targeting qualitative factors such as voluntariness of exposure and controllability in predicting and explaining the use of a technology,7–9 these approaches have typically used perceived risk – where risk refers to the danger of death or injury – and acceptance as the main outcomes of analysis. To take the example of GM foods, while deemed safe by governing bodies, they have been perceived as risky by the general public, a situation that has generated social tension.10 But in addition to harboring toxicological and environmental concerns about genetically modified organisms (GMOs), members of the public have also raised more metaphysical questions: the need for GMOs’ creation, the legitimacy of their creation, and the nature of trends implicit in technological development – all considerations that exceed the limitations of traditional approaches to assessment.11 In this light, it has become clear that there is a need to develop a fresh approach to impact perception, acceptance, and acceptability in relation to new technologies.
Building on the case of GM foods, what can we expect when it comes to nanomedicine applications such as the use of drug-delivery nanocarriers? Empirical studies have been published on the public’s impact perception, acceptance, and acceptability judgments regarding nanotechnologies.12–16 One finding emerging from research with respondents having little familiarity with these technologies is that the attitude of uninformed members of the public is significantly influenced by their affective and emotional responses.16 Existing associations with more familiar technologies, functioning as cognitive shortcuts and often fed by information conveyed by the mass media, can also influence perceptions of nanotechnologies’ risks and benefits.12 Individual values influence the perception of nanotechnologies more heavily when respondents are more familiar with the technologies.16 Generally, members of the public display a high degree of enthusiasm about nanotechnologies’ positive impacts and a low degree of concern about negative impacts,15 which they deem less prevalent than the benefits; thus, the public is more hopeful than anxious on this subject.14 It is important, however, to nuance this generalization with a cultural perspective: the American public tends to be enthusiastic about nanotechnologies, while the European community tends to adopt a more pragmatic attitude.13 Few studies, however, have examined responses to nanomedicine applications as part of health care delivery.17–19 A study on perceptions of nanotechnologies and nanomedicine as displayed in social media revealed that nanomedicine is expected by most users to have an economic impact and that nearly 50% of users consider theranostics to be the optimal nanomedicine application.19 It has also been found that a medication’s frequency of administration can have a greater impact on attitude toward a treatment than whether a treatment is of a nano-nature.17 At the same time, it has been shown that when four scenarios were presented, the scenario involving conventional treatment repeatedly administered and the scenario involving a nanotreatment administered just once were more negatively perceived. That is, the adverse effects of conventional treatment are deemed to be important in long-term use, whereas the positive effects of a nanotreatment are deemed to override adverse effects in long-term use. As with nanotechnologies in general, nanomedicine technologies appear to be associated with public optimism tinged with concerns relating not just to health but also to impacts on the environment and on social considerations.18 A big picture has emerged for impact perception and acceptance in relation to nanomedicine, but the lack of data on the acceptability of specific applications raises important questions about developments in this field. Will these technologies and their applications conform to individual and societal values?3 How will nanomedicine applications be accepted by the general public, and will patients understand the issues surrounding nanomedicine?20 An understanding of lay opinion is essential to ensure the success of any new technology.21
Furthermore, advocacy for or against the use of nanotechnologies often comes from researchers involved in the development of new technologies. The process of conveying scientific knowledge and opinion to the public is complex.22 Across numerous fields of study, these opinions can shape lay perception.23–25 The relationship between public and expert opinion makes it important to investigate researchers’ perceptions and acceptability judgments in the field of new technologies. Work on experts’ views about societal responses, risk perception, and acceptance of nanotechnologies26–29 reveals diverse points of view on the risks and benefits of the outcomes of research in nanotechnology. Confidence in government agencies also seems to be a significant predictor of risk perceptions about nanotechnology applications.27 While the environment and health concerns are the issues most often raised as potentially susceptible to the greatest negative impact and as requiring more stringent government regulation, researchers in nanotechnology are also currently raising concerns about social risks.28 The nature of applications also appears to influence researchers’ expectations around impact perceptions and social acceptance.26 Nevertheless, major variations in assessment frameworks and risk perceptions have been reported among researchers from different settings, the likelihood being that researchers are influenced by differing disciplinary backgrounds29 and diverse epistemologies.30 Some work done on nanomedicine as seen through the prism of experts’ perceptions of ethical issues31 suggests that nanomedicine practitioners are interested in reflecting on ethical issues in their work but overall do not consider ethical issues associated with nanomedicine to be new issues.31 No studies so far have directly reported on researchers’ perceptions, acceptance, and acceptability judgments in relation to specific applications in nanomedicine. The objectives of the study reported on here were to examine, among researchers active in the field of new technologies, impact perception, acceptance, and acceptability judgments regarding two kinds of drug-delivery nanocarriers (one made of carbon, the other of synthetic DNA) in two different contexts of use (treatment of lung cancer, treatment of seasonal flu), and to explore the relationships between impact perception, acceptance, and acceptability judgments.
Materials and methods
Study design
Given the emergent nature of the area of research and the research questions to be answered, an exploratory design with sequential data triangulation was preferred.32 A mixed-methods approach (QUANTITATIVE → qualitative) allowed for the initial collection of quantitative data from responses to a web-based questionnaire with the aim of studying the following: impact perception, acceptance, and acceptability judgments; the relationships among these variables; and the influence of respondents’ profiles on the variables. Then, to complement the information regarding the initial research objectives and deepen the exploration of the concept of acceptability, preliminary conclusions about the quantitative data were used in developing an interview guide focused mainly on acceptability. In this paper, only the project’s quantitative phase will be addressed.
Conceptual framework and variables
Impact perception, acceptance, and acceptability are key concepts in the study of new technologies and their applications, for example, targeted drug delivery in nanomedicine. In our study, these concepts were operationalized from an applied ethics perspective and within a conceptual framework developed by an interdisciplinary research group (InterNE3LS),11 took the form of a web-based questionnaire on two scenarios for the use of nanocarriers. Briefly, the framework defines impact perception (where an impact is defined as a positive or negative consequence resulting from the development or use of a new technology), acceptance, and acceptability (Table 1 provides the conceptual definitions). The framework is based on the premise that a decision about acceptance and acceptability rests not only on risks to be avoided but also on a weighting of the negative and positive impacts for certain social issues. Acceptance and acceptability can be weighted from an individual perspective (personal intention of use and individual justification) or a social perspective (level of development of a technology desirable for society, justification).
  | Table 1 Definition of main concepts, related variables, abbreviations, and operationalization |
Questionnaire based on conceptual framework
The web-based questionnaire was introduced by a short video describing nanotechnologies, nanomedicine, and targeted drug-delivery treatments. An informational section also presented two kinds of drug-delivery nanocarriers (one composed of carbon, the other of synthetic DNA) in two different contexts of use (lung cancer treatment, seasonal flu treatment). The questionnaire proposed closed-ended questions based on the operationalization of the core concepts for each scenario presented (questionnaire is given in Figure S1). Questions about impact perception and acceptability were based on a list of positive and negative impacts of the treatments, presented as better chances of a cure and fewer adverse effects, cell toxicity, greater environmental friendliness, environmental toxicity, decreased costs to the health care system in the long run, and unequal patient access to treatments (Table 2 provides a complete description). Data reduction of responses to these questions yielded scores on five variables: perception index (PI), individual acceptability index (IndAI), social acceptability index (SocAI), individual issue of preponderant concern (IndPIssue), and social issue of preponderant concern (SocPIssue). The section on acceptability included a subquestion about the treatments’ individual and social perceived usefulness (Useful/Ind, Useful/Soc), a core concept of the Technology Assessment Model33 that was revealed as important to include by the pretest phase of the questionnaire (described in the “Results” section). Questions about individual acceptance (IndAtce) were based on a four-point Likert scale, with respondents being asked to indicate whether or not they would use the nanocarriers presented (carbon composition, synthetic DNA composition) in each context of use (to treat lung cancer, to treat seasonal flu). Questions were formulated to measure social acceptance (SocAtce) by asking whether or not respondents deemed it desirable to have widespread use of the two kinds of nanocarriers in the two clinical contexts of use. See Table 1 for details of conceptual and operational definitions and data reduction.
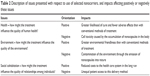  | Table 2 Description of issues presented with respect to use of selected nanocarriers, and impacts affecting positively or negatively these issues |
Prior to the posting of the questionnaire online, an iterative pretest using a cognitive interviewing approach34 was conducted to check instructions, key concepts, the presentation of the two kinds of nanocarriers, questions and response options, and the adequacy of response options, as well as to optimize comprehension. Three rounds of interviews were conducted with individuals representing the targeted participants (first round, n=8 participants; second round, n=17 participants; third round, n=10 participants). After answering the questionnaire, participants were debriefed to identify problems. Changes were made between rounds and validated in the next round until a final version was established for implementation online. In the last round, in order to test the design and improve user experience, usability tests were conducted on the questionnaire assessing completion time, ease of administration, and visual considerations. Mean respondent completion times for every section and for the questionnaire as a whole during the last round were used as standard times to establish quality cutoffs. Time stamps for login and completion of the questionnaire were automatically collected for every participant.
Study participants and recruitment
The study was approved by a research ethics board of the Centre Hospitalier Universitaire de Sherbrooke. Participation in the study was anonymous (no personal identifiers) and by invitation only. It consisted solely in responding to the web-based questionnaire. Consent for participation was considered as given by completing the questionnaire. Researchers were identified as potential participants after an exhaustive literature review of numerous databases using combinations of keywords such as nanotechnology, nanomedicine, ethics, social sciences, and new technologies. A language limitation restricted the geographical areas of recruitment to French-speaking regions of Europe and Canada. The electronic addresses of corresponding authors were recorded in a private database; other authors’ addresses were found online on the institutional or personal websites of the identified researchers. French and Canadian thematic research groups involved in research in nanotechnology and nanomedicine were also targeted for recruitment using institutions such as NE3LS Network on Nanotechnology35 (Canada) and Pacte – Social Science Research Laboratory36 (France). In total, a list of 1,527 potential participants, including graduate research trainees, was established; for 207, addresses were no longer valid. An email invitation to participate, containing a link to the web-based questionnaire, was sent out in September 2013 to 1,320 potential participants. Following initial contact, a first reminder was sent in October 2013 and a final reminder in December 2013. Data from respondents who did not meet the minimum completion quality outcome, defined a priori (all questions completed, questionnaire completed in more than 11 minutes), were also excluded from the analyses.
Statistical analysis
The influence of nanocarrier composition on variables related to impact perception, acceptance, and acceptability and the influence of context of use on acceptance and acceptability were assessed using the Wilcoxon signed-rank test, McNemar’s test, the McNemar–Bowker test, and the Pearson test. A multiple correspondence analysis (MCA) was then performed to examine relationships between core variables. Logistic and ordinal regression models were calculated to assess the predictive values of core variables in relation to each other. All statistical tests used an alpha of 0.05. Analyses were carried out using SPSS Statistics (V20) (IBM Corporation, Armonk, NY, USA).
Results
An invitation to participate in an anonymous web-based questionnaire study was sent via email to 1,320 active researchers and graduate research trainees in the field of new technologies. The questionnaire was available online from 4 September 2013 to 15 March 2014 (192 days). By the end of this period, 585 of persons contacted had accessed the questionnaire (44.32% access rate), and 214 had completed it (16.21% response rate meeting quality criteria). Average completion time (excluding extreme values) from access to the last question was 25 minutes 38 seconds (±14 minutes 47 seconds; minimum 11 minutes 7 seconds to maximum 89 minutes 32 seconds). Respondents (n=214) identified themselves as researchers (71%) or graduate students (29%) in the fields of the natural sciences (67%) or the social sciences and humanities (33%). Male respondents accounted for 63% of the sample. Sixty-six percent of the respondents were from Europe (France =58%; Belgium =5%; Switzerland =2%; Italy =1%), and the rest from Canada.
Comparisons among nanocarriers and contexts of use
Response frequencies for carbon and synthetic DNA nanocarriers on scales for impact perception, acceptance, and acceptability are presented in Table 3. Comparison of the PI for carbon and synthetic DNA nanocarriers reveals a significant difference (P<0.01, Wilcoxon signed-rank test) in the way respondents identified and assessed the impacts of the two drug-delivery carriers. Impacts of the synthetic DNA nanocarrier on health, the environment, and social cohabitation were perceived more positively (38.3%) as compared with the impacts of the carbon nanocarrier (27.1%). In contrast, impacts of the carbon nanocarrier on health, the environment, and social cohabitation were perceived more negatively (33.9%) as compared with the impacts of the synthetic DNA nanocarrier (29%). For both nanocarriers, clear differences in individual and social acceptance are observed as between the two contexts of use (P<0.05, McNemar’s test). No significant difference is observed based on nanocarrier composition. To treat lung cancer, 92.5% of respondents reported that they would use a treatment delivered by carbon nanocarrier and 90.7% that they would use a treatment delivered by synthetic DNA nanocarrier. To treat seasonal flu, only 19.2% of respondents reported that they would rely on carbon nanocarrier delivery and 19.6% that they would use synthetic DNA nanocarriers.
The concept of acceptability is divided into two variables, acceptability index (AI) and preponderant issue (PIssue). When explaining why they would or would not use a treatment relying on carbon nanocarriers to treat lung cancer (IndAI), 76.2% of respondents cited positive impacts, 15.4% were ambiguous, and 8.4% mentioned negative impacts. In the same clinical context, to explain why they would or would not use a treatment relying on synthetic DNA nanocarriers, the same percentage of respondents cited positive impacts, 12.6% were ambiguous, and 11.2% mentioned negative impacts. When explaining why they would or would not use a treatment relying on carbon nanocarriers to treat seasonal flu, 23.8% of respondents cited positive impacts, 21.5% were ambiguous, and 54.7% mentioned negative impacts. In the same clinical context, to explain why they would or would not use a treatment relying on synthetic DNA nanocarriers, the same percentage of respondents cited positive impacts, 23.4% were ambiguous, and 52.8% mentioned negative impacts. Here too, significant differences (P<0.05) are observed between contexts of use. In contrast, no difference is observed based on nanocarrier composition. Similar percentages were obtained for SocAI (Table 3), with no significant differences between IndAI and SocAI.
Analysis of preponderant issues (Figure 1) reveals that the individual acceptability of carbon and synthetic DNA nanocarriers for treating lung cancer mainly centers on health issues: for both carbon and synthetic DNA nanocarriers, 82.2% of respondents based their acceptability judgment on health issues. In this context of use, respondents were less inclined to take into account environmental (carbon =1.9%, DNA =1.9%) and social (carbon =2.8%, DNA =2.3%) issues as a priority in justifying their acceptance. In the context of the treatment of seasonal flu, the individual acceptability of carbon and synthetic DNA nanocarriers proved much more complex: in addition to health issues (carbon =45.8%, DNA =49.1%), respondents considered issues related to the environmental (carbon =9.8%, DNA =7.5%) and social cohabitation (carbon =11.2%, DNA =10.3%). Similar results were observed for social acceptability: health issues preponderated, and respondents were more likely to accept both carbon and synthetic DNA nanocarriers to treat lung cancer than to treat seasonal flu. Respondents also raised environmental and social cohabitation issues in relation to social acceptability. Significant differences (P<0.05, McNemar–Bowker test) were observed between contexts of use, but no difference was noted based on composition.
Relations among variables
Logistic and ordinal regression models
As a first step in preparing to describe relationships between variables, logistic and ordinal regression models helped determine which factors shape the core variables. Five models were tested for prediction of the main variables (PI, IndAtce, IndAI, SocAtce, and SocAI). Six factors were included, comprising the core variables plus perceived usefulness (Useful/Ind and Useful/Soc) as a sub-variable of acceptability. No interaction parameter was tested in the models. Tests showed no multicollinearity between independent variables in any of the five regression models. As shown in Table 4, all five models were significant (P<0.05). IndAtce and SocAtce logistic regression models presented a high proportion of variance explained (associations between variables are reported by Nagelkerke pseudo-R2 measures): 67.6% of variance is explained for the IndAtce model and 71.4% for the SocAtce model. They also both present high classification accuracy: 90.7% for the IndAtce model and 91.6% for the SocAtce model. In the IndAtce model, of the six predicting factors, only three were statistically significant (P<0.05, two-tailed t-test): IndAI (odds ratio [OR] =2.52), SocAtce (OR =8.02), and Useful/Ind (OR =14.6). In the SocAtce model, of the six predicting factors, again, only three were statistically significant: IndAtce (OR =7.36), SocAI (OR =3.61), and Useful/Soc (OR =13.1). The PI, IndAI, and SocAI ordinal regression models presented a lower proportion of variance explained (PI, R2 =21.9%; IndAI, R2 =37.2%; SocAI, R2 =40.9%). In the PI model, of the six predicting factors, only two were statistically significant: IndAI (negative IndAI, OR =0.37) and SocAI (negative SocAI, OR =0.30; neutral SocAI, OR =0.28). In the IndAI model, of the six predicting factors, only three were statistically significant: PI (negative PI, OR =0.40), IndAtce (OR =0.21), and SocAI (negative SocAI, OR =0.17). In the SocAI model, of the six predicting factors, only three were statistically significant: PI (negative PI, OR =0.32; neutral PI, OR =0.44), IndAI (negative IndAI, OR =0.21), and SocAtce (OR =0.14).
Multiple correspondence analysis
In order to look more closely at the relationships between impact perception, individual and social acceptance, and individual and social acceptability, a single scenario was chosen (carbon nanocarrier to treat seasonal flu) which presented great variability in answers for all variables. The relationships between these categorical variables were studied using MCA. This data reduction approach, used both in the social sciences and in clinical and epidemiological research, is designed for the study of associations within one set of variables.37 MCA is part of a family of descriptive methods (such as clustering and factor analysis and principal components analysis) that reveal patterning in complex data sets but are specific to categorical variables and enable the visualization of independent clusters on a multidimensional plane. The application of MCA to the data (n=214) from the chosen scenario showed that the total inertia explained is equal to 97.5%, with 56.8% due to the first dimension and 40.7% to the second dimension. The first dimension corresponds to the orientation of modalities (positive/negative) and the second to their polarization (low/strong). The analysis does not take into account the potential effect of a third dimension. Cronbach’s alpha (α=0.825) indicates high internal consistency for all measured items. IndAtce (D1 =71.9%; D2 =67.4%), Useful/Ind (D1 =66.6%; D2 =69%), SocAtce (D1 =76.7%; D2 =59%), and Useful/Soc (D1 =73.5%; D2 =81.4%) presented strong correlations with dimension 1 and dimension 2, as observable in the percentages noted in parentheses. All other variables were weakly correlated with both dimensions.
A visualization of the MCA results is presented in Figure 2. A look at the coordinate graph of modalities reveals six clusters:
- Cluster 1: There is a strong correlation on both dimensions between IndAtce, SocAtce, Useful/Ind, and Useful/Soc. Researchers who strongly agreed with individual use of a treatment for seasonal flu relying on carbon nanocarriers strongly agreed with its widespread use in society and deemed this treatment to be really useful to them and to society.
- Cluster 2: There is a strong correlation on both dimensions between PI, IndAI, and SocAI. Respondents who perceived positive impacts for a seasonal flu treatment relying on carbon nanocarriers were more likely to justify their individual and social acceptance by citing positive impacts.
- Cluster 3: As with cluster 1, a strong correlation is observed on both dimensions between IndAtce, SocAtce, Useful/Ind, and Useful/Soc. Researchers who agreed with individual use of a treatment for seasonal flu relying on carbon nanocarriers agreed with its widespread use in society and deemed this treatment useful to them and to society.
- Cluster 4: As with clusters 1 and 3, this cluster presents a strong correlation on both dimensions between IndAtce, SocAtce, Useful/Ind, and Useful/Soc. Researchers who disagreed with individual use of a treatment for seasonal flu relying on carbon nanocarriers disagreed with its widespread use in society and deemed this treatment useless to them and to society.
- Cluster 5: A relationship is observable between neutral modalities for PI, IndAI, and SocAI. However, the possibilities for interpretation are limited because of the proximity of the modalities to the center of the plot.
- Cluster 6: There appears to be a global correlation of negative modalities for all variables. Thus respondents who strongly disagreed with the individual and social acceptance of seasonal flu treatment relying on carbon nanocarriers deemed such treatment totally useless to themselves and to society. As was seen in clusters 2 and 5, there is a correlation between PI, IndAI, and SocAI: respondents who perceived negative impacts for the use of a seasonal flu treatment relying on carbon nanocarriers were more likely to justify their individual and social acceptance by citing negative impacts.
Discussion
This study aimed to answer three questions: Do nanocarrier composition and medical context of use influence researchers’ impact perception, acceptance, and acceptability judgments in relation to treatments? Are impact perception, acceptance, and acceptability related to each other? If so, how?
Overall, the results showed that without regard for the context of use, nanocarrier composition does not seem to affect individual and social acceptance or individual and social acceptability. Within a precise context of use of nanocarriers – the treatment of lung cancer, for example – the fact that the pharmacological agent delivered by both kinds of nanocarriers was perceived as the same may explain why composition is not a relevant concern. However, it should be noted that the details on the exact composition of the nanocarriers (type, methodology of preparation) were not provided to the participants and could have influenced their judgment. A medical context in which what matters to patients is usually effectiveness and nothing else may partly account for the drug carrier’s composition having little or no influence on acceptance and acceptability. In contrast to what is observed for cosmetology – a context of use in which a great deal of importance is assigned to nanoparticle composition and public perception38,39 – it has been reported that in medical contexts with health outcomes, treatment composition (nanoformulated drug versus regular drug) seems not to govern product acceptance.17 On the other hand, impact perception differs significantly as between the two kinds of nanocarriers. The very different biochemical structures may account for the differences in impact perception: a carbon nanocarrier may be perceived as being more costly to produce and less ecofriendly, while synthetic DNA may appear less threatening in terms of biological compatibility and environmental degradability. As well, currently concerns are being aired about the toxicity of carbon nanostructures, which may account for researchers’ less optimistic view of the carbon nanocarrier. Nevertheless, although impact perception differed as between different nanocarrier compositions, composition does not appear to be a factor governing and accounting for clinical decisions.
It has been reported that when little is known about a new technology, as is the case for nanoparticle use in the food industries and food packaging, the context of use itself seems to be an important factor in attitude and acceptance.40 In contrast to what we found regarding nanocarrier composition, our results showed that the clinical context significantly influences individual and social acceptance and individual and social acceptability in relation to nanocarriers. Respondents did not really accept seasonal flu treatment as a suitable context of use of nanocarriers. This rejection may largely be accounted for by the fear of negative outcomes for health and social cohabitation. In contrast, lung cancer received high acceptance as a suitable context of use, perhaps because of its horrifying consequences and the lack of effective and safe treatments for it. Our results on this score confirm what we found in the literature, namely that the nature and context of applications can influence impact perception and social acceptance.26
Along with context of use, perceived usefulness of treatments appears to be a significant factor influencing acceptance. This relationship between acceptance and perceived usefulness has been documented in the literature on technology acceptance models.41,42 Perceived usefulness, defined as the individual’s perception that using a technological system will enhance job performance,43 has been reported as an important factor in accounting for a technology’s acceptance. This may also apply to clinical contexts: a lung cancer treatment may meet with quick acceptance if the person to whom it is offered fears dying of cancer and is prepared to fight the adverse effects but be rejected if treatment is offered as a third-line therapy. Nanocarrier treatment for the seasonal flu may be accepted by a self-employed person who does not want to miss a day of work; it may be rejected by another person who believes the human body benefits from fighting viruses. The significant relationship between perceived usefulness and acceptance again suggests a consideration of the user’s personal assessment of the clinical situation as well as consideration of the context of use.
The MCA revealed a relationship between impact perception and acceptability whose strength was unexpected, given that perceived impacts can differ from impacts invoked in making an acceptability judgment. The relationship between these variables may have been accentuated by the process of operationalizing the framework for these two related but distinct concepts. Little is known about the relationship between impact perception and acceptability, and our result points toward continuing the research on the general public’s perception of the impact of new technologies. Our study is in line with previously reported findings19,29 that, even if efforts are invested in reducing the toxicity of new technologies such as nanocarriers and in maximizing their economic benefits, many other factors, such as concerns about social cohabitation and the environment, play a role in the formation of individual and social acceptability judgments. These issues must be taken into consideration in ensuring the responsible development and implementation of use of nanocarriers in medicine.
Regression analyses on core variables from a single case study (using the synthetic DNA nanocarrier to treat the seasonal flu) demonstrated the differences in predicting these variables. The two models related to IndAtce and SocAtce were the more accurate ones (67.6% and 71.4% of variance explained, 90.7% and 91.6% classification accuracy) and exceeded expectations, given that the range of explained variances in the recent literature is 29%–70%.43 The high percentages of variance explained for the IndAtce and SocAtce regression models reveal that a considerable amount is already known about the concept of acceptance and that the questionnaire we developed may help predict usage of the two kinds of nanocarriers in the contexts presented. Impact perception and models for individual and social acceptability, on the other hand, presented lower percentages of variance explained (21.9%, 37.2%, and 40.9%) and were less accurate in predicting the variables studied. Fewer independent variables in regression models were significant in predicting PI, IndAI, and SocAI when compared with the IndAtce and SocAtce models. This may suggest that although certain factors do help predict impact perception and individual and social acceptability, these concepts are more complex than acceptance and may reflect more complex contextual factors that were not included in the present study. But it may also suggest that the predictive approach used with acceptance and implicit in the traditional psychosocial paradigm may not be adequate for the study of these variables and there is a need to go further.13 The latter interpretation is in fact supported by the results of our analysis of the preponderant issues, which reveals that the acceptability judgment is characterized by a certain depth produced by the variability in the nature of the impacts on the different issues taken into account in an acceptability judgment. While acceptability judgment can be treated in a scalar manner by categorizing it as positive, neutral, or negative, it would gain from a deepened approach and by being treated not as a variable subject to prediction but as a whole approach allowing for a greater understanding of users’ motivations and fears in the face of a new technology. The nanocarriers described in both scenarios are examples of envisioned targeted drug-delivery applications. They were specifically chosen in order to provide an initial analysis of perceived impacts and acceptability of these nanocarrier-based envisioned applications. Conclusions from this exploratory study are drawn to open discussion on nanocarriers’ composition and contexts of use, but the results might not be used to infer any general conclusions regarding all nanocarriers for targeted drug-delivery applications or nanomedicine in general.
In order to go beyond the limitations of traditional approaches to predicting behaviors, which have reflected the assumption that behavior is guided mainly by economic and toxicological considerations, the survey questionnaire developed for this study was based on impact assessment and the balancing of impacts against each other to yield an acceptability judgment guided mainly by ethical considerations. This constitutes a previously unexplored avenue of research that could lead, through an understanding of users’ value judgments, to a better understanding of the issues surrounding the nanocarriers studied. Although we have here presented only the quantitative phase of this mixed-methods project, the semi-directed interviews we conducted made possible a deepened exploration of the main concepts. We intend to produce further publications to present these results. The exploratory context of the study design accounts for certain limitations in the project. Although the questionnaire we employed was pretested in successive rounds of cognitive interviews, we cannot claim that it could be considered to have been validated. Future research could be conducted to this end. Furthermore, in recruiting participants, we preferred to rely on convenience sampling, which entails selection bias; for example, only Francophone researchers active in their fields were included in the study. Thus, it is somewhat hard to transfer our results to the general research community of Canada and Europe. Finally, the length of the questionnaire, the recurrence of questions, and the cognitive load entailed by the complexity of the concepts under examination could also constitute a limitation on the quality of the data gathered.
Conclusion
Nanotechnology applications offer the potential for many improvements in the spheres of health care and medical treatment. Integrating patients’ values and social concerns into the process of developing new medical technologies and products could help nanomedicine to flourish by ensuring clinical compliance and enhancing the general acceptability of treatments. Though it has been reported that, because its applications often consist of forms of medical intervention, nanomedicine comes under heavier scrutiny than does nanotechnology in general,44 our study’s results suggest that context of use must also be taken into account in any assessment in the field of nanomedicine. Our study highlights researchers’ degree of acceptance of both kinds of nanocarriers from the perspectives of both composition and context of use, and it reveals what factors account for researchers’ positions. Using the same approach with other targeted populations could lead to a better understanding of the concerns of all stakeholders, including the general public, about a wide range of technological and medical products.
Acknowledgments
This study was supported by NE3LS thematic scholarship from the Fond Québécois de Recherche en Santé and by InterNE3LS grant from the Canadian Institutes for Health Research (#43854). Région Rhône-Alpes and the Ministry of Education, Leisure and Sports are acknowledged for Vanessa Chenel’s mobility grants. CNRS and École Centrale de Lyon are acknowledged for the delegation of Jean-Pierre Cloarec at Université de Sherbrooke. We also thank Georges-Auguste Legault (Sherbrooke, Canada) and Céline Verchère (Grenoble, France) for their helpful comments.
Disclosure
The authors report no conflicts of interest in this work.
References
Farokhzad OC, Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev. 2006;58:1456–1459. | ||
Wagner V, Dullaart A, Bock A-K, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217. | ||
Bawa R, Johnson S. Emerging issues in nanomedicine and ethics. In: Allhoff F, Lin P, editors. Nanotechnology and Society – Current and Emerging Ethical Issues. New York, NY: Springer; 2009:207–223. | ||
Moghimi MS, Hunter CA, Murray CJ. Nanomedicine: current status and future prospects. FASEB J. 2005;12(3):311–330. | ||
Mnyusiwalla A, Daar AS, Singer PA. ‘Mind the gap’: science and ethics in nanotechnology. Nanotechnology. 2003;14:R9–R13. | ||
Gaskell G, Allum N, Wagner W, et al. GM foods and the misperception of risk perception. Risk Anal. 2004;24(1):185–194. | ||
Fischhoff B, Slovic P, Lichtenstein S, Read S, Combs B. How safe is safe enough? A psychometric study of attitudes toward technological risks and benefits. Pol Sci. 1978;9:14. | ||
Slovic P, Fischhoff B, Lichtenstein S. Rating the risks. Environment. 1979;21(3):14–20, 36–39. | ||
Slovic P. Perception of risk. Science. 1987;236:280–285. | ||
Roco M, Renn O, Jäger A. Nanotechnology risk governance. In: Renn O, Walker K, editors. Global Risk Governance: Concept and Practice Using the IRGC Framework. Vol. 1. Netherlands: Springer; 2008:201–227. | ||
Patenaude J, Legault GA, Beauvais J, et al. Framework for the analysis of nanotechnologies’ impacts and ethical acceptability: basis of an interdisciplinary approach to assessing novel technologies. Sci Eng Ethics. 2014. | ||
Scheufele DA, Lewenstein BV. The public and nanotechnology: how citizens make sense of emerging technologies. J Nanopart Res. 2005;7:659–667. | ||
Burri RV, Bellucci S. Public perception of nanotechnology. J Nanopart Res. 2008;10(3):387–391. | ||
Cobb MD, Macoubrie J. Public perceptions about nanotechnology: risks, benefits and trust. J Nanopart Res. 2004;6:395–405. | ||
Bainbridge WS. Public attitudes toward nanotechnology. J Nanopart Res. 2002;4:561–570. | ||
Kahan DM, Slovic P, Braman D, Gastil J, Cohen G. Nanotechnology Risk Perceptions: The Influence of Affect and Values; 2007. Report conducted by the Cultural Cognition Project at Yale Law School. | ||
Nerlich B, Clarke DD, Ulph F. Risks and benefits of nanotechnology: how young adults perceive possible advances in nanomedicine compared with conventional treatments. Health Risk Soc. 2007;9(2):159–171. | ||
Bottini M, Rosato N, Gloria F, et al. Public optimism toward nanomedecine. Int J Nanomedicine. 2011;6:3473–3485. | ||
Sechi G, Bedognetti D, Sgarrella F, et al. The perception of nanotechnology and nanomedicine: a worldwide social media study. Nanomedicine (London). 2014;9(10):1475–1486. | ||
Berube DM. The public acceptance of nanomedicine: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:2–5. | ||
Sylvester DJ, Abbott KW, Marchant GE. Not again! Public perception, regulation, and nanotechnology. Regul Governance. 2009;3:165–185. | ||
Corley EA, Kim Y, Scheufele DA. Leading US nano-scientists’ perceptions about media coverage and the public communication of scientific research findings. J Nanopart Res. 2011;13:7041–7055. | ||
Cobb MD. Framing effects on public opinion about nanotechnology. Sci Commun. 2005;27(2):221–239. | ||
Satterfield T, Conti J, Harthorn BH, Pidgeon N, Pitts A. Understanding shifting perceptions of nanotechnologies and their implications for policy dialogues about emerging technologies. Sci Public Policy. 2013;40:247–260. | ||
te Kulve H, Konrad K, Palavicino CA, Walhout B. Context matters: promises and concerns regarding nanotechnologies for water and food applications. Nanoethics. 2013;7:17–27. | ||
Gupta N, Saji G, Fischer ARH, Frewer LJ. Expert views on societal responses to different applications of nanotechnology: a comparative analysis of experts in countries with different economic and regulatory environments. J Nanopart Res. 2013;15:1838. | ||
Siegrist M, Keller C, Wiek A, Frey S, Kastenholz H. Laypeople’s and experts’ perception of nanotechnology hazards. Risk Anal. 2007;27(1):59–69. | ||
Besley JC, Kramer VL, Priest SH. Expert opinion on nanotechnology: risks, benefits, and regulation. J Nanopart Res. 2008;10:549–558. | ||
Powell MC. New risk or old risk, high risk or no risk? How scientists’ standpoints shape their nanotechnology risk frames. Health Risk Soc. 2007;9(2):18. | ||
Althaus CE. A disciplinary perspective on the epistemological status of risk. Risk Anal. 2005;25(3):567–588. | ||
Silva Costa H, Sethe S, Pêgo AP, Olsson AS. Scientists’ perception of ethical issues in nanomedicine: a case study. Nanomedicine (London). 2011;6(4):681–691. | ||
Creswell JW. Research Design – Qualitative, Quantitative, and Mixed Methods Approaches. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2009. | ||
Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 1989;13(3):319–340. | ||
Willis BG. Cognitive Interviewing – A Tool for Improving Questionnaire Design. Thousand Oaks, CA: SAGE; 2005. | ||
NetWork NL. NE3LS – Network Overview; 2014. Available from: http://www.ne3ls.ca/ne3ls-overview/?lang=en. Accessed October 29, 2014. | ||
PACTE. Pacte – Social Science Research Laboratory; 2014. Available from: http://www.pacte-grenoble.fr/english/. Accessed October 29, 2014. | ||
Greenacre M. Correspondance Analysis in Practice. Boca Raton, FL: Taylor & Francis Group; 2007. | ||
Chaudhry Q, Sanner T, van Engelen J, et al. Guidance on the Safety Assessement of Nanomaterials in Cosmetics. Brussels, Belgium: Scientific Committee on Consumer Safety; June 27th 2012. | ||
FDA. Guidance for Industry – Safety of Nanomaterials in Cosmetic Products. U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition; June 2014. | ||
Siegrist M, Cousin M-È, Kastenholz H, Wiek A. Public acceptance of nanotechnology food and food packaging: the influence of affect and trust. Appetite. 2007;49:459–466. | ||
Venkatesh V, Bala H. Technology Acceptance Model 3 and a research agenda on interventions. Decision Sciences. 2008;39(2):273–315. | ||
Davis FD. A Technology Acceptance Model for empirically testing new end-user information systems : theory and results. Cambridge: Management Science, Massachusetts Institute of Technology; 1985. | ||
Holden RJ, Karsh B-T. The Technology Acceptance Model: its past and its future in health care. J. Biomed. Inf. 2010;43:159–172. | ||
Pautler M, Brenner S. Nanomedicine: promises and challenges for the future of public health. International Journal of Nanomedicine. 2010;5:803–809. |
Supplementary materials
  | Figure S1 Survey questionnaire. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




