Back to Archived Journals » International Journal of Interferon, Cytokine and Mediator Research » Volume 6
TGR5 ligands as potential therapeutics in inflammatory diseases
Authors Eggink H, Soeters M, Pols T
Received 2 April 2014
Accepted for publication 1 May 2014
Published 21 August 2014 Volume 2014:6 Pages 27—38
DOI https://doi.org/10.2147/IJICMR.S40102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Video abstract presented by Hannah M Eggink
Views: 811
Hannah M Eggink,1 Maarten R Soeters,1 Thijs WH Pols2
1Department of Endocrinology and Metabolism, 2Department of Medical Biochemistry, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
Abstract: Takeda G protein-coupled receptor 5 (TGR5), also known as Gpbar1, membrane-type bile acid receptor (M-BAR), or GPR131 is a G protein-coupled receptor that is best known for its activation by bile acids. TGR5 has been found to regulate a number of specific processes, including energy expenditure and glucagon-like peptide-1 release. Other actions in which TGR5 is implied range from regulating bile acid homeostasis to playing a role in the nervous system. The receptor is increasingly associated with the regulation of inflammatory responses in a number of cells that are relevant to the immune response. TGR5 exerts antiinflammatory actions by decreasing adhesion molecule expression in endothelial cells and inhibiting proinflammatory cytokine production in macrophages. A number of animal models also hint toward the antiinflammatory actions of TGR5. These include models of atherosclerosis, colitis, and inflammation-driven liver diseases. In the current review, we provide a comprehensive overview of TGR5 with a focus on its role in inflammation. We furthermore describe the currently known agonists of TGR5 and discuss some of the recent findings on TGR5 signaling. The potential drawbacks, as well as the encouraging prospects, of TGR5 will be discussed in view of TGR5 as a therapeutic target in diseases with inflammatory facets.
Keywords: metabolism, inflammation, macrophages, G protein-coupled receptor, bile acids, Takeda G protein-coupled receptor 5
Physiological expression and function of TGR5
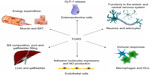
Takeda G protein-coupled receptor 5 (TGR5), also known as Gpbar1, membrane-type bile acid receptor (M-BAR), or GPR131, is one of ~800 human G protein-coupled receptors (GPCRs) that impact all levels of biology by transducing signals from a diverse spectra of events.1 TGR5 belongs to the family A group of GPCRs, also known as the rhodopsin-like subfamily of GPCRs. The receptor is conserved among mammals, and is also found in lower vertebrates, such as fish.1 TGR5 was discovered as a membrane receptor responsive to bile acids (BAs).2–4 Since then, TGR5 has been investigated in several cell types, such as muscle cells, adipocytes, endothelial cells (ECs), and macrophages.1,5 The functions of TGR5, which are dependent on cell type, range from glucagon-like peptide-1 (GLP-1) release and mitochondrial energy homeostasis to the control of inflammatory responses (see Figure 1).6–9
In the current review, we provide an extensive introduction to TGR5 and its function in different tissues, with a focus on the effects of TGR5 in inflammation. We describe the possible problems and promising outlook of TGR5 as a therapeutic target in diseases with inflammatory aspects.
Signaling of TGR5
TGR5 binds and responds to several BA species with different affinities,3,4 which results in the upregulation of cyclic adenosine monophosphate (cAMP) levels. Other compounds that have been described to activate TGR5 include triterpenoids, bile alcohols, and certain steroid hormones, including neurosteroids.10–13 A large number of semisynthetic and synthetic agonists have also been developed to target TGR5.14 These are more elaborately described in the section titled, “TGR5 agonists”.
The receptor has no known sorting motifs and is synthesized in the endoplasmic reticulum, after which it translocates to the plasma membrane to function. This translocation process was addressed by Spomer et al,15 who demonstrated that this trafficking depends on the membrane proximal C-terminal alpha helix of TGR5. In contrast to most GPCRs, TGR5 does not traffic to endosomes, but relocates to plasma microdomains where it elevates cAMP levels for a prolonged period.16 This is in line with the weak interaction of TGR5 with GPCR kinases or β-arrestin-1/2.16
It is well described that TGR5 is able to interact with G protein αS (GαS).3,4 Upon the binding of agonists to the ligand-binding pocket of TGR5, GαS is activated and regulates adenylyl cyclase activity. This results in the elevation of cAMP levels (among others) and further downstream signaling.3,4 TGR5 is also able to bind Gαi3 and GαQ in the FLO cancer cell line, as well as in the human AGS gastrinoma cancer cells.17,18 These G proteins respectively dampen cAMP activity and increase phospholipase activity.19 Additionally, TGR5 couples to GαS in H69 nonciliated cholangiocytes, but couples to Gαi in ciliated H69 cholangiocytes,20 which underlines the binding of TGR5 to different G proteins. TGR5 also activates ERK1/2 (extracellular signal-regulated kinases-1/2) in an epidermal growth factor receptor (EGFR)-dependent manner.16,21 Finally, it has been demonstrated that TGR5 induces JNK (c-Jun-N terminal kinase) activation, and it has been proposed to play a role in hepatocyte apoptosis.22 Of interest, a human study has revealed a number of genetic variants of TGR5 that show abrogated cAMP signaling.23
Taken together, TGR5 is able to bind to multiple G proteins and subsequently induces distinct downstream signaling cascades in several cell types. This could suggest that the downstream signaling of TGR5 is dependent on cell type and its differentiation state.
Physiological role of TGR5 in BA homeostasis
TGR5 is expressed in many cells that are relevant to the homeostasis of BA metabolism. These cells include gallbladder epithelial cells, gallbladder smooth muscle cells, as well as cholangiocytes.20,23–27 A role of TGR5 in BA metabolism is underlined by findings that the BA pool size is decreased in Tgr5−/− mice.28,29 In gallbladder epithelial cells, TGR5 is demonstrated to increase chloride secretion via activation and translocation of the cystic fibrosis transmembrane conductance regulator, and as such to modulate bile composition.24 In cholangiocytes, TGR5 is proposed to function as a sensor to couple biliary BA concentration to ductular bile formation and bile flow.20,23 TGR5 is also demonstrated to relax gallbladder smooth muscle cells, leading to gallbladder filling.26,27 In short, evidence suggests that TGR5 regulates bile composition and bile flow.
Physiological role of TGR5 in metabolic tissues
Accumulating evidence suggests that TGR5 influences glucose homeostasis and energy expenditure.7,8 Activation of TGR5 by BAs triggers the release of GLP-1 in murine intestinal L-cells.6,7 GLP-1 is an incretin with beneficial effects on glucose homeostasis, such as increased insulin secretion and thus improved glucose tolerance.7 Upon treatment of obese mice with BA sequestrants that increase the BA pool size, GLP-1 secretion increases in a TGR5-dependent manner, with improved glucose tolerance when compared to controls.7,30,31
TGR5 increases energy expenditure in thermogenic tissues such as mouse brown adipose tissue and human skeletal muscle.8 Stimulation of the TGR5 pathway in these tissues triggers the thyroid hormone-activating enzyme type 2 iodothyronine deiodinase (D2), converting inactive thyroxine (T4) into the active iodothyronine (T3) form, thereby increasing energy expenditure.8 Mice on a high-fat diet were protected from obesity and insulin resistance when fed a BA-supplemented diet, whereas this effect was not observed in D2−/− mice, which underlined the role of D2 in this response.8 Plasma BA levels have also been associated with thyroid hormone control and energy expenditure in humans.32 Thus, TGR5 plays an important metabolic role by which it positively influences glycemic control via GLP-1 release and increases energy expenditure by activating D2.
TGR5 in the nervous system
TGR5 is present in the enteric nervous system, as well as in the central nervous system.3,12,28,33 In the murine enteric nervous system, TGR5 was found in inhibitory motor neurons and descending interneurons, but not in glial cells.33 Stimulation of TGR5 with the BA deoxycholic acid (DCA) inhibited intestinal motility and secretion, suggesting a neural role for TGR5 in digestion.33 In humans, a link has been made between a TGR5 single-nucleotide polymorphism and bowel transit, although this relationship was not very strong.34 In addition, the receptor has been proposed to mediate itch and analgesia.35
In the central nervous system, TGR5 messenger ribonucleic acid (mRNA) expression has been detected in mice, rats, and humans.3,12,28 The TGR5 protein is expressed throughout the rat brain in both neurons and astrocytes.12 However, the function of TGR5 in the brain remains enigmatic. It is possible that TGR5 plays a role as a neurosteroid receptor because TGR5 can be activated in vitro by several neurosteroids, predominantly by 5β-pregnan-3α-ol-20-one.12 Stimulation of astrocytes with 5β-pregnan-3α-ol-20-one leads to an increase in cAMP and calcium, and to the downregulation of Tgr5 mRNA.12 Interestingly, BAs with moderate TGR5 affinity are also present in the human and rat brain.36,37 In the rat brain, only the unconjugated primary BAs – chenodeoxy cholic acid (CDCA) and cholic acid (CA) – and secondary BA (DCA) have been detected, while their tauro-conjugated forms and other BAs were also found in peripheral tissue.37 In rats, as well as in humans, BAs are able to pass the blood–brain barrier.38,39 So, although BAs seem to be common ligands for TGR5 in the periphery, this might not be the case in the central nervous system.
TGR5 in the immune system
TGR5 is also expressed in several cells of the immune system, and it has been demonstrated to regulate immune responses. These cells include monocytes, macrophages, ECs, and dendritic cells (DCs). We discuss the function of TGR5 in these cells in detail in the section titled, “TGR5 in the immune response”.
TGR5 agonists
Development of TGR5 agonists
Endogenous BAs are physiological ligands that activate TGR5, albeit with different affinities.3,4 There has been a rush to design and validate new compounds that are able to modulate the activity of the TGR5 receptor and, until now, this research has focused on TGR5 ligands that improve glucose homeostasis and limit body weight gain. The design and synthesis of TGR5 agonists has especially been deemed necessary because endogenous BAs generally have a lower affinity and selectivity for TGR5, and they have specific biological properties with regard to localization and cycling. The hierarchy of BA affinity for TGR5 is as follows: lithocholic acid (LCA) > DCA > CDCA > CA.40 In line with computational methods that have been used to investigate BA binding to TGR5, a recent TGR5 mutation study confirmed key residues within the TGR5 binding pocket that are involved in BA binding to TGR5.41,42
Different research groups and companies have used step-by-step strategies to design selective agonists via the extensive screening of BA derivatives and the subsequent modification of their individual structures. Medicinal chemistry strategies yielded potent BA (steroidal) derivatives via modification of the BA scaffold and screening of chemical libraries. Subsequent hit-to-lead optimization produced potent nonsteroidal heterocyclic compounds, as reviewed earlier.14 Evidently, the steroidal agonists contain a steroidal core that includes BAs, steroid hormones, and semisynthetic derivatives, whereas this is not the case for the nonsteroidal agonists. Besides the selective TGR5 agonists, compounds have been developed with dual affinity for both TGR5 and the nuclear BA receptor, farnesoid X receptor (FXR).43 Although many compounds have been identified and tested for affinity, efficacy, and selectivity, only a limited number have made it to preclinical testing in the fields of atherosclerosis, colitis, and liver diseases. Most compounds were investigated with respect to specific aspects of obesity and type 2 diabetes mellitus.14
Semisynthetic bile acids as TGR5 agonists
One of the best known semisynthetic BA TGR5 agonists might be 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777), which was developed via the incorporation of crucial 6α-ethyl and 23(S)-methyl moieties in the scaffold of CA.44 Likewise, other semisynthetic BA receptor ligands have been developed solely with FXR (INT-747) or with combined FXR/TGR5 (INT-767) activity.43,45,46 The TGR5 agonist, INT-777, stimulates receptor activation and GLP-1 release from enteroendocrine L-cells, increases energy expenditure, reduces hepatic steatosis, improves insulin sensitivity, and stimulates insulin release in cultured pancreatic MIN6 cells and human pancreatic islets.6–8,47 Furthermore, INT-777 stimulates bile flow, gallbladder filling, and gallbladder relaxation.26,27 In 2011, it became clear that INT-777 was also able to inhibit macrophage inflammation and atherosclerosis, which is discussed in the sections titled, “TGR5 function in monocytes and macrophages” and “Atherosclerosis”.5 In addition, INT-777 decreases basal secretory tone and inhibits cholinergic-induced secretory responses in the colon via TGR5 activation.48 Other semisynthetic BAs include multiple ursodeoxycholic acid analogs studied by Iguchi et al,49 among which 7α-methylated ursodeoxycholic acid (3α,7β-dihydroxy-7α-methyl-5β-cholanoic acid) had a remarkably high affinity to bind to and activate TGR5.
Triterpenoid TGR5 agonists
The screening of a library of plant extracts for TGR5 activity led to the discovery of oleanolic acid (OA) as a triterpenoid TGR5 agonist.40 OA was known as an olive leaf-derived oleanane-type triterpenoid and has weak antiinflammatory and anticarcinogenic effects.40,50 Multiple studies, primarily on glucose metabolism, have shown the biological action of OA as a TGR5 agonist. OA improved glucose tolerance in mice fed a high-fat diet.40 Additionally, OA increases pancreatic beta cell insulin release via TGR5 and improves prokinetic action (motility) of the colon.35,47
Betulinic acid (BTA) was identified as a triterpenoid able to activate TGR5 by a biological screening of a collection of naturally occurring triterpenoids.51 In this study, triterpenoid derivatives were generated, that were also potent TGR5 agonists.51 Even the most potent BTA derivative, however, showed marginal effects on glucose metabolism, despite its beneficial toxicity profile; these effects may have been due to its low bioavailability.51 Other findings indicate that BTA may play a role in intraluminal duodenal HCO3− secretion after luminal administration.52 BTA also inhibits high glucose-induced vascular smooth muscle cell proliferation and migration.53 Finally, BTA decreased body weight, as well as blood glucose and lipid levels, while increasing insulin and leptin in mice fed a high-fat diet.54 However, none of these studies definitely proved a TGR5-mediated mechanism, leaving the relevance of TGR5 in these effects of BTA uncertain.
The same holds true for the triterpenoid, ursolic acid (UA).51 Before the discovery of UA as a TGR5 agonist, UA was shown to improve glucose metabolism without affecting body weight.55 Later on, these findings were more or less confirmed in other studies that showed ameliorated glucose metabolism, decreased abdominal obesity, and improved hepatic steatosis.56–58 Of relevance, TGR5 dependence was not investigated in these studies.
Other TGR5 agonists
A number of other TGR5 agonists have been published that show therapeutic potential. One of those agonists is SB-756050 (1,4-bis[{3,4-bis(methyloxy)phenyl} sulfonyl]hexahydro-1H-1,4-diazepine). SB-756050 is a selective TGR5 agonist, and its use in humans has been researched published.59 Chronic enteral administration of SB-756050 improved glucose metabolism in diabetic rats; however, these effects were not reproduced in humans.59 This could indicate a difference between rat and human TGR5 targeting.
Zambad et al60 screened ~400 compounds for TGR5 activation in vitro and identified TRC210258 (N-[4-chlorophenyl]-2-[4-fluorophenoxy]-N-methylimidazo [1, 2-a] pyrimidine-3-carboxamide) as a new TGR5 activator. TRC210258 efficiently activated TGR5 and improved many metabolic parameters, such as GLP-1 secretion, energy expenditure, cholesterol, and glucose metabolism in diet-induced obese mice and hamsters.
The 3-aryl-4-isoxazolecarboxamides have been shown to activate TGR5 as well.61,62 These specific compounds demonstrated improved GLP-1 secretion after intraluminal administration in the colon of dogs together with a concomitant glucose challenge.61 Another class of compounds is the class of specific 2-aryl-3-aminomethylquinones. These molecules are demonstrated to activate TGR5, increase GLP-1 secretion, and increase glucose clearance in diet-induced obese mice.62
Other synthetic TGR5 agonists include 6-methyl-2-oxo-4-thiophen-2-yl-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid benzyl ester and (S)-1-(6-fluoro-2-methyl-3,4-dihydroquinolin-1[2H]-yl)-2-(isoquinolin-5-yloxy)ethanone, although, follow-up on their biological activity has never been published.63 Another group of TGR5 agonists are the 5-phenoxy-1,3-dimethyl-1H-pyrazole-4-carboxamides.64
The fluoroquinolone, ciprofloxacin, has also been shown to activate TGR5.65 Before its discovery as a TGR5 agonist, ciprofloxacin was already known to increase cAMP in monocytes and macrophages, and to counterregulate cytokine production.66 Cipriani et al65 demonstrated that ciprofloxacin functions as a TGR5 agonist, as determined by in silico screening, docking calculation, and in vitro experiments.
Finally, the TGR5 agonist, XL-475, has been reported to beneficially alter blood glucose, glucose tolerance, hepatic steatosis, and hepatic and plasma lipid levels in animal models.14 XL-475 was designed to selectively target the intestines, which could limit potential adverse effects by preventing systemic exposure.14
In summary, the interest in TGR5 as a target receptor has led to the identification and synthesis of several compounds. Many of these compounds did not advance to clinical application.14 However, much research is currently ongoing, and clinical trials have started to test XL-475 for its benefit in preventing diabetes in humans, as reviewed earlier.14
TGR5 in the immune response
TGR5 function in monocytes and macrophages
Upon its initial discovery as a BA responsive receptor, Kawamata et al3 already linked TGR5 to the regulation of immune responses. In this study, high TGR5 expression was demonstrated in human monocytes, as well as in rabbit alveolar macrophages.3 Tumor necrosis factor (TNF)α secretion of human monocytic THP-1 cells overexpressing TGR5 was inhibited by several BA species and correlated to cAMP levels – an effect not observed in control THP-1 cells.3 Concordantly, TGR5 is expressed in rat Kupffer cells (resident liver macrophages), which show a decrease in cytokine production in response to TGR5 agonist exposure.67 To prove the physiological relevance and contribution of TGR5 in this response, primary mouse macrophages were isolated from Tgr5+/+ and Tgr5−/− mice. It was demonstrated that, in contrast to Tgr5+/+ macrophages, Tgr5−/− macrophages were deficient in inducing cAMP or a calcium flux upon treatment with the TGR5-specific agonist, INT-777 (see the section titled, “Semisynthetic bile acids as TGR5 agonists”).5 Furthermore, Tgr5−/− macrophages showed an increased production of cytokines, thereby further establishing the role of TGR5 in regulating immune responses in macrophages (see Figure 2A), an effect confirmed in the different mouse macrophage subsets by different research groups.5,65,68 Additionally, human macrophages express TGR5 and show a similar inhibition of cytokine production upon treatment with compounds that are able to stimulate TGR5.69–71
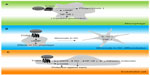  | Figure 2 Simplified drawing of proposed action of TGR5 in cells relevant to the immune response. |
A number of different downstream signaling routes have been proposed to contribute to the antiinflammatory properties of TGR5, which seem to converge on the inhibition of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB).9 The precise contributions of these pathways have not been investigated.
TGR5 in dendritic cells
A recent study addressed the role of TGR5 during the differentiation of monocytes into DCs in vitro.72 In this study, it was confirmed that TGR5 mRNA levels are high in monocytes, consistent with previous findings.3 Furthermore, monocytes exposed to BAs showed activation of the CREB (cAMP response element-binding protein), indicating functional TGR5 downstream signaling.72 Upon the differentiation of monocytes into DCs by granulocyte-macrophage colony-stimulating factor and interleukin (IL)-4, TGR5 gradually decreases but remains detectable.72 Differentiation of DCs in the presence of a TGR5 compound results in DCs that are less reactive, as measured by low IL-12 and TNFα expression (see Figure 2B). DCs are able to regulate inflammation by interacting with T-cells and by secreting several cytokines.72 These findings indicate that TGR5 affects the adaptive (specific) immune response by modulating DC function.
TGR5 in endothelial cells
ECs form the lining between blood and tissue. These cells play a major role in inflammation, as they are involved in the trafficking of leukocytes to sites of inflammation via adhesion molecule expression. TGR5 expression has been investigated in ECs, and it was found that the receptor was present in several EC subsets, including rat sinusoidal ECs and bovine aortic ECs.73,74 Also, human ECs were shown to express TGR5, including ECs derived from human umbilical cord, the pulmonary artery, and the dermal microvasculature.73,74 In ECs, TGR5 was demonstrated to regulate phosphorylation of endothelial nitric oxide (NO) synthase (eNOS), resulting in the production of NO.73,74 The NO produced has been proposed to subsequently inhibit adhesion molecule expression and monocyte adhesion by the inhibition of NF-κB (see Figure 2C).74 NO also protects ECs from oxidative stress which, together with the demonstration that TGR5 induces cluster of differentiation (CD)95 phosphorylation in ECs, acts in an antiapoptotic manner and protects ECs from damage (see Figure 2C).73,74 These observations are in line with the notion that TGR5 is suggested to protect against gastric and intestinal injury caused by acetylsalicylic acid and nonsteroidal antiinflammatory drugs via eNOS enzymes.75 In addition, NO plays a role in vasodilation, which could suggest that TGR5 plays a beneficial role in the regulation of blood pressure. This has been addressed by Fryer et al,76 who found that activation of TGR5 using a novel synthetic compound induces vasodilatation in dogs without changes in NO production, but by activation of the large-conductance Ca2+-activated potassium channel, KCa1.1. Lastly, TGR5 has been suggested to improve EC barrier function via cAMP–protein kinase A/Rac1-dependent cytoskeletal rearrangement.77 Thus, TGR5 has several actions in ECs, including regulating eNOS activity, modulating adhesion molecule expression, and protecting them from cell death.
Clinical potential of TGR5 activation in inflammatory disease
The common denominator of diseases like atherosclerosis, colitis, hepatic disease, and the metabolic syndrome is inflammation.78 As detailed in the section titled, “TGR5 in the immune response”, activation of TGR5 has been shown to dampen the inflammatory response.5,29,68
Atherosclerosis
The expression of functional TGR5 in immune cells could suggest that TGR5 activation may play a protective role against atherosclerosis and coronary artery disease (CAD). Pols et al5 showed that INT-777 lowered proinflammatory cytokine production via cAMP signaling and NF-κB inhibition and, as a consequence, decreased atherosclerosis in atherosclerosis-susceptible Ldlr−/−Tgr5+/+ mice, but not in Ldlr−/−Tgr5−/− double-knockout mice. The authors found that INT-777 treatment resulted in less plaque formation, less inflammation, and less macrophage infiltration. Furthermore, INT-777 did not show effects on atherosclerosis in mice with leukocyte-specific deletion of Tgr5, whereas mice that normally express TGR5 in leukocytes did reveal an antiatherogenic effect of INT-777. The latter finding emphasized the role of TGR5 in leukocytes in the antiatherogenic effects of INT-777. This concept has yet to be proven in humans. Of interest, Steiner et al79 showed that elevated secondary BA levels were associated with protection against CAD in humans. In support of this, Duboc et al80 demonstrated that subjects with CAD have lower levels of the secondary BA, LCA. LCA has a high affinity for the TGR5 receptor, possibly suggesting a protective role for TGR5 in human CAD.40
Colitis
TGR5 has been suggested to play a role in the integrity of the colon.65 The receptor is expressed in enteric ganglia, the muscolaris externa, and the muscolaris mucosa, in enterocytes and in mononuclear cells migrated into the lamina propria.65 Cipriani et al65 showed that Tgr5−/− mice have altered gut architecture with lower colon cellularity and crypt distortion compared to wild-type mice. Moreover, Tgr5−/− animals had worse dextran sodium sulfate-induced colitis with higher colonic macroscopic injury scores, more severe intestinal permeability, and diarrhea. The fluoroquinolone, ciprofloxacin, may be of special interest in the case of inflammatory colitis because of both its antibiotic and TGR5 agonistic properties.65 Additionally, Yoneno et al70 showed that macrophage colony-stimulating factor and interferon-γ-stimulated macrophages expressed more TGR5 compared to other types of differentiated macrophages. Also, lamina propria mononuclear cells from Crohn’s disease patients expressed more TGR5 compared to controls. In support of this, Fryer et al76 found that rats showed faster recovery of 2,4-dinotrobenzene sulfonic acid-induced colitis when cotreated with a TGR5 agonist. This was demonstrated by lower colon weight and lower overall damage. Thus, TGR5 is protective in colitis-related diseases via the inhibition of inflammatory responses and by improving intestinal barrier function.
Liver diseases
Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis are part of the metabolic syndrome and are characterized by an inflammatory response.78 Moreover, these conditions may progress to liver cirrhosis. Kupffer cells may play an important role in nonalcoholic fatty liver disease.81 Wang et al68 showed that Tgr5−/− mouse macrophages, Kupffer cells, and livers have more NF-κB activation in both a quiescent and activated state after lipopolysaccharide (LPS), as compared to control mice. Furthermore, the higher expression of NF-κB-regulated proinflammatory genes, such as monocyte chemo-attractant protein-1 and interferon-γ, was observed in the immune cells and livers of Tgr5−/− mice as compared to controls.68 Immunohistochemistry staining also showed elevated Kupffer cell numbers in Tgr5−/− mice compared to Tgr5+/+ mice, even in the absence of LPS. Moreover, treatment of animals with the TGR5 ligand, 23(S)-mCDCA, a specific semisynthetic TGR5 agonist, lowered these responses in Tgr5+/+ mice, but not in the Tgr5−/− animals.68,82 McMahan et al83 treated obese db/db mice with a dual FXR/TGR5 agonist (INT-767) and found improved nonalcoholic steatohepatitis with increased intrahepatic antiinflammatory monocytes and a gene expression profile indicating alternative macrophage activation, thus pointing to an antiinflammatory response.
Cholestasis is another area in which TGR5 targeting may be beneficial. Keitel et al67 showed that TGR5 is present in isolated Kupffer cells, and that these cells are responsive to BAs. Subsequently, BAs lowered LPS-induced cytokines via the established TGR5–cAMP pathway. TGR5 immunoreactivity in rat liver Kupffer cells increased after bile duct ligation, pointing to a possible protective role in obstructive cholestasis.67
After a partial hepatectomy, Tgr5−/− mice showed severe hepatocyte necrosis, cholestasis, a severe inflammatory response, and delayed regeneration in contrast to control mice.84 Comparable severe liver injury was observed after bile duct ligation, which was worse in Tgr5−/− animals compared to controls.84 It was proposed that TGR5 mediates these effects via the control of bile hydrophobicity and via inhibition of cytokine secretion of IL-1β, IL-6, and TNFα.84
Adverse effects of TGR5 ligands
As discussed in the section titled, “Physiological expression and function of TGR5”, TGR5 is expressed in several organs such as the gut, liver, immune cells, kidney, spleen, brown adipose tissue, and the nervous system. This is important because targeting TGR5 for a specific diseased target site will result in TGR5 activation in other tissues as well.
Stimulation of TGR5 is demonstrated to result in modulated gastrointestinal motility.33,85 Poole et al33 showed that DCA lowered spontaneous phasic activity in segments of the ileum and colon, and it delayed gastric emptying and small intestinal transit. TGR5 slows down gastric motility, as shown by the treatment of gastric smooth muscle cells with OA, resulting in increased cAMP levels and relaxation of the carbachol-induced contraction of gastric muscle cells in wild-type, but not Tgr5−/−, animals.86 Interestingly, another study indicated that Tgr5−/− mice had slower colon and whole-gut transit, indicating that TGR5 agonism reduced peristalsis in the colon.85 To what extent TGR5 agonism results in nausea or constipation in humans remains to be elucidated.
Limited data indicate that TGR5 agonism may affect the incidence of biliary stones (see the section titled, “Physiological role of TGR5 in BA homeostasis”). TGR5 is expressed in the gallbladder and may play a role in gallbladder emptying.87 Tgr5−/− mice are protected from the development of gallstones when placed on a high-fat lithogenic diet.27 This may be due to a reduced BA pool size or reduced CYP7a1 activity that reduces biliary cholesterol accumulation.27 TGR5 ribonucleic acid (RNA) expression in human gallbladders has been shown to correlate with the incidence of gallstones, but this relationship was not found for the TGR5 protein.24 Although the above data suggest a role for TGR5 in gallbladder physiology, the causal link to gallstones may be premature.
Another side effect that has been proposed is itching (pruritus).35 This was observed with the TGR5-selective agonist, OA, which induced hyperexcitability of the dorsal root ganglia neurons and stimulated the release of the itch-related transmitters, gastrin-releasing peptide and leucine-enkephalin.35 Intradermal injection of the same agonist induced scratching behavior in Tgr5+/+ mice, but not in Tgr5−/− mice.35
The infusion of TGR5 agonists reduces vascular tone and blood pressure in dogs, but not in rats, combined with reflex tachycardia and an inotropic response, resulting in enhanced cardiac output.76 These effects were proposed to be mediated through the activation of the large-conductance Ca2+-activated potassium channel, KCa1.1.76
Finally, some very limited in vitro evidence could suggest that TGR5 agonism induces (cancer) cell proliferation. Casaburi et al88 suggested the involvement of TGR5 in endometrial cancer risk in obesity/type 2 diabetes. They used an in vitro approach in which low concentrations of CDCA stimulated the growth of an endometrial cancer cell line via a TGR5-dependent pathway. Perhaps more relevant is the finding of Yasuda et al,21 who demonstrated that TGR5 signaling may be implicated in gastrointestinal tract cell carcinogenesis via the EGFR–extracellular signal-regulated kinase 1/2 pathway. This pathway was activated in human gastric carcinoma cells by BAs. Here, small interfering RNAs that target TGR5 mRNA suppressed phosphorylation of EGFR thereby inhibiting BA-induced activation. Additionally, TGR5 is proposed to increase in vitro cell growth of FLO cancer cells and AGS gastric adenocarcinoma cells.17,18 It has also been observed that the methylation of TGR5 DNA is a hallmark of hepatitis B virus-associated hepatocellular carcinoma in humans.89
Although these possible side effects of TGR5 agonists in development should be taken into consideration, all data are based on animal and in vitro studies. While these preclinical models provide important clues, clear clinical evidence of the side effects of therapeutic TGR5 targeting is very limited at this point in time.
Future research required for therapeutic TGR5 ligands
As outlined in the previous sections, TGR5 may be a promising receptor in the adjunct treatment of a number of diseases, including inflammatory disorders. With regard to future research, it should be underlined that many actions of TGR5 in different cells are not fully resolved or described. Studies elucidating the biological actions of TGR5 and its potential adverse effects are crucial, especially with the purpose of clinical application. Additionally, more translational and human studies investigating TGR5 will provide essential information regarding therapeutic ligands. An important step is to define the therapeutic window in which TGR5 activation is an effective treatment without resulting in adverse effects. So far, this has been done in one animal study that failed to show a therapeutic window between the inhibition of colitis and the TGR5 agonist’s effects on vasodilation.76
Depending on the potential adverse effects of TGR5 signaling in humans, a number of approaches can be undertaken to minimize adverse effects. Three options are worth investigating, which include: 1) the biochemical properties of the ligand; 2) biased ligands; and 3) tissue-specific drug delivery, as will be discussed.
Firstly, a number of different synthetic agonists have been described that have different scaffolds.14 These agonists differ in tissue distribution and metabolism, which will result in different properties. One example of such an agonist is XL-475 (discussed in the section titled, “Other TGR5 agonists”), which localizes to the intestine, thereby limiting systemic exposure.14 This could potentially increase the therapeutic window. Other compounds that could have a beneficial tissue distribution are specific semisynthetic BA derivatives (see the section titled, “Semisynthetic bile acids as TGR5 agonists”), which cycle in the enterohepatic circulation and are consequently enriched in areas such as the colon and the liver.90 If these latter tissues are desired target areas, these compounds could also have less adverse effects and consequently have a larger therapeutic window, as compared to TGR5 agonists that are also present throughout the body.
Secondly, a ligand of TGR5 can be designed in such a manner that downstream signaling is biased, which will result in a TGR5 agonist that has more specificity toward the desired signaling pathway.91 This can potentially lead to a TGR5 ligand that discriminates between therapeutic and adverse effects, thereby enhancing the therapeutic window.
Finally, one could opt to target TGR5 in a tissue-specific manner, which could include a vehicle that directs TGR5 ligands to specific cells and tissues. Examples of these are liposomes, micelles, and biodegradable microparticles.92 Via these vehicles, the TGR5 compound can be specifically routed to the desired sites and consequently reduce adverse effects in other tissues.
Conclusion
TGR5 is a G protein-coupled BA receptor present in a number of tissues and cell types throughout the body. The receptor functions in several processes, including metabolism and energy homeostasis. More recently, the role of TGR5 in suppressing immune responses has been highlighted, as outlined in this review. TGR5 dampens immune responses via different routes in immune-relevant cell types such as macrophages, DCs, as well as ECs. In line with these notions, TGR5 is involved in inflammatory diseases such as atherosclerosis, colitis, and liver diseases, and it could present an attractive target for therapeutic use. Next to its natural BA ligands, several semisynthetic agonists have been found that can activate TGR5 and subsequently exert positive effects on energy balance, glucose homeostasis, and inflammation. Further research is warranted to elucidate and map all actions of TGR5, especially in humans where information on TGR5 function is still scarce. Evidence indicates that TGR5 signaling may depend on the cell differentiation state and could differ between cell types. TGR5 downstream signaling should therefore be carefully dissected in immune cells, which could pave the way for biased signaling ligands to minimize potential adverse effects.
In our opinion, further studies, especially in humans, will be crucial to further validate TGR5. We are, however, optimistic that TGR5 is a promising target for adjunct intervention in inflammatory diseases. Innovative developments, such as biased signaling and tissue-specific drug targeting, could circumvent potential adverse effects of TGR5 targeting.
Acknowledgments
MRS is supported by a grant from the Dutch Diabetes Foundation (Diabetes Fonds, Ruby – grant 2011.80.1423). TWHP is supported by a personal grant from The Netherlands Organization of Scientific Research (NWO VENI – grant 016.136.083), as well as by a Follow-up Research Fund from the Federation of European Biochemical Societies (FEBS).
Disclosure
The authors report no conflicts of interest in this work.
References
Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7(8):678–693. | |
Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. | |
Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–9440. | |
Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298(5):714–719. | |
Pols TW, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14(6):747–757. | |
Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–390. | |
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. | |
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. | |
Pols TW. TGR5 in inflammation and cardiovascular disease. Biochem Soc Trans. 2014;42(2):244–249. | |
Sato H, Macchiarulo A, Thomas C, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51(6):1831–1841. | |
Iguchi Y, Yamaguchi M, Sato H, Kihira K, Nishimaki-Mogami T, Une M. Bile alcohols function as the ligands of membrane-type bile acid-activated G protein-coupled receptor. J Lipid Res. 2010;51(6):1432–1441. | |
Keitel V, Görg B, Bidmon HJ, et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58(15):1794–1805. | |
Ono E, Inoue J, Hashidume T, Shimizu M, Sato R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem Biophys Res Commun. 2011;410(3):677–681. | |
Gioiello A, Rosatelli E, Nuti R, Macchiarulo A, Pellicciari R. Patented TGR5 modulators: a review (2006 – present). Expert Opin Ther Pat. 2012;22(12):1399–1414. | |
Spomer L, Gertzen CG, Schmitz B, Häussinger D, Gohlke H, Keitel V. A membrane-proximal, C-terminal α-helix is required for plasma membrane localization and function of the G Protein-coupled receptor (GPCR) TGR5. J Biol Chem. 2014;289(6):3689–3702. | |
Jensen DD, Godfrey CB, Niklas C, et al. The bile acid receptor TGR5 does not interact with β-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. J Biol Chem. 2013;288(32):22942–22960. | |
Hong J, Behar J, Wands J, et al. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut. 2010;59(2):170–180. | |
Cao W, Tian W, Hong J, et al. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G322–G327. | |
Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. NatRev Mol Cell Biol. 2002;3:639–650. | |
Masyuk AI, Huang BQ, Radtke BN, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304(11):G1013–G1024. | |
Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354(1):154–159. | |
Yang JI, Yoon JH, Myung SJ, et al. Bile acid-induced TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced caspase 8 activation in hepatocytes. Biochem Biophys Res Commun. 2007;361(1):156–161. | |
Hov JR, Keitel V, Laerdahl JK, et al; IBSEN Study Group. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5(8):e12403. | |
Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Häussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50(3):861–870. | |
Vassileva G, Golovko A, Markowitz L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398(3):423–430. | |
Lavoie B, Balemba OB, Godfrey C, et al. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol. 2010;588(Pt 17):3295–3305. | |
Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25(6):1066–1071. | |
Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191(1):197–205. | |
Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54(6):1263–1272. | |
Harach T, Pols TW, Nomura M, et al. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep. 2012;2:430. | |
Potthoff MJ, Potts A, He T, et al. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G371–G380. | |
Ockenga J, Valentini L, Schuetz T, et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab. 2012;97(2):535–542. | |
Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22(7):814–825, e227. | |
Camilleri M, Vazquez-Roque MI, Carlson P, Burton D, Wong BS, Zinsmeister AR. Association of bile acid receptor TGR5 variation and transit in health and lower functional gastrointestinal disorders. Neurogastroenterol Motil. 2011;23(11):995–999, e458. | |
Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123(4):1513–1530. | |
Ogundare M, Theofilopoulos S, Lockhart A, et al. Cerebrospinal fluid steroidomics: are bioactive bile acids present in brain? J Biol Chem. 2010;285(7):4666–4679. | |
Mano N, Goto T, Uchida M, et al. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J Lipid Res. 2004;45(2):295–300. | |
Kitazawa T, Terasaki T, Suzuki H, Kakee A, Sugiyama Y. Efflux of taurocholic acid across the blood-brain barrier: interaction with cyclic peptides. J Pharmacol Exp Ther. 1998;286(2):890–895. | |
Parry GJ, Rodrigues CM, Aranha MM, et al. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol. 2010;33(1):17–21. | |
Sato H, Genet C, Strehle A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362(4):793–798. | |
Macchiarulo A, Gioiello A, Thomas C, et al. Molecular field analysis and 3D-quantitative structure-activity relationship study (MFA 3D-QSAR) unveil novel features of bile acid recognition at TGR5. J Chem Inf Model. 2008;48(9):1792–1801. | |
Macchiarulo A, Gioiello A, Thomas C, et al. Probing the binding site of bile acids in TGR5. ACS Med Chem Lett. 2013;4(12):1158–1162. | |
Rizzo G, Passeri D, De Franco F, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78(4):617–630. | |
Pellicciari R, Gioiello A, Macchiarulo A, et al. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52(24):7958–7961. | |
Rizzo G, Disante M, Mencarelli A, et al. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol Pharmacol. 2006;70(4):1164–1173. | |
Pellicciari R, Gioiello A, Sabbatini P, et al. Avicholic acid: a lead compound from birds on the route to potent TGR5 modulators. ACS Med Chem Lett. 2012;3(4):273–277. | |
Kumar DP, Rajagopal S, Mahavadi S, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427(3):600–605. | |
Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil. 2013;25(8):708–711. | |
Iguchi Y, Nishimaki-Mogami T, Yamaguchi M, Teraoka F, Kaneko T, Une M. Effects of chemical modification of ursodeoxycholic acid on TGR5 activation. Biol Pharm Bull. 2011;34(1):1–7. | |
Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7(5):357–369. | |
Genet C, Strehle A, Schmidt C, et al. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem. 2010;53(1):178–190. | |
Inoue T, Wang JH, Higashiyama M, et al. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G810–G816. | |
Yoon JJ, Lee YJ, Kim JS, Kang DG, Lee HS. Betulinic acid inhibits high glucose-induced vascular smooth muscle cells proliferation and migration. J Cell Biochem. 2010;111(6):1501–1511. | |
de Melo CL, Queiroz MG, Arruda Filho AC, et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J Agric Food Chem. 2009;57(19):8776–8781. | |
Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). Journal of agricultural and food chemistry. 2006;54(1):243–248. | |
Jang SM, Kim MJ, Choi MS, Kwon EY, Lee MK. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism. 2010;59(4):512–519. | |
Kunkel SD, Elmore CJ, Bongers KS, et al. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One. 2012;7(6):e39332. | |
Rao VS, de Melo CL, Queiroz MG, et al. Ursolic acid, a pentacyclic triterpene from Sambucus australis, prevents abdominal adiposity in mice fed a high-fat diet. J Med Food. 2011;14(11):1375–1382. | |
Hodge RJ, Lin J, Vasist Johnson LS, Gould EP, Bowers GD, Nunez DJ; SB-756050 Project Team. Safety, pharmacokinetics, and pharmacodynamic effects of a selective TGR5 agonist, SB-756050, in type 2 diabetes. Clinical Pharmacology in Drug Development. 2013;2(3):213–222. | |
Zambad SP, Tuli D, Mathur A, et al. TRC210258, a novel TGR5 agonist, reduces glycemic and dyslipidemic cardiovascular risk in animal models of diabesity. Diabetes Metab Syndr Obes. 2013;7:1–14. | |
Evans KA, Budzik BW, Ross SA, et al. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J Med Chem. 2009;52(24):7962–7965. | |
Herbert MR, Siegel DL, Staszewski L, et al. Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg Med Chem Lett. 2010;20(19):5718–5721. | |
Eng H, Niosi M, McDonald TS, et al. Utility of the carboxylesterase inhibitor bis-para-nitrophenylphosphate (BNPP) in the plasma unbound fraction determination for a hydrolytically unstable amide derivative and agonist of the TGR5 receptor. Xenobiotica. 2010;40(6):369–380. | |
Londregan AT, Piotrowski DW, Futatsugi K, et al. Discovery of 5-phenoxy-1,3-dimethyl-1H-pyrazole-4-carboxamides as potent agonists of TGR5 via sequential combinatorial libraries. Bioorg Med Chem Lett. 2013;23(5):1407–1411. | |
Cipriani S, Mencarelli A, Chini MG, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6(10):e25637. | |
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. | |
Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372(1):78–84. | |
Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54(4):1421–1432. | |
Haselow K, Bode JG, Wammers M, et al. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J Leukoc Biol. 2013;94(6):1253–1264. | |
Yoneno K, Hisamatsu T, Shimamura K, et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology. 2013;139(1):19–29. | |
Keitel V, Spomer L, Marin JJ, et al. Effect of maternal cholestasis on TGR5 expression in human and rat placenta at term. Placenta. 2013;34(9):810–816. | |
Ichikawa R, Takayama T, Yoneno K, et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology. 2012;136(2):153–162. | |
Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45(3):695–704. | |
Kida T, Tsubosaka Y, Hori M, Ozaki H, Murata T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1663–1669. | |
Cipriani S, Mencarelli A, Bruno A, et al. Activation of the bile acid receptor GPBAR1 protects against gastrointestinal injury caused by non-steroidal anti-inflammatory drugs and aspirin in mice. Br J Pharmacol. 2013;168(1):225–237. | |
Fryer RM, Ng KJ, Nodop Mazurek SG, et al. G protein-coupled bile acid receptor 1 stimulation mediates arterial vasodilation through a K(Ca)1.1 (BK(Ca))-dependent mechanism. J Pharmacol Exp Ther. 2014;348(3):421–431. | |
Kida T, Omori K, Hori M, Ozaki H, Murata T. Stimulation of G protein-coupled bile acid receptor enhances vascular endothelial barrier function via activation of protein kinase A and Rac1. J Pharmacol Exp Ther. 2014;348(1):125–130. | |
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. Epub 2014 Apr 13. | |
Steiner C, Othman A, Saely CH, et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS One. 2011;6(11):e25006. | |
Duboc H, Aelion H, Rainteau D, et al. Crosstalk between the hepatologist and the cardiologist: a future place for the lithocholic acid as a coronary atheroma risk factor? Hepatology. 2012;56(6):2426. | |
Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51(1):212–223. | |
Pellicciari R, Sato H, Gioiello A, et al. Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J Med Chem. 2007;50(18):4265–4268. | |
McMahan RH, Wang XX, Cheng LL, et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem. 2013;288(17):11761–11770. | |
Péan N, Doignon I, Garcin I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58(4):1451–1460. | |
Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144(1):145–154. | |
Rajagopal S, Kumar DP, Mahavadi S, et al. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am J Physiol Gastrointest Liver Physiol. 2013;304(5):G527–G535. | |
Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Häussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50(3):861–870. | |
Casaburi I, Avena P, Lanzino M, et al. Chenodeoxycholic acid through a TGR5-dependent CREB signaling activation enhances cyclin D1 expression and promotes human endometrial cancer cell proliferation. Cell Cycle. 2012;11(14):2699–2710. | |
Han LY, Fan YC, Mu NN, et al. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 (TGR5) is a potential biomarker for hepatitis B virus associated hepatocellular carcinoma. Int J Med Sci. 2014;11(2):164–171. | |
Gioiello A, Rosatelli E, Nuti R, Macchiarulo A, Pellicciari R. Patented TGR5 modulators: a review (2006 - present). Expert Opin Ther Pat. 2012;22(12):1–16. | |
Liu JJ, Horst R, Katritch V, Stevens RC, Wüthrich K. Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335(6072):1106–1110. | |
Tiwari G, Tiwari R, Sriwastawa B, et al. Drug delivery systems: an updated review. Int J Pharm Investig. 2012;2(1):2–11. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.