Back to Journals » Open Access Emergency Medicine » Volume 12
Testicular Torsion in the Emergency Room: A Review of Detection and Management Strategies
Authors Laher A , Ragavan S , Mehta P , Adam A
Received 3 April 2020
Accepted for publication 21 September 2020
Published 12 October 2020 Volume 2020:12 Pages 237—246
DOI https://doi.org/10.2147/OAEM.S236767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Hans-Christoph Pape
Abdullah Laher,1 Shavania Ragavan,2 Puja Mehta,2 Ahmed Adam2
1Department of Emergency Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 2Division of Urology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Correspondence: Abdullah Laher
Department of Emergency Medicine, Faculty of Health Sciences, University of the Witwatersrand, 7 Jubilee Road, Parktown, Johannesburg 2193, South Africa
Tel +27 84 840 2508
Email [email protected]
Abstract: Testicular torsion is a challenging and time-sensitive diagnosis that is encountered frequently in daily practice, especially in the emergency room. A thorough history, the presence of a painful and swollen testis and testicular ultrasonography plays a vital role in the prompt diagnosis of testicular torsion. Prompt diagnosis is essential to prevent complications of testicular torsion which include testicular infarction, necrosis, and sub/infertility. This can be challenging as there are various other conditions that may mimic the presentation of testicular torsion. Since testicular torsion is an extremely time-sensitive diagnosis, it may also be a subject of many medicolegal challenges. This review article serves as a guide for clinicians involved with the diagnosis and management of testicular torsion. We review and discuss detection and management strategies based on their validity, statistical significance, and effectiveness in enabling prompt diagnosis and management of testicular torsion. Medicolegal implications of testicular torsion are also highlighted.
Keywords: testis torsion, scrotal pain, acute scrotum, emergency room, orchidectomy
Introduction
Testicular torsion is an urological emergency necessitating emergent intervention. It occurs when the testicle rotates around its spermatic cord leading to impaired blood supply and possible permanent ischemic testicular damage.1 The severity of ischemia varies and is dependent on the time period of torsion as well as the extent of rotation of the cord.2
Testicular torsion can occur at any age but commonly occurs soon after birth or between the ages of 12–18 years with a peak in incidence at age 13–14 years. The incidence of torsion in males below the age of 25 years is approximately 1 in 4000.3 A study based in the USA reported that testicular torsion was diagnosed in 10–15% of pediatric patients presenting with acute scrotal pain and an orchidectomy was performed on 42% of patients undergoing scrotal exploration for testicular torsion. The study also noted that the time interval for the development of ischemia after the onset of testicular torsion was 4–8 hours.4
The early recognition of testicular torsion has been associated with an increase in the rates of testicular salvage and the prevention of complications such as testicular infarction and infertility.3 By analyzing the available literature, this review article discusses predisposing factors, patient presentation, detection strategies including predictive scoring, differential diagnosis, diagnostic modalities, management options and medicolegal aspects pertaining to patients presenting with suspected testicular torsion. The role, effectiveness, and statistical significance of diagnostic and management strategies to determine which strategies should be utilized in the emergency setting for the rapid diagnosis and management of testicular torsion are also reviewed.
A search strategy was formulated to identify publications relating to testicular torsion. The databases searched included PubMed, Web of Science, Scopus, Cochrane Database of Systematic Reviews, BMJ and Google Scholar. The following search terms were used: testicular torsion, acute scrotum, spermatic cord torsion, familial torsion, TWIST, epididymo-orchitis, orchidectomy, and orchidopexy. Relevant articles including additional articles identified from references cited in the original search articles were reviewed. Relevant sections of pertinent manuscripts were identified, assessed, and evaluated prior to writing this article. A total of 56 articles relevant to the contents of this article were reviewed and analyzed.
Discussion
Predisposing Factors
In general, the underlying etiology of testicular torsion is not easily identifiable. However, there are a number of factors including genetic factors, environmental factors, preceding trauma and the clapper-bell deformity that may predispose a patient to testicular torsion.5
Although evidence relating to familial inheritance is limited, a meta-analysis suggested that there is a familial link, especially with the occurrence of bilateral testicular torsion.6
A study conducted by Cunningham reviewed 6 brothers within a family, each of whom developed spermatic cord torsion within a period of 3 years. Each of the brothers were found to have testicular hypermobility predisposing them to testicular torsion.5 While the literature on familial inheritance is limited, there is a likely link between structural deviation and familial predisposition. Another study assessed 70 patients with testicular torsion and found that eight had family members that were previously diagnosed with testicular torsion.7
With regards to environmental factors, there is a recognizable association between a hyperactive cremasteric reflex, cold weather and testicular torsion.8 A Brazilian study that assessed the association between testicular torsion and weather conditions among 21,289 patients between 1992 and 2010 reported that the incidence of testicular torsion was substantially higher during the winter months.1 Decreasing humidity is also considered a possible risk factor for testicular torsion and has been associated with a higher incidence of testicular torsion.9
The clapper-bell deformity is commonly noted in patients with testicular torsion. Normally, the tunica vaginalis attaches directly to the posterolateral part of the testis. With the clapper-bell deformity the epididymis, distal spermatic cord and testis are entirely surrounded by the tunica vaginalis, allowing the testis to hang freely with the ability to turn and swing inside the tunica vaginalis and thereby predisposing the individual to testicular torsion.10 A study based on autopsies that were conducted in 51 male patients found a 12% incidence of clapper-bell deformity, suggesting that in addition to the clapper-bell deformity other factors that may increase the risk of testicular torsion.11
Clinical Presentation
A thorough history is an essential component when evaluating a patient for the probability of testicular torsion. Key features on history that may heighten the index of suspicion include age, a sudden onset of severe unilateral testicular pain of less than a 24-hour duration associated with one or more of the following; nausea, vomiting, scrotal swelling, testicular tenderness, erythema, a high riding testicle (Brenzel sign) and retraction of the scrotal skin (Ger’s sign). However, pain that has been ongoing for more than 24 hours does not rule out the presence of a testicular torsion.4,12 A recent study identified the presence of increasing testicular pain, nausea, vomiting, abnormal position of the testicle and skin changes of the scrotum as highly predictive of testicular torsion. Testicular pain may also be intermittent in nature and in general there is a lack of pain relief on elevation of the affected side (Prehn’s sign).13
A retrospective study spanning over a period of 23 years, which included patients with surgically confirmed testicular torsion and torsion of the appendix testis, assessed the prevalence of various presenting. Overall, 70% had been diagnosed with testicular torsion and the other 30% with torsion of the appendix testis.2 It was noted that 87.8% of patients with testicular torsion reported a duration of pain of less than 24 hours. The study also reported that more patients with testicular torsion reported or presented with onset of pain during sleep (67.3% vs 33%), testicular swelling (79.6% vs 52.4%) and a high-riding testicle (61.2% vs 33.3%).2
Another retrospective study conducted in pediatric patients with a median age of 10 years and presenting with acute scrotum reported that 19 out of 138 patients were diagnosed with testicular torsion. Patients with testicular torsion in this study were on average older age than patients presenting with other causes of acute scrotum. This study also found that the presence of nausea, vomiting, abnormal cremasteric reflex, pain of less than 24 hours and a high position of the affected testis were strongly associated with testicular torsion.12
Another retrospective study that included 118 patients with testicular torsion reported that 92.4% of patients complained of pain, 88.1% presented with scrotal swelling, 94.1% presented with tenderness and 94.9% had an absent cremasteric reflex.14 This study however reported that only 26.3% of patients had vomiting as part of their presenting complaint. This study did not specifically review the duration of pain, but a more general view of the duration of symptoms was analyzed and showed a median duration of 64 hours. It was also noted that two patients had normal cremasteric reflexes suggesting that the presence of a normal cremasteric reflex does not completely rule out the diagnosis of testicular torsion.
Atypical presentations of testicular torsion can occur, and symptoms may be non-specific causing a challenge in diagnosis. This can lead to a delay in treatment with a higher risk of complications. Hence, clinicians should be aware of atypical presentations.15 A case study from Pennsylvania which reviewed a patient with mesorchial testicular torsion highlighted that intermittent pain and a present cremasteric reflex can also present as atypical presentations of testicular torsion. The report suggested that if atypical pain is persistent, further investigations should be conducted.16
Atypical presentation of testicular torsion can also occur in the setting of cryptorchidism. A retrospective study found eight cases of torsion of the undescended testis (within the inguinal canal) out of 84 patients that received surgical management for testicular torsion between 1999 and 2012. These patients presented with inguinal swelling and an inguinal mass that was tender. Torsion of the undescended testis can occur in the inguinal canal as well as intra-abdominally and these patients may present with abdominal or groin pain. Although torsion of the undescended testis is not common, it is essential that clinicians are aware of these presentations to avoid misdiagnosis and increase the chance of testicular salvage. This study also highlighted the importance of examining the external genitalia in patients that present with abdominal and inguinal pain.17 Since it is not always possible to rule-out testicular torsion on history and examination alone, radiological imaging or immediate exploration is often required.18
Clinical Decision Scoring
Utilizing clinical scores when assessing patients with an acute scrotum can provide guidance in identifying patients who may require scrotal ultrasonography, urological consultation or urgent scrotal exploration.12 Various clinical decision tools have been described to assist the clinician in the diagnosis and work-up of testicular torsion.
The Testicular Workup for Ischemia and Suspected Torsion (TWIST) score focuses on five criteria from the history and clinical examination to estimate the likelihood of testicular torsion. This scoring system was initially validated by Barbosa et al and was utilized in their study. Criteria for this scoring system include testicular swelling (2 points), presence of a hard testicle (2 points), absent cremasteric reflex (1 point), nausea/vomiting (1 point) and a high-riding testicle (1 point). A score of 0–2 is deemed low risk and is associated with a 100% negative predictive value for torsion. In general, ultrasonography and urological consultation are not required in patients in this category. A score of 3–4 is deemed intermediate risk and warrants ultrasonography and possible urological consultation, whereas a score of 5 or more is classed as high risk and is associated with a 100% positive predictive value for testicular torsion.19 Patients in this category do not require ultrasonography but rather urgent urological consultation and surgery with a view for testicular salvage.20
A study was conducted to evaluate the validity of the TWIST scoring system when used by non-urologists’ medical professionals on a patient sample of 128 patients. Although slightly different cut off values for low, intermediate, and high risk were used (0, 1–5, more than 6, respectively), the authors reported that there were no cases of testicular torsion in the group of patients that were classified as low risk (indicating a 100% negative predicative value). The positive predictive value of patients within the Tanner stages of 3–5 with a TWIST score of ≥6 was 100%; however, an accurate diagnosis was difficult in patients in Tanner stages 1–2. It therefore suggests that this scoring tool can potentially be used in the emergency room setting by non-urologists but advises that if patients are in Tanner stages 1–2, an ultrasound is also recommended including in those with a high-risk TWIST score.20 Other scoring tools, although not widely used, have also shown promise.13,21,22
Differential Diagnosis
The differential diagnosis of testicular torsion is broad. Various scrotal-related pathologies may clinically mimic testicular torsion and present with an acute scrotum. These include scrotal cellulitis, gangrene, edema, scrotal abscess, and fat necrosis secondary to trauma. Pathology of surrounding structures such as rupture of the tunica albuginea, spasm of the cremasteric muscle, torsion of the spermatocele, hydrocele and pyocele may also present with an acute scrotum.23
Testicular pathologies that may mimic testicular torsion include torsion of testicular appendages, epididymo-orchitis, mumps orchitis, testicular infarct, polyorchidopathia, trauma, ischemic necrosis, tumour-related hemorrhage and myofibroblastic pseudotumor. An infarcted spermatic chord, hematoma and thrombophlebitis associated with varicocele represent conditions affecting the spermatic cord which may mimic testicular torsion. Various systemic conditions can also present with symptoms in keeping with the presentation of testicular torsion. These include polyarteritis nodosa, hypersensitivity angiitis, thromboangitis obliterans, Henoch-Schonlein purpura and familial Mediterranean fever. Abdominal and retroperitoneal pathologies such as incarcerated strangulated hernia, pancreatic tumour, hemoperitoneum, acute appendicitis, and rarely adrenal hemorrhage in neonates can also mimic testicular torsion.23
Torsion of the testicular appendages and epididymo-orchitis is the most common mimics of testicular torsion.24 Clinical characteristics of these pathologies are described in Table 1. Torsion of any of the four testicular appendages [appendix testis (remnant of paramesonephric duct), appendix epididymis (remnant of the mesonephric duct), paradidymis (organ of Giraldes) and vas aberrans (organ of Haller)] may present with an acute scrotum, especially in children.25 The appendix testis is usually located at the superior testicular pole in the groove between the testicle and epididymis. Ninety-five percent of cases of torsion of the appendages are attributed to the appendix testis. Although torsion of the testicular appendages is a benign process, it poses a major clinical challenge due to its similarities with the presentation of testicular torsion. In contrast, epididymo-orchitis is defined as inflammation of the epididymis and testes with or without associated infection. Compared to testicular torsion, symptoms may be more insidious with pain persisting for days to months. Most cases occur between the ages of 18 to 35 years. Concurrent symptoms such as dysuria, hematuria, urinary urgency, fever, and tachycardia are frequently encountered. The presence of a normal cremasteric reflex and a thickened spermatic chord with increased doppler wave pulsation on ultrasonography are both useful in distinguishing epididymo-orchitis from testicular torsion.26
 |
Table 1 Clinical Characteristics That May Assist with Distinguishing Testicular Torsion from Torsion of the Testicular Appendix and Epididymo-Orchitis |
Diagnostic Modalities
Over the years, various diagnostic modalities including ultrasonography, nuclear imaging, orthogonal polarization spectral (OPS) imaging, near-infrared spectroscopy (NIRS), magnetic resonance imaging (MRI) and surgical exploration have been considered in the diagnosis of testicular torsion.
Ultrasonography
Due to its portability and lack of ionizing radiation, ultrasonography is regarded as the preferred choice of imaging modality in the evaluation of an acute scrotum.27 Like all diagnostic ultrasonography imaging, scrotal ultrasonography is also subject to operator dependency, hence findings must be interpreted in conjunction with the clinical presentation.21
It is important to note that ultrasonography findings may vary according to the degree of blood flow that is compromised.28 Color Doppler is widely used to assess testicular vascular flow in patients where testicular torsion is suspected. In patients with complete testicular torsion, there is an absence of flow which can be seen on Doppler ultrasound; however, in incomplete torsion, Doppler ultrasound is known to have a high false-negative rate.29 Conventional color Doppler ultrasonography can also lead to a misdiagnosis as it only assesses the macrovascular blood flow in the testes.30 Misdiagnosis in younger patients is also common due to detection challenges with regards to a smaller testes volume.31
A study conducted in Oman that included 33 patients with surgically confirmed testicular torsion assessed the sensitivity of color Doppler ultrasonography and high-resolution ultrasonography. It found that the sensitivity of color Doppler ultrasound was 84.85% while that of high-resolution ultrasonography was 93.94%. The authors suggested that if a combination of both these types of ultrasonography are used, a higher sensitivity may be achieved.32
The use of contrast-enhanced ultrasonography can be utilized to assess microvascular testicular blood flow. However, it has also been found to be of limited value in the pediatric patient population.30 Power Doppler sonography is a tool that can be used to aid standard color Doppler since it has higher sensitivity than standard color Doppler, especially when determining low-velocity flow.33 Power Doppler is utilized to determine if there is testicular blood flow while spectral Doppler allows differentiation between venous and arterial flow. The settings for both power and spectral Doppler should be changed to the most sensitive settings to allow a minimal amount of artefact. It is also suggested that the Pulse Repetition Frequency (PRF) should be set as low as possible to ensure optimal imaging.28
Superb Microvascular Imaging (SMI) is a recently developed Doppler modality which assesses microvascular blood flow and addresses the challenges associated with standard Doppler ultrasonography.34 It has the ability to assesses low-velocity flow with less tissue movement artefacts and signals.30 A prospective study of 156 patients compared the testicular blood flow findings between conventional Doppler imaging and SMI (both color and monochrome). In patients of all age groups, it was found that the SMI technology provided more information regarding the vascular structure of the testis and was able to assess thin vessels and blood flow within the testes more accurately when compared to conventional Doppler imaging. The study also found that the monochrome SMI technique showed stronger statistical evidence than color SMI in evaluating blood flow in the testis. It concluded that monochrome SMI should be utilized in pediatric patients including patients with cryptorchidism, patients with a small testis volume and in patients where there is suspicion for testicular torsion.35
Another features that may be identified on ultrasonography is the presence of a scrotal whirlpool sign (Figure 1), which can be described as a spiral-like pattern of the spermatic cord and is virtually pathognomonic of testicular torsion. It is seen as a twist in the course of the spermatic cord. This sign can be visualized with either standard ultrasonography, high-resolution ultrasonography or color Doppler ultrasonography. The sign however is not easily identified in neonates.36 A recent meta-analysis to determine the accuracy of the whirlpool sign observed a pooled sensitivity of 92% (95% CI, 0.70–0.98), a pooled specificity of 99% (95% CI, 0.95–1.00) and a summary estimate log odds ratio of 5.32 (95% CI, 1.59–9.05; p=0.001) in adults and non-neonatal pediatric patients.37
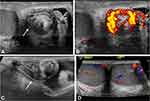 |
Figure 1 “Whirlpool sign” of the spermatic cord. (A) Gray-scale transverse US image of upper left scrotal sac shows an eddy swirl (arrow) of the spermatic cord suggesting torsion of the cord. This 12-year-old boy woke with acute left testicular pain and experienced nausea and vomiting along with the pain. (B) Power Doppler US image of the same twisted cord shows concentric pattern of preserved flow in the vessels of the twisted cord. The flow in the left testis (not shown) was minimally decreased compared to the right side and bilateral bell clapper deformity was found during orchiopexy along with complete torsion of the left testis with 360° twist. (C) Gray-scale longitudinal US image of the left scrotum in a 13-year-old boy with 1 day of left-side pain shows abrupt spiral twisting of the spermatic cord (arrow) at the external inguinal ring, creating a whirlpool sign. (D) Color Doppler transverse image of the testes in the same boy as in (C) shows preserved and symmetrical flow bilaterally. After manual detorsion in the emergency room, he underwent orchiopexy and was diagnosed with intermittent torsion. (Image obtained from Bandarkar AN, Blask AR. Testicular torsion with preserved flow: key sonographic features and value-added approach to diagnosis. Pediatr Radiol. 2018;48(5):735–744. doi:10.1007/s00247-018-4093-0.27 Distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). No changes have been made to the images or the image description). |
Another sign that may be visualized on ultrasonography is the redundant spermatic cord sign, where the spermatic cord appears torturous, indicating an abnormality in the attachment of the tunica vaginalis.27 In the setting of an acute scrotum, this sign may be seen as a boggy pseudo mass or a torsion knot (Figure 2).21,38 The presence of the horizontal lie (Figure 3) of the testicle may also be visualized on ultrasound and is indicative of the clapper-bell deformity.27
 |
Figure 2 Intermittent torsion in a 17-year-old boy who presented with 5 h of acute right testicular pain after a game of football. He had experienced 6–7 similar episodes in the last 2 years where the pain had spontaneously resolved. Cremasteric reflex was absent on the right. (A) Gray-scale transverse US image of the right testis shows a redundant spermatic cord (arrow) occupying the medial half of the scrotal sac, with a mildly edematous epididymis (E) adjacent to it. The echogenic mediastinum testis faced medially instead of posterolaterally, which was concerning for altered testicular lie. (B) Color Doppler longitudinal image of the right scrotum shows excess and tortuous spermatic cord bunched up in the scrotal sac superior to the testis and formation of a pseudomass, suggesting torsion of the spermatic cord. Note that this extratesticular pseudomass is not hyperemic and should not be confused with epididymitis. Orchiopexy was recommended; however, the family chose to wait because his pain had improved. Elective orchiopexy was performed 7 months later and bilateral bell clapper anomaly was noted; he was diagnosed with intermittent torsion. (Image obtained from Bandarkar AN, Blask AR. Testicular torsion with preserved flow: key sonographic features and value-added approach to diagnosis. Pediatr Radiol. 2018;48(5):735–744. doi:10.1007/s00247-018-4093-0.27 Distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). No changes have been made to the images or the image description). |
 |
Figure 3 Testicular lie—normal and abnormal. (A) Color Doppler transverse US image of the testes in a 12-year-old boy with mild groin pain demonstrates normal vertical lie with the testes seen in round cross-section and with the mediastinum testis (arrows) directed posterolaterally. (B) Color Doppler transverse US image of both testes in a 14-year-old boy who woke with acute right scrotal pain demonstrates abnormal horizontal lie of the right testis (arrow) with slightly decreased intratesticular flow compared to the normal left side. He had experienced similar episodes of pain in the past and was diagnosed with intermittent torsion. During orchiopexy 12 h later, a bell clapper deformity was noted bilaterally. (C) Gray-scale transverse US image of both testes in a 16-year-old boy with right testicular pain demonstrates abnormal oblique lie of the right testis (arrows), which is oriented diagonally compared to the normal left side. Intermittent torsion was diagnosed intraoperatively. (Image obtained from Bandarkar AN, Blask AR. Testicular torsion with preserved flow: key sonographic features and value-added approach to diagnosis. Pediatr Radiol. 2018;48(5):735–744. doi:10.1007/s00247-018-4093-0.27 Distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). No changes have been made to the images or the image description). |
Computed Tomography
Scrotal computed tomography (CT) imaging is rarely performed in the evaluation of an acute scrotum. However, it is the choice investigation in the assessment of scrotal hernia involving the ureters, Fournier’s gangrene, acute trauma and in cancer staging. CT is also of value in detecting a perfusion insult in indeterminate cases. It is important to note that CT imaging involves ionizing radiation and should only be used for scrotal imaging where absolutely necessary.39 This modality is also difficult to use in pediatric patients and it is not cost-effective.30 CT is not the modality of choice in the setting of acute scrotum when there is a need to evaluate testicular perfusion.
Nuclear Imaging
Testicular nuclear imaging has an overall accuracy of 95% and is useful in differentiating testicular torsion from epididymo-orchitis when the diagnosis is in doubt. However, this imaging modality is not always easily accessible and may result in delays in the setting of acute scrotum, suggesting that it should not be utilized as the initial modality of choice.40,41
Orthogonal Polarization Spectral (OPS) Imaging
Disturbances in testicular microcirculation occurs in the late stages of torsion. Hence, OPS imaging can be utilized to assess changes in the microcirculation of the testis after the ischemia-reperfusion phase.42 However, OPS imaging is not readily available.
Near Infrared Spectroscopy (NIRS)
This modality utilizes infrared light to estimate tissue saturation of oxygen in superficial body tissues of up to several centimeters. Evidence regarding near-infrared spectroscopy and diagnosing testicular torsion is limited. A recent systematic review concluded that NIRS was unreliable in the diagnosis of testicular torsion.43 Hence, this modality is currently not recommended in the work-up of testicular torsion.
Magnetic Resonance Imaging (MRI)
Contrast or non-contrast-enhanced MRI is useful when the diagnosis of incomplete testicular torsion is suspected.44 A retrospective study indicated that contrast-enhanced MRI in the evaluation of torsion had an associated sensitivity and specificity of 100% and 93%, respectively.45 However, MRI is not as easily available as ultrasonography, it is difficult to use in pediatric patients and it is not cost-effective.30 MRI may have some value in selected cases but it is not recommended as routine.
Surgical Exploration
Although surgical exploration is invasive, it remains the gold standard in the diagnosis of testicular torsion.44 Since testicular salvage is time dependent and clinical examination as well as the various imaging modalities described above are unable to exclude the diagnosis of testicular torsion in all cases, early surgical exploration must be given consideration when the index of suspicion for testicular torsion is high.46
Preoperative Mean Platelet Volume (MPV) and Leucocyte Count
The mean platelet volume indicates the level of platelet activity and forms part of a full blood count investigation. It can assess for the presence of inflammation as part of a preoperative assessment. A retrospective study based in Turkey compared the MPV value and leucocyte count of 51 healthy patients to 50 patients with surgically confirmed testicular torsion. The study showed that the mean MPV and the mean leucocyte count were remarkably higher in patients with testicular torsion compared to the healthy patient group. The MPV has been shown to be increased in vascular pathologies of the urological system, although literature is limited. Although the MPV has a low sensitivity in the diagnosis of testicular torsion, it may be useful to support the presence of testicular torsion.47
Management
The maximum duration for testicular salvage after torsion has been highly debated. A study found that 89% of testes operated on between 7 and 12 hours were salvaged.48 Another study noted that rates of testicular salvage decreased from 100% to 90% when time to testicular surgery had been delayed by 4–8 hours.49
The Role of Manual Detorsion
Manual detorsion was first described in 1893 to reverse ischemia and provide instantaneous pain relief. It can be considered a “time buying procedure” and a prelude to surgical intervention that may limit testicular infarction while preparations are being made for surgical exploration. For torsion of the left side, the testicle should initially be rotated 180 degrees clockwise. The procedure may need to be repeated 2–4 times, as torsion can involve rotations of 180–720 degrees. Repeat attempts of manual detorsion should be guided by instantaneous resolution of pain and re-establishment of blood flow on doppler ultrasonography. The method is similar for torsion of the right testicle, except that the testicle is rotated counterclockwise. Manual detorsion should be followed by surgical exploration and orchidopexy.50
A recent study reported that 20 out of 26 patients were successfully detorted by manual detorsion with elective surgery thereafter being performed at a median of 10 (0–45) days later. None of the patients had recurrent episodes of torsion while awaiting surgery. The median ischemia-free time gained was 90 (20–240) minutes compared to the group of patients that underwent surgical exploration without manual detorsion.50
Patient compliance can be a limiting factor, as manual detorsion is generally performed without the administration of anesthesia. This is to allow for monitoring of pain which can be used as a surrogate marker of successful detorsion or recurrence of torsion. Contraindications to manual detorsion include scrotal wall thickening, inflammation and reactive hydrocele.51
Surgical Exploration
In the suspected diagnosis of torsion, urgent scrotal exploration is the treatment of choice to salvage a possible ischemic testis.23 Non-viable testis should be removed (orchiectomy) to prevent formation of anti-sperm antibodies and thus compromising the functionality of the healthy contralateral testis. Testicular viability during surgical exploration can be determined on clinical grounds in most instances. In doubtful cases, fluorescent dye may be used to assess for the presence of ischemia.9 A viable testis should be fixed to the inner scrotal wall to prevent re-torsion (orchidopexy). In addition, orchidopexy of the opposite testis should also be performed.52
Predictors of Viability
A history of more than 10 hours between onset of symptoms and surgery is a very strong indicator of testicular non-viability with an associated sensitivity and specificity of 62% and 100%, respectively. A recent study conducted in Italy that enrolled 15 boys found that absence of testicular blood flow on color Doppler ultrasound had a sensitivity and specificity of 88% and 86%, respectively. With regards to the intraoperative bleeding test, all patients with grade 3 bleeding (major bleeding that requires multiple hemoclips and sessions of hemocoagulation) required orchidectomy and had necrotic tissue on histopathology. Majority of patients with grade 2 intraoperative bleeding required only orchidopexy.53 Regarding long-term viability, unilateral torsion affected spermiogenesis in about 50% of patients and produced borderline impairment in another 20%.54
Long-Term Recovery After Torsion
Despite prompt diagnosis and orchidopexy, infertility remains a major issue after the treatment of testicular torsion. A study looking at the late endocrine profile, seminal parameters and anti-sperm antibody levels after testicular torsion showed that sperm motility after orchiectomy was higher than in patients who had an orchidopexy. The endocrine levels and anti-sperm antibody levels were otherwise unremarkable in both groups. Higher levels of anti-sperm antibodies were found in all patients regardless of whether the patient underwent orchiectomy or orchidopexy. No correlation was found between anti-sperm antibody levels and age of torsion, ischemia time, seminal parameters or treatment applied. Testicular fate did not have any correlation with the formation of anti-sperm antibodies. Further studies are required to clarify whether maintenance of a severely ischemic testis may impair testicular function.55
Medicolegal Aspects of Testicular Torsion
Due to delays in diagnosis, treatment and resultant testicular loss, testicular torsion is a relatively frequent cause of medical malpractice litigation.56 A retrospective study that analyzed litigation claims filed over an 18-year period reported that indemnity payments were made in 67% of cases, half of which were in favor of the claimant. Testicular torsion was misdiagnosed as epididymitis in 61% of cases and medicolegal outcome was not affected by late presentation. Major liabilities for paid claims were an error in diagnosis (74%), a delay in or lack of referral (48%), failure to order radiological investigations (19%), delayed surgical exploration (13%), error in surgical technique or judgment (13%) and falsified records (10%). The median indemnity payment was $45,000 while the total cost for 39 cases including payments, legal expenses and indemnity was $2,286,528.00.57
Conclusion
Testicular torsion is a time-sensitive diagnosis that requires prompt surgical intervention to avoid testicular ischemia, infertility, and unwanted litigation. When imaging is required, the recommended and most available modality to detect torsion is ultrasonography. In pediatric patients, the use of SMI is supported. This holds high significance in countries where waiting for other imaging modalities may produce unfavorable outcomes. Rates of testicular salvage are better when surgery is performed within 7–12 hours of symptom onset. Clinicians must be aware of the presentation, broad differential diagnosis, available diagnostic modalities, and management of testicular torsion. Due to the high associated medico-legal risk, clinicians must have a sound knowledge regarding testicular torsion. There is a need for further research with regards to testicular torsion in the emergency room in resource limited settings as a lack of resources may influence timeous diagnosis, imaging modalities and time to surgery.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors hereby certify that all authors are free from any financial or other conflicts of interest for this work.
References
1. Korkes F, Cabral PRDA, Alves CDM, Savioli ML, Pompeo ACL. Testicular torsion and weather conditions: analysis of 21,289 cases in Brazil. Int Braz J Urol. 2012;38(2):222–229. doi:10.1590/S1677-55382012000200010
2. Fujita N, Tambo M, Okegawa T, Higashihara E, Nutahara K. Distinguishing testicular torsion from torsion of the appendix testis by clinical features and signs in patients with acute scrotum. Res Reports Urol. 2017;9:169–174. doi:10.2147/RRU.S140361
3. Ogunyemi OI. Testicular Torsion; Published 2018. Available from: https://emedicine.medscape.com/article/2036003-overview#a7.
4. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835–840.
5. Cunningham RF. Familial occurrence of testicular torsion. JAMA. 1960;174(10):1330. doi:10.1001/jama.1960.63030100018026b
6. Shteynshlyuger A, Yu J. Familial testicular torsion: A meta analysis suggests inheritance. J Pediatr Urol. 2013;9(5):683–690. doi:10.1016/j.jpurol.2012.08.002
7. Cubillos J, Palmer JS, Friedman SC, Freyle J, Lowe FC, Palmer LS. Familial testicular torsion. J Urol. 2011;185(6S):2469–2473. doi:10.1016/j.juro.2011.01.022
8. Mabogunje OA. Testicular torsion and low relative humidity in a tropical country. BMJ. 1986;292(6517):363–364. doi:10.1136/bmj.292.6517.363
9. Srinivasan AK, Freyle J, Gitlin JS, Palmer LS. Climatic conditions and the risk of testicular torsion in adolescent males. J Urol. 2007;178(6):2585–2588. doi:10.1016/j.juro.2007.08.049
10. Dogra V. Bell-Clapper Deformity. Am J Roentgenol. 2003;180(4):1176–1177. doi:10.2214/ajr.180.4.1801176
11. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformityin an autopsy series. Urology. 1994;44(1):114–116. doi:10.1016/S0090-4295(94)80020-0
12. Boettcher M, Bergholz R, Krebs TF, Wenke K, Aronson DC. Clinical predictors of testicular torsion in children. Urology. 2012;79(3):670–674. doi:10.1016/j.urology.2011.10.041
13. Srinivasan A, Cinman N, Feber KM, Gitlin J, Palmer LS. History and physical examination findings predictive of testicular torsion: an attempt to promote clinical diagnosis by house staff. J Pediatr Urol. 2011;7(4):470–474. doi:10.1016/j.jpurol.2010.12.010
14. Yang C, Song B, Tan J, Liu X, Wei G. Testicular torsion in children: a 20-year retrospective study in a single institution. Sci World J. 2011;11:362–368. doi:10.1100/tsw.2011.39
15. Jansen R, Fooks H, Zaslou S. An atypical presentation of testicular torsion: a case report. W V Med J. 2013. Published online.
16. Chan JL, Knoll JM, Depowski PL, Williams RA, Schober JM. Mesorchial testicular torsion: case report and a review of the literature. Urology. 2009;73(1):83–86. doi:10.1016/j.urology.2008.06.053
17. Pogorelić Z, Mrklić I, Jurić I, Biočić M, Furlan D. Testicular torsion in the inguinal canal in children. J Pediatr Urol. 2013;9(6):793–797. doi:10.1016/j.jpurol.2012.10.013
18. Kaplan G Testicular torsion - BMJ best practice; Published 2018. Available from: https://bestpractice.bmj.com/topics/en-gb/506?utm_source=google&utm_medium=cpc&utm_campaign=sales&utm_content=bau&utm_term=&gclid=Cj0KCQjwmpb0BRCBARIsAG7y4zaKI7gXPlSjSr6yJOKp44dmK9-pjkSQR-0I5W6-GqlvEIxmrjfjksUaAtMOEALw_wcB.
19. Barbosa JA, Tiseo BC, Barayan GA, et al. Development and initial validation of a scoring system to diagnose testicular torsion in children. J Urol. 2013;189(5):1859–1864. doi:10.1016/j.juro.2012.10.056
20. Sheth KR, Keays M, Grimsby GM, et al. Diagnosing testicular torsion before urological consultation and imaging: validation of the twist score. J Urol. 2016;195(6):1870–1876. doi:10.1016/j.juro.2016.01.101
21. Boettcher M, Krebs T, Bergholz R, Wenke K, Aronson D, Reinshagen K. Clinical and sonographic features predict testicular torsion in children: a prospective study. BJU Int. 2013;112(8):1201–1206. doi:10.1111/bju.12229
22. Karmazyn B, Steinberg R, Kornreich L, et al. Clinical and sonographic criteria of acute scrotum in children: a retrospective study of 172 boys. Pediatr Radiol. 2005;35(3):302–310. doi:10.1007/s00247-004-1347-9
23. Pentyala S, Lee J, Yalamanchili P, Vitkun S, Khan SA. Testicular torsion: a review. J Low Genit Tract Dis. 2001;5(1):38–47. doi:10.1046/j.1526-0976.2001.51008.x
24. Fehér ÁM, Bajory Z. A review of main controversial aspects of acute testicular torsion. J Acute Dis. 2016;5(1):1–8. doi:10.1016/j.joad.2015.06.017
25. Rakha E. Torsion of the testicular appendix: importance of associated acute inflammation. J Clin Pathol. 2006;59(8):831–834. doi:10.1136/jcp.2005.034603
26. Skoglund RW, Mcroberts JW, Ragde H. Torsion of testicular appendages: presentation of 43 new cases and a collective review. J Urol. 1970;104(4):598–600. doi:10.1016/S0022-5347(17)61790-7
27. Bandarkar AN, Blask AR. Testicular torsion with preserved flow: key sonographic features and value-added approach to diagnosis. Pediatr Radiol. 2018;48(5):735–744. doi:10.1007/s00247-018-4093-0
28. Blaivas M, Brannam L. Testicular ultrasound. Emerg Med Clin North Am. 2004;22(3):723–748. doi:10.1016/j.emc.2004.04.002
29. Sanelli PC, Burke BJ, Lee L. Color and spectral doppler sonography of partial torsion of the spermatic cord. Am J Roentgenol. 1999;172(1):49–51. doi:10.2214/ajr.172.1.9888737
30. Karaca L, Oral A, Kantarci M, et al. Comparison of the superb microvascular imaging technique and the color Doppler techniques for evaluating children’s testicular blood flow. Eur Rev Med Pharmacol Sci. 2016;20(10):1947–1953.
31. Singhal A, Agarwal A, Metuge J, Olsavsky T. Neonatal testicular torsion with an unusual sonographic feature. J Clin Ultrasound. 2012;40(4):243–246. doi:10.1002/jcu.21889
32. Selim YARM, Albroumi SA. Acute torsion of the testis in children and young adults: role of high resolution and color doppler ultrasonography. Egypt J Radiol Nucl Med. 2015;46(1):151–157. doi:10.1016/j.ejrnm.2014.11.018
33. Babcock DS, Patriquin H, LaFortune M, Dauzat M. Power Doppler sonography: basic principles and clinical applications in children. Pediatr Radiol. 1996;26(2):109–115. doi:10.1007/BF01372087
34. Hata J Seeing the unseen, new techniques in vascular imaging: superb micro-vascular imaging; Published 2014. Available from: https://eu.medical.canon/wp-content/uploads/sites/2/2015/01/Seeing-the-unseen-2014-SMI-on-Aplio-500.pdf.
35. Durmaz MS, Sivri M. Comparison of superb micro-vascular imaging (SMI) and conventional Doppler imaging techniques for evaluating testicular blood flow. J Med Ultrason. 2018;45(3):443–452. doi:10.1007/s10396-017-0847-9
36. Esposito F, Di Serafino M, Mercogliano C, Vitale V, Sgambati P, Vallone G. The “whirlpool sign”, a US finding in partial torsion of the spermatic cord: 4 cases. J Ultrasound. 2014;17(4):313–315. doi:10.1007/s40477-014-0095-4
37. McDowall J, Adam A, Gerber L, et al. The ultrasonographic “whirlpool sign” in testicular torsion: valuable tool or waste of valuable time? A systematic review and meta-analysis. Emerg Radiol. 2018; 1–12. doi:10.1007/s10140-018-1579-x.
38. Munden MM, Williams JL, Zhang W, Crowe JE, Munden RF, Cisek LJ. Intermittent testicular torsion in the pediatric patient: sonographic indicators of a difficult diagnosis. Am J Roentgenol. 2013;201(4):912–918. doi:10.2214/AJR.12.9448
39. Parenti GC, Feletti F, Carnevale A, Uccelli L, Giganti M. Imaging of the scrotum: beyond sonography. Insights Imaging. 2018;9(2):137–148. doi:10.1007/s13244-017-0592-z
40. MacDonald A, Burrell S. Infrequently performed studies in nuclear medicine: part 1. J Nucl Med Technol. 2008;36(3):132–143. doi:10.2967/jnmt.108.051383
41. Haynes BE, Bessen HA, Haynes VE. The diagnosis of testicular torsion. JAMA. 1983;249(18):2522–2527. doi:10.1001/jama.1983.03330420068040
42. Bajory Z, Szabo A, Deak G, Varga R, Pajor L. Orthogonal polarization spectral imaging: a novel tool for examination of microcirculatory changes in the testis. J Androl. 2012;33(3):499–504. doi:10.2164/jandrol.111.013599
43. Laher A, Swart M, Honiball J, Perera M, Lawrentschuk N, Adam A. Near‐infrared spectroscopy in the diagnosis of testicular torsion: valuable modality or waste of valuable time? A systematic review. ANZ J Surg. 2019;
44. Gotto GT, Chang SD, Nigro MK. MRI in the diagnosis of incomplete testicular torsion. Br J Radiol. 2010;83(989):e105e107. doi:10.1259/bjr/95900989
45. Terai A, Yoshimura K, Ichioka K, et al. Dynamic contrast-enhanced subtraction magnetic resonance imaging in diagnostics of testicular torsion. Urology. 2006;67(6):1278–1282. doi:10.1016/j.urology.2005.12.021
46. Hyun GS. Testicular Torsion. Rev Urol. 2018;20(2):104–106. doi:10.3909/riu0800
47. Cicek T, Togan T, Akbaba K, Narci H, Aygun C. The value of serum mean platelet volume in testicular torsion. J Int Med Res. 2015;43(3):452–459. doi:10.1177/0300060514558898
48. Anderson JB, Williamson RCN. Testicular torsion in Bristol: A 25-year review. Br J Surg. 1988;75(10):988–992. doi:10.1002/bjs.1800751015
49. Watkin NA, Reiger NA, Moisey CU. Is the conservative management of the acute scrotum justified on clinical grounds? BJU Int. 1996;78(4):623–627. doi:10.1046/j.1464-410X.1996.16321.x
50. Demirbas A, Demir DO, Ersoy E, et al. Should manual detorsion be a routine part of treatment in testicular torsion? BMC Urol. 2017;17(1):84. doi:10.1186/s12894-017-0276-5
51. Cornel K. Manual derotation of the twisted spermatic cord. BJU Int. 2001;83(6):672–674. doi:10.1046/j.1464-410x.1999.00003.x
52. Thakare N, O’Flynn KJ, Pearce I. Testicular torsion: a urological emergency. Trends Urol Men’s Heal. 2010;1(1):31–34. doi:10.1002/tre.161
53. Cimador M, DiPace MR, Castagnetti M, DeGrazia E. Predictors of testicular viability in testicular torsion. J Pediatr Urol. 2007;3(5):387–390. doi:10.1016/j.jpurol.2007.01.194
54. Visser AJ, Heyns CF. Testicular function after torsion of the spermatic cord. BJU Int. 2003;92(3):200–203. doi:10.1046/j.1464-410X.2003.04307.x
55. Arap MA, Vicentini FC, Cocuzza M, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007;28(4):528–532. doi:10.2164/jandrol.106.002097
56. Hadway P, Reynard JM. The six-hour rule for testis fixation in testicular torsion: is it history? J Clin Urol. 2013;6(2):84–88. doi:10.1177/2051415812472676
57. Matteson JR, Stock JA, Hanna MK, Arnold TV, Nagler HM. Medicolegal aspects of testicular torsion. Urology. 2001;57(4):783–786. doi:10.1016/S0090-4295(00)01049-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
