Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
TET2 Promotes Keloid Hyperplasia by Regulating 5hmC Modification in the TGFβ Promoter Region
Received 22 February 2023
Accepted for publication 12 April 2023
Published 21 April 2023 Volume 2023:16 Pages 1063—1070
DOI https://doi.org/10.2147/CCID.S409621
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Changying Niu,1 Shenxing Tan2
1Dermatological Department, Affiliated Hospital of Weifang Medical University, Weifang, People’s Republic of China; 2Plastic Surgery, Affiliated Hospital of Weifang Medical University, Weifang, People’s Republic of China
Correspondence: Shenxing Tan, Tel +8618754411279, Email [email protected]
Introduction: As a kind of human unique benign skin tumour, keloid has caused great trouble to the physical and mental health of patients and is unfavourable for beautiful. The abnormal proliferation of fibroblasts is one of the main causes of keloid formation. TET2 (Ten eleven translocation 2) catalyzes the oxidation of cytosine 5mC to 5hmC which process plays important role in cell proliferation. However, the molecular mechanism of TET2 in keloids is not well-researched.
Methods: qPCR was used to detect the mRNA levels and Western blot was used to detect the protein level. DNA Dot blot was used to detect the level of 5hmC. CCK8 was used to examine the cell proliferation rate. EDU/DAPI staining was used to evaluate the living cells’ proliferation rate. DNA IP and PCR were used to detect the accumulation of DNA at the target site after 5hmC enrichment.
Results: We found that TET2 was highly expressed in keloid tissue. Interestingly, TET2 expression was increased in fibroblasts that were isolated and cultured in vitro compared to the tissue of origin. Knocking down TET2 expression can effectively decrease the modification level of 5hmC and inhibit the proliferation of fibroblasts. Notably, overexpression of DNMT3A inhibited fibroblast proliferation by decreasing 5hmC. The 5hmC-IP assay showed that TET2 could affect the expression of TGFβ by regulating the 5hmC modification level in the promoter region. And by this way, TET2 regulates the proliferation of fibroblasts.
Conclusion: This study found new epigenetic mechanisms for keloid formation.
Keywords: keloid, TET2, 5hmC, TGFβ
Introduction
Keloid is a benign fibroproliferative tumour of the skin that is unique to humans. It usually occurs after trauma and is caused by abnormal wound healing.1 Keloid formation in this pathological state is usually characterized by fibroblast hyperplasia and abnormal deposition of collagen fibres.2 Although keloids are benign tumours, they can cause deformities and induce skin dysfunction by invading adjacent normal tissues.3,4 Keloid is often accompanied by severe pain, pruritus, and even joint motor dysfunction, affecting the physical and mental health of patients. No treatment provides satisfactory results due to the high rate of recurrence after keloid treatment. Therefore, it is particularly urgent to explore the molecular mechanism of keloid formation and fibroblast dysplasia.
The TET (Ten eleven translocation) protein family has three members and is a kind of dioxygenase. TET2 can catalyze the oxidation of cytosine 5mC to 5hmC.5 The methylation and demethylation processes of cytosine are closely related to a variety of biological functions, such as embryonic stem cell development,6 neurogenesis,7 stem cell fate determination,8 etc. The occurrence and development of cancer are also accompanied by the demethylation of the promoter and enhancer regions of proto-oncogenes.9 Therefore, the abnormal expression of TETs and the abnormal modification of 5hmC has also become a new research hotspot of tumorigenesis.
The occurrence of keloid is largely caused by the abnormal proliferation of fibroblasts.2 TGFβ/Smad signalling pathway plays an important role in the abnormal proliferation of fibroblasts and the occurrence of keloid.10 Previous studies have shown that turning on or off of this pathway greatly affects the proliferation and differentiation process of fibroblasts.11 For example, LncRNA COLIA2-AS1 can regulate the proliferation of fibroblasts by adsorbing miRNA-21.12 Similarly, the knockdown of eIF3A and NLRC5 can also effectively increase the inhibitory state of the TGFβ/Smad signalling pathway, thereby inhibiting the proliferation of fibroblasts.13,14 All the proteins and RNAs mentioned above can be used as new therapeutic targets and drugs for the research and development of therapeutic methods.
In this study, we found that the TET2 protein was highly expressed in keloid tissue. TET2 is involved in the regulation of the TGFβ signalling pathway by regulating the 5hmC modification site and modification level. This study is the first to investigate the molecular mechanism of keloid from epigenetics. Furthermore, this study provides a new idea for the development of new therapeutic methods and drug research.
Materials
Tissue Samples
The keloid tissue samples were collected from 12 patients who underwent plastic surgery in the affiliated hospital of Weifang Medical University between January 2018 and December 2020. At the same time, normal skin tissue was obtained from 12 cases of circumcision. The age of all the patients are over 18. The study was approved by the affiliated hospital of Weifang medical university and all the patients signed the informed consent before the study. All the research procedures are conformed to the principle of the Declaration of Helsinki.15
Isolation and Culture of Primary Fibroblasts
According to the previous literature, human keloid fibroblasts (HDFs) and human dermal fibroblasts (HKFs) were isolated from the newly surgically resected keloid tissue and normal skin tissue by collagenase digestion method (Pandamooz et al, 2012). Primary fibroblasts culture with DMEM/F12 (Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA), penicillin and streptomycin (100 IU/mL) in 37 °C, 5% CO2 incubator. HDFS and HKFS could be used for further experiments after 5 passages.
Transfection
Lipo2000 (Invitrogen, USA) was diluted with 500μL Opti-Mem (Gibco, USA) and incubated at room temperature for 10 min. The above-mentioned mixed droplets were added into the fibroblasts with a confluence rate of 70%. The medium was replaced with a fresh medium after 24h. The sequence of ShTET2 is “CCTCAGAGATATTGTGGGTTT”.
Q-Pcr
1ug total RNA was extracted from tissue or cell samples with Trizol (Invitrogen, USA), extracted with chloroform, precipitated with isopropanol, and washed with ethanol. Let dry and dissolve in 200μL ddH2O. Subsequently, cDNA was reverse transcribed with a reverse transcription kit (Vizyme, China) and quantitative fluorescence detection was performed with an SYBR Green kit (Vizyme, China).
Dot Blot
Cell or tissue samples were beaten and mixed with lysate and then lysed at 56°C for 16 h after adding Proteinase K, followed by adding RNase A to remove RNA at 37°C. DNA was extracted with phenol: chloroform: isoamyl alcohol =25:24:1 and dissolved with ddH2O.
200 ng DNA was heated at 99°C for 10 min, and then quickly placed in an ice bath to cool for 10 min. DNA was dropped onto PVDF membrane (Millipore, USA), dried at 80°C for 30 min, and incubated overnight with 5hMC (Active motif, USA) primary antibody. The second day was incubated with HRP-labeled secondary antibodies at room temperature for 1h, and the chromatographic statistics were performed.
Western Blot
The cells or tissues were lysed in the ice bath with RIPA (Tiangen, China) for 30 min, and the supernatant was retained after centrifugation at 12000 rpm for 30 min. After boiling the supernatant for 5 minutes, SDS-PAGE was performed with a total of 30μg protein. The protein was then transferred to the PVDF membrane at 90V. After the primary antibody was incubated overnight, the secondary antibody with an HRP label was incubated and the colour was developed. Primary antibodies: mouse anti-TET2 antibody (Abcam, ab243323, Cambridge, MA, USA), Anti-GAPDH antibody (Abcam, ab125247, Cambridge, MA, USA), Anti-TGF beta 1 antibody (Abcam, ab215715, Cambridge, MA, USA). Anti-5-hydroxymethylcytosine (5-hmC) (Abcam, ab106918, Cambridge, MA, USA). Anti-5-methylcytosine (5-mC) (Abcam, ab10805, Cambridge, MA, USA).
Cck-8
Cells were seeded into 96-well plates with 3000 cells per well, and cultured for 36h with 100 μL CCK-8 solution (Tiangen, China). It was incubated in an incubator at 37°C for 2h. Then the enzyme plate analyzer was used for detection.
5 - Ethynyl - 2 ‘- Deoxyuridine Staining
Cells were plated on cell culture slides, and 5-acetyl-2 ‘-deoxyuridine (EDU) was added after 24h of culture. After 6 hours of incubation, DNA Proliferation In Vitro Detection Kit (Ruibo Biological Technology, China) was used for incubation. Then they were photographed and counted under a fluorescence microscope.
Immunoprecipitation (IP)
4μg of DNA was broken into about 400bp fragments by ultrasound. 5μg of 5hmC antibody (Active motif, USA) was added and incubated overnight at 4°C. Add 30μL Protein A Beads to the mixture and incubate for 6h at 4°C. Wash the Beads 3 times with IP Buffer, then elution the DNA and discard the beads. The eluted DNA was purified. Q-PCR was used to detect the accumulation of DNA at the target site after 5hmC enrichment.
Results
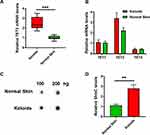
Tet2 is Highly Expressed in Keloid Tissue
We collected tissue samples from a total of 12 Keloid patients who had undergone plastic surgery in our hospital in the past two years. After the consent of the patient, we detected the mRNA level of TET2 in keloid tissue and we found TET2 is higher than that in the normal tissue (Figure 1A). However, the expression differences between TET1 and TET3 were not significant, and their expression levels were both lower than that of TET2 (Figure 1B). TETs promote the modification and oxidation of 5mC to 5hmC and could be detected by dot blotting. Dot blotting results showed that the level of 5hMC in keloid tissue is higher than in normal skin. (Figure 1C and D). Considering TET2 levels are higher in keloid tissue, 5hMC level is positively correlated with TET2 in skin tissue.
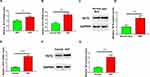
TET2 Expression Levels Increased in HDF and HKF Than in Their Isolated Tissues
We isolated human keloid fibroblasts (HKFs) and human dermal fibroblasts (HDFs) simultaneously. Q-PCR showed that the TET2 mRNA level in HKFs was slightly higher than that in HDFs (Figure 2A). Interestingly, the expression level of TET2 in HDFs cultured in vitro was higher than that in normal skin before isolation (Figure 2B–D). When we compared the expression level of HKF with keloids, the difference was more significant. (Figure 2E–G). Based on this phenomenon We hypothesized that TET2 in HKF may be involved in the regulation of cell proliferation.
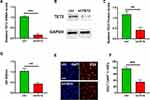
Low TET2 Expression Inhibits HKFs Proliferation
We constructed a TET2 knockdown plasmid named shTET2 and transfected it into HKFs. Figures 3A and B showed the level of TET2 in HKFs after shTET2 was transfected. CCK-8 assay was used to detect the proliferation of the cells 48 hours after transfection (Figure 3C). The experimental results showed that the low expression of TET2 could decrease the proliferation ability of HKFs (Figure 3D). Subsequently, we used EDU+/DAPI+ to evaluate the living cells’ proliferation rate. EDU incorporation experiment also showed that the EDU+/DAPI+ in the TET2 knockdown group was about 35.87%, which was much lower than that in the control group (78.23%, Figure 3E and F).
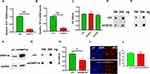
Low DNA 5hmC Modification Inhibits HKFs Proliferation and Was Recovered by TET2
We then knocked down the expressions of TET1 and TET3 respectively in HDFs (Figure 4A and B). However, no significant reduction in proliferation was detected by CCK8 (Figure 4C).
For checking the relation of 5hmc and TET2 in HKFs, TET2 was knocked down in the HKF. The level of 5hmC in the cells was significantly decreased (Figure 4D) and the level of 5mC was increased (Figure 4E). Overexpression of cytosine methylase DNMT3A (Figure 4F) in HKFs also resulted in the restricted proliferation of HKFs (Figure 4H) detected by CCK8 while increasing the level of 5mC (Figure 4G). Furthermore, when we over-expressed TET2 and in the DNMT3A over-expressed HKFs, the proliferation restriction was recovered (Figure 4I and J).
TET2 Regulates the 5hmC in the TGFβ Promoter Region
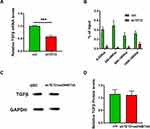
We also examined TGFβ expression, which affects the proliferation ability of keloid cells. Q-PCR results showed that TGFβ expression was inhibited when TET2 was knocked down, while other expressions were affected (Figure 5A). We then co-immunoprecipitated DNA with 5mC and 5hmC antibodies, respectively. The levels of methylation and hydroxymethylation in the −2000bp region of the TGFβ transcription start site (TSS) were detected. Q-PCR results showed that, after TET2 knockdown, the hydroxymethylation level of multiple sites in the TGFβ promoter region was decreased (Figure 5B). TGFβ expression level in DNMT3A over-expressed level HKFs was similar to the control when knockdown TET2 cells in it (Figure 5C and D). This proved that TET2 and DNMT3A regulate the TGFβ levels by methylation and hydroxymethylation at the TSS of TGFβ.
Discussion
As an important organ of the human body, the skin plays a wide role in protecting our health. However, when the skin was injured deeply, the keloid would appear and will also be unfavourable for the beauty of patients. The development of keloids is usually the result of the abnormal proliferation of fibroblasts after trauma or surgery.
In this study, 12 pairs of clinical samples were detected, and it was found that the expression level of TET2 was significantly higher than that of control tissues. More interestingly, TET2 expression was significantly increased in HDFs and HKFs when they were cultured in vitro. This strongly suggested that TET2 regulates keloid growth by the proliferation of HKFs. Similarly, previous studies have also found that TET2 is involved in the occurrence of breast cancer,16 glioma17 and other cancers. Furthermore, TET2 affects tumour formation by regulating the proliferation process of cancer cells.
One of the main functions of TET2 is to participate in the oxidation process of cytosine. When 5hmC is enriched in the promoter region of genes, it can promote gene expression by mediating the recognition and binding of transcription factors. In order to further explore how TET2 regulates keloid growth, we detected the function of TET2 on HKFs proliferation. We also regulated the expression of three TETs on HKF respectively. Interestingly, only TET2 knockdown significantly affected the proliferation of HKFs. Previous studies have also found that in the same tissue or cell, three TETs have different 5hmC modification sites and also play different biological functions.18 That’s why only TET2 has functions on HKFs proliferation. In order to verify the above conclusions, we also reduced the modification of 5hmC by overexpressing DNMT3A in HKFs. Proliferation experiments showed that both TET2 knockdown and DNMT3A overexpression affected the proliferation of HKFs. This also suggests that TET2 can affect proliferation by regulating the 5hmC modification of specific genes of HKFs.
After clearing the TET2 functions on the proliferation of HKFs, the next question is which gene would be the target of TET2. There are over 70 pathways in cells responsible for proliferation and TGFβ/Smad signalling pathway is the main pathway affecting the abnormal proliferation of HDFs.19,20 We found that low TET2 expression reduced TGFβ expression. The 5hmC-IP assay showed that TET2 could affect the 5hmC modification of the TGFβ promoter region. These results proved that TET2 affected the expression of TGFβ by regulating the oxidation process of cytosine in the promoter region. Furthermore, it affects the proliferation of HDFs and the formation of keloids.
This is the first epigenetics study to explore the molecular mechanisms of TET2 regulating keloids. TET2 and 5hmC modifications may serve as a new target for the development of therapeutic and diagnostic methods for keloid. However, this is a very preliminary work which still needs to be further explored. We need to further explore the further molecular mechanisms that TET2 regulates TGFβ expression, such as the binding of transcription factors and the activation of genes, and even the structural changes of heterochromatin. Also, in this work, we only explored the regulation of TETs on the TGFβ/Smad signalling pathway. Other proliferation pathways also need to be explored. But, answering these questions could lead to a variety of treatments for keloids.
Author Contributions
All authors made a significant contribution to the work reported, SXT contributed to the conception and study design. CYN contributed in the execution, acquisition of data, analysis and interpretation, SXT and CYN took part in drafting, revising or critically reviewing the article.
SXT and CYN gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Development Plan for Medical and Health Science and Technology Projects in Shandong Province (202204100932).
Disclosure
Both of the authors declared that there is no competing interest.
References
1. Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. The keloid disorder: heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. 2020;8:360. doi:10.3389/fcell.2020.00360
2. Rippa AL, Kalabusheva EP, Vorotelyak EA. Regeneration of dermis: scarring and cells involved. Cells. 2019;8(6):607. doi:10.3390/cells8060607
3. Berman B, Maderal A, Keloids RB. Hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–s18. doi:10.1097/DSS.0000000000000819
4. Har-Shai Y, Brown W, Labbé D, et al. Intralesional cryosurgery for the treatment of hypertrophic scars and keloids following aesthetic surgery: the results of a prospective observational study. Int J Low Extrem Wounds. 2008;7(3):169–175. doi:10.1177/1534734608322813
5. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–378. doi:10.1111/bjd.13954
6. Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333(6047):1300–1303. doi:10.1126/science.1210597
7. Blaschke K, Ebata KT, Karimi MM, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500(7461):222–226. doi:10.1038/nature12362
8. Seritrakul P, Gross JM. Tet-mediated DNA hydroxymethylation regulates retinal neurogenesis by modulating cell-extrinsic signaling pathways. PLoS Genet. 2017;13(9):e1006987–e1006987. doi:10.1371/journal.pgen.1006987
9. Sardina JL, Collombet S, Tian TV, et al. Transcription factors drive Tet2-mediated enhancer demethylation to reprogram cell fate. Cell Stem Cell. 2018;23(5):727–741.e729. doi:10.1016/j.stem.2018.08.016
10. Rodríguez-Aguilera JR, Ecsedi S, Goldsmith C, et al. Genome-wide 5-hydroxymethylcytosine (5hmC) emerges at early stage of in vitro differentiation of a putative hepatocyte progenitor. Sci Rep. 2020;10(1):7822. doi:10.1038/s41598-020-64700-2
11. Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24(2):215–222. doi:10.1111/wrr.12398
12. Nong Q, Li S, Wu Y, LncRNA LD. COL1A2-AS1 inhibits the scar fibroblasts proliferation via regulating miR-21/Smad7 pathway. Biochem Biophys Res Commun. 2018;495(1):319–324. doi:10.1016/j.bbrc.2017.11.027
13. Zhang Y-F, Wang Q, Luo J, Yang S, Wang J-L, Li H-Y. Knockdown of elF3a inhibits collagen synthesis in renal fibroblasts via Inhibition of transforming growth factor-β1/Smad signaling pathway. Int J Clin Exp Pathol. 2015;8(8):8983–8989.
14. Luan P, Jian W, Xu X, et al. NLRC5 inhibits neointima formation following vascular injury and directly interacts with PPARγ. Nat Commun. 2019;10(1):2882. doi:10.1038/s41467-019-10784-y
15. Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
16. Zhang Z, Jin Y, Zhang W, et al. Values of 5mC, 5hmC, and TET2 for identifying the presence and progression of breast precancerous lesion. J Clin Lab Anal. 2020;34(5):e23162–e23162.
17. Chen B, Lei Y, Wang H, et al. Repression of the expression of TET2 by ZEB1 contributes to invasion and growth in glioma cells. Mol Med Rep. 2017;15(5):2625–2632. doi:10.3892/mmr.2017.6288
18. Melamed P, Yosefzon Y, David C, Tsukerman A, Pnueli L. Tet enzymes, variants, and differential effects on function. Front Cell Dev Biol. 2018;6:22. doi:10.3389/fcell.2018.00022
19. Ren Y, Song Z, Rieser J, et al. USP15 represses hepatocellular carcinoma progression by regulation of pathways of cell proliferation and cell migration: a system biology analysis. Cancers. 2023;15(5):1371. doi:10.3390/cancers15051371
20. Murphy-Marshman H, Quensel K, Shi-Wen X, et al. Antioxidants and NOX1/NOX4 inhibition blocks TGFβ1-induced CCN2 and α-SMA expression in dermal and gingival fibroblasts. PLoS One. 2017;12(10):e0186740. doi:10.1371/journal.pone.0186740
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.