Back to Journals » Clinical Ophthalmology » Volume 17
Terson Syndrome – Clinical Presentation, Management, and Visual Outcomes in a Tertiary Centre
Authors Lima-Fontes M , Leuzinger-Dias M , Rodrigues R, Barros-Pereira R, Falcão M , Fernandes V, Alves-Faria P, Falcão-Reis F, Rocha-Sousa A
Received 16 November 2022
Accepted for publication 9 January 2023
Published 25 January 2023 Volume 2023:17 Pages 351—359
DOI https://doi.org/10.2147/OPTH.S396781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mario Lima-Fontes,1 Mariana Leuzinger-Dias,1 Rita Rodrigues,1 Ricardo Barros-Pereira,1 Manuel Falcão,1,2 Vítor Fernandes,1 Pedro Alves-Faria,1,2 Fernando Falcão-Reis,1,2 Amândio Rocha-Sousa1,2
1Ophthalmology Department, Centro Hospitalar Universitário São João, Porto, 4200-319, Portugal; 2Department of Surgery and Physiology, Faculty of Medicine, University of Porto, Porto, 4200-319, Portugal
Correspondence: Mario Lima-Fontes, Department of Ophthalmology, Alameda Prof. Hernâni Monteiro, Porto, 4200-319, Portugal, Email [email protected]
Purpose: The purpose of this study was to characterize the clinical presentation, management strategy and visual outcomes of patients diagnosed with Terson syndrome and followed in a tertiary centre in Portugal.
Patients and Methods: A single-centre retrospective study was performed, based on the survey review of the medical records of every consecutive patient diagnosed with Terson syndrome and followed from January 2018 to August 2021. The change in best-corrected visual acuity (BCVA) from baseline to the final evaluation was the primary outcome.
Results: Fifteen eyes from 8 patients (50% female) were included. The mean age at diagnosis was 55± 7 years. The neurological event was traumatic brain injury in 37.5% (n=3) and subarachnoid haemorrhage in 62.5% of the patients (n=5). Bilateral intraocular haemorrhage occurred in 875% (n=7) of the patients. Vitreous and preretinal haemorrhages occurred each in 66.7% (n=10), intraretinal in 30% (n=3) and subretinal in 13.3% (n=2) of the eyes. In 40% of the eyes (n=6), spontaneous resolution of intraocular haemorrhage occurred, while PPV was performed in the remaining 60% (n=9). Ocular haemorrhage detection occurred 58.47 ± 40.94 days after the neurological event (range 11 to 121 days). Baseline BCVA was 1.11 ± 1.01 logMAR and improved to 0.32 ± 0.69 logMAR in the follow-up period (p=0.004). A positive correlation was found between initial and final BCVA (Spearman’s rho = 0.643, p=0.01). Baseline BCVA of eyes undergoing PPV was lower than of those conservatively managed (1.84± 0.72 vs 0.20± 0.28 logMAR, p< 0.001). However, there were no statistically significant differences in final BCVA after surgery or observation (0.56 ± 0.90 vs 0.04 ± 0.04 logMAR, p=0.149). Longer periods between the neurological and the ophthalmological diagnosis were correlated with worse final BCVA (Spearman’s rho = 0.688, p=0.005).
Conclusion: Terson syndrome is a potential cause of irreversible visual loss. Diagnosis delay may affect visual prognosis. PPV is indicated when intraocular haemorrhage is dense and does not resolve spontaneously or when visual acuity at presentation is low, allowing for good visual outcomes with minimal complications.
Keywords: Terson syndrome, management, vitrectomy, visual outcomes, clinical presentation
Introduction
Terson syndrome was first described by Moritz Litten in 1881 and later named by Albert Terson in 1900, as an association between subarachnoid haemorrhage and vitreous hemorrhage.1,2 This definition has evolved over the years and, nowadays, it comprises various forms of ocular haemorrhage, including vitreous, preretinal, intraretinal, or subretinal bleeding, more commonly identified in patients with intracranial haemorrhage or traumatic brain injury.3 However, it can also be associated with other causes of acute elevation in intracranial pressure, such as following epidural saline injection, neurosurgical third ventriculostomy, papilledema with development of optociliary shunt vessels and cases of pseudotumor cerebri.4
Its reported incidence ranges from 12.5% to 40% of individuals with intracranial haemorrhage, being particularly frequent in cases of aneurysmal subarachnoid hemorrhage.5–7
Retinal haemorrhages are most commonly seen in the macula and surrounding the optic disc and are found with decreasing density in the peripheral retina. Haemorrhages may be unilateral or bilateral, and they can be present within 1 hour after the subarachnoid haemorrhage. Retinal haemorrhages may be associated with increased mortality.5,8
In almost 150 years since its description, the physiopathology of Terson syndrome is still controversial. The most accepted theory is that the sudden increase in intracranial pressure closes the central retinal vein and its choroidal anastomoses, halting venous blood flow from the retina and raising intraluminal pressure within all branches of the vein. When coupled with unimpeded arterial blood flow into the retina, the resultant high intraluminal pressure ruptures the veins and causes bleeding into the retina.9–12
Alternative theories suggest that subarachnoid blood may pass into the cerebrospinal fluid surrounding the optic nerve or cause veins within the optic nerve sheath to rupture and then progress through the retinal layers, internal limiting membrane (ILM), and posterior hyaloid membrane,8 or that subarachnoid blood in skull base cisterns near the optic nerve is refluxed into the globe through glymphatic channels, leading to intraocular hemorrhage.3
The diagnosis of Terson syndrome is usually delayed, given the presence of concomitant neurological deficits and depressed conscious state, which limit formal assessment of visual acuity loss.13
In many patients, ocular haemorrhage resolves spontaneously after a period of observation. If after conservative management, vitreous and/or preretinal haemorrhage does not clear and visual deficits persist, surgical treatment with pars plana vitrectomy (PPV) is recommended.13–15
The purpose of this study was to characterize the clinical presentation, management strategy and visual outcomes of patients diagnosed with Terson syndrome and followed in a tertiary centre in Portugal.
Materials and Methods
An observational retrospective study was performed, based on the survey review of electronic medical records of every consecutive patient diagnosed with Terson syndrome and followed at the ophthalmology department of Centro Hospitalar Universitário de São João (CHUSJ) from January 2018 to August 2021. The study was conducted according to the tenets of the Declaration of Helsinki the study adhered to the tenets of the Declaration of Helsinki and was approved by the local Ethics Committee of CHUSJ (project n° 227/22). Informed consent was waived due to the retrospective nature of the study and the absence of reported data that can identify single patients.
Data collected included sex, age at diagnosis, type of neurological event, ocular presentation, the period between neurological and ophthalmological diagnosis, baseline best-corrected visual acuity (BCVA), chosen management strategy and timing of PPV, surgical complications, and visual outcomes at final evaluation. Best-corrected visual acuity was measured using Snellen charts and converted to the logarithm of the minimum angle of resolution (logMAR) for analysis. To facilitate analysis, light perception, hand movement, and finger counting were recorded as 2.6, 2.3 and 1.85 logMAR, respectively.16 Snellen equivalents were added in the main text to aid in the interpretation of the results.
Head computerized tomography (CT) scans requested during the monitorization of the neurological condition were reviewed to determine if a crescent sign in the posterior pole of the eye was present, which would constitute evidence of sub-ILM haemorrhage.8
Patients with dense vitreous haemorrhage and low BCVA underwent PPV. Unless there was some concern about the patient’s systemic status, the surgery was performed within two weeks of the diagnosis. If no progressive absorption was observed during consecutive assessments, pre-retinal haemorrhages affecting the central macula were also surgically managed.
The primary outcome was the change in BCVA from baseline to final evaluation. Secondary outcomes included a comparison of final BCVA between patients conservatively managed and those submitted to PPV and correlations between baseline and final BCVA, and between ophthalmologic diagnosis delay and final BCVA.
In the description of the sample’s characteristics, data are presented as counts and proportions for categorical variables, and as mean and standard deviation the continuous variables. When reporting time intervals, range was also included.
Statistical analysis was performed using the IBM® SPSS® Statistics software (version 27.0 for Windows; SPSS Inc., Chicago, IL, USA). Variables’ normal distribution was verified by skewness, kurtosis, and Kolmogorov–Smirnov test. Parametric or non-parametric tests were used for variables comparison, according to the data distribution. The level of significance was established at a P-value of <0.05.
Results
Sample Description
Fifteen eyes from 8 patients were included, 4 (50%) being female. The mean age at diagnosis of Terson syndrome was 55±7 years. No relevant past ophthalmology history was found for any patient. Dyslipidaemia and hypertension were the most frequent comorbidities in 50% (n=4) and 37.5% (n=3) of the patients, respectively. The mean follow-up time was 36.8 ± 54.8 months. Table 1 summarizes the demographics and clinical characteristics of the sample.
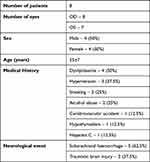 |
Table 1 Demographic and Clinical Characteristics of Cohort |
Neurological Cause
The neurological event was traumatic brain injury in 37.5% (n=3) and subarachnoid haemorrhage in 62.5% of the patients (n=5). Of the cases of traumatic brain injury, 2 were associated with subdural/subarachnoid haemorrhage. Concerning the cases of non-traumatic subarachnoid haemorrhage, 2 were linked to aneurysmatic rupture (right anterior cerebral artery and anterior communicating artery, respectively) and 1 to vascular dissection of the right vertebral artery.
Ocular Presentation
Bilateral intraocular haemorrhage occurred in 87.5% (n=7) of the patients. Vitreous and preretinal haemorrhage occurred each in 66.7% (n=10), intraretinal in 30% (n=3), and subretinal in 13.3% (n=2) of the eyes. Figure 1 shows two examples of fundoscopic presentations in our sample. BCVA at presentation was 1.11 ± 1.01 logMAR (20/257 Snellen) and it was worse in eyes with vitreous haemorrhage (1.60 ± 0.83 vs 0.40 ± 0.02 logMAR, p=0.001). Male patients presented worse baseline BCVA (1.77 ± 0.75 vs 0.46 ± 0.79 logMAR, p=0.006) but also more cases of vitreous haemorrhage (100% vs 37.5%, p=0.026). Terson syndrome diagnosis occurred 58.47 ± 40.94 days after the neurological event (range 11 to 121 days). However, when reviewing the head CT scans requested for re-evaluation of the neurological condition, a crescent sign in the posterior pole of the eye was evident before the ophthalmological diagnosis in two cases (Figure 2).
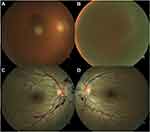 |
Figure 1 Fundoscopic presentation of two patients with bilateral Terson syndrome. (A and B) show, respectively, the right and left eyes of a patient with multiple peripapillary pre-retinal haemorrhages after traumatic brain injury (patient 6 in Table 3). The second set of images belongs to a patient with a subarachnoid haemorrhage due to rupture of an anterior communicating artery aneurysm (patient 8 in Table 3). The right eye (C) presented with a discrete vitreous haemorrhage and a dense pre-retinal macular haemorrhage, while the left eye (D) shows a very dense vitreous haemorrhage. |
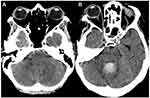 |
Figure 2 Head CT scan of patients 3 and 5 (Table 3) showing a positive crescent sign in both right eyes ((A and B), respectively). In the first case, the sign is present 1 day after the diagnosis of a subarachnoid haemorrhage due to an intradural dissection of the right vertebral artery, and 10 days before the ophthalmological diagnosis. In the second clinical picture, the sign is visible 11 days after the diagnosis of a subarachnoid haemorrhage due to the rupture of a right anterior cerebral artery aneurysm and 109 days before the diagnosis of Terson syndrome was made. |
Management Strategy
In 40% of the eyes (n=6), spontaneous resolution of intraocular haemorrhage occurred in 2 months, while PPV was performed in the remaining 60% (n=9; 4 with combined phacoemulsification). The timing of PPV after neurological and ophthalmologic diagnosis was 91.22 ± 52.07 (range 44–189) days and 24.33 ± 24.11 (range 5–69) days, respectively.
The only intraoperative complication observed was one posterior capsule rupture with herniation of the lens fragment in the vitreous cavity in a combined phacoemulsification and PPV. The lens fragment was removed during PPV, and an intraocular lens was successfully placed in the sulcus. However, a recurrence of vitreous haemorrhage was noticed in this eye during follow-up and, after being submitted to a second PPV, a total retinal detachment occurred.
Two other eyes developed cataract in the post-operative period and later underwent phacoemulsification and intraocular lens implantation.
The two eyes with subretinal haemorrhage developed macular atrophy (Figure 3). In this patient, the bilateral dense vitreous haemorrhage prevented the visualization of pre- and subretinal bleeding before surgery. During PPV, these lesions were already dehemoglobinized with a yellow appearance, so intravitreal tissue plasminogen activator (tPA) was not used.
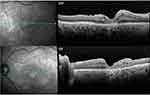 |
Figure 3 Optimal coherence tomography (OCT) scan of the right and left eye (OD and OS, respectively) of patient 4 (see Table 3), showing outer retina atrophy in the foveal area and some intraretinal fluid, after a bilateral subretinal haemorrhage. |
There was a recurrence of vitreous haemorrhage in the eye with posterior capsule rupture and, after being submitted to a second PPV, a total retinal detachment occurred. Two other eyes developed cataract in the post-operative period and later underwent phacoemulsification and intraocular lens implantation.
Table 2 describes the ocular presentation, baseline BCVA, management strategy adopted, complications and visual outcomes for each patient/eye.
Visual Outcomes
BCVA improved to 0.32 ± 0.69 logMAR (20/42 Snellen) in the follow-up period (p=0.004). Initial BCVA positively correlated with final BCVA (Spearman’s rho = 0.643, p=0.01).
Despite the discrepancies in baseline BCVA, no statistical differences were found in final BCVA between genders (male: 0.62 ± 0.94 vs female: 0.05 ± 0.07 logMAR, p=0.161) and between presence or absence of vitreous haemorrhage (0.46 ± 0.81 vs 0.02 ± 0.03 logMAR, respectively, p=0.114).
Baseline BCVA of eyes undergoing PPV was lower than of those conservatively managed (1.84 ± 0.72 vs 0.20 ± 0.28 logMAR, p<0.001). However, BCVA improvement was higher in the surgical group than in the observation group (−1.29 ± 1.01 vs −0.08 ± 0.16, p=0.007) and there were no statistically significant differences in final BCVA after surgery or observation (0.56 ± 0.90 vs 0.04 ± 0.04 logMAR, p=0.149).
Longer periods between the neurological and the ophthalmological diagnosis were correlated with worse final BCVA (Spearman’s rho = 0.688, p=0.005).
Table 3 summarizes the visual acuity data described.
 |
Table 2 Clinical Characteristics of Patients with Terson Syndrome |
 |
Table 3 Summary of Visual Acuity Data |
Discussion
Even though the first cases of Terson syndrome were reported in the 19th century, there is still debate regarding underdiagnosis and timely surgical management.
In similarity with other series, the sample described in this paper is composed of predominantly middle-aged patients with no relevant ophthalmological history.13,15,17 It is particularly important to prevent long-term visual sequela in this group of patients, since it may affect their neurological recovery and their capacity to return to an active and productive life.
The majority of patients (7 in 8 patients) presented with subarachnoid haemorrhage, whether related to traumatic brain injury or not, which is the most frequent cause of Terson syndrome.18
Ophthalmological presentation is variable, as blood can be located from the subretinal space to the vitreous cavity. In this sample, vitreous and pre-retinal haemorrhage were the most frequent presentations (66.7% of the eyes, each), which are usually indications for PPV if there is no spontaneous reabsorption of the blood, while intraretinal haemorrhages cannot be managed surgically.13
BCVA at presentation was markedly decreased (1.11 ± 1.01 logMAR, corresponding to 20/257 Snellen), particularly in eyes with vitreous haemorrhage.
Male patients exhibited lower baseline BCVA, which was possibly explained by a higher proportion of eyes with vitreous haemorrhage. No references were found in the literature regarding differences in presentation and outcomes between genders.
We reported a mean difference of 60 days between the neurological and the ophthalmological diagnosis. In 2000, Gnanaraj et al reported a mean interval between visual symptoms and referral to an ophthalmologist of 5.2 months for the nine unilateral cases and 4.9 months for the eight bilateral cases of Terson syndrome.19 Since this delay in diagnosis was correlated with final BCVA in our sample, it is crucial to identify potential causes and adopt measures to minimize this temporal gap.
Moreover, in two cases a positive crescent sign in the posterior pole of the eye was evident in the head CT scans, before ophthalmology evaluation was requested, which is a highly sensitive and specific marker for high risk vision loss in Terson syndrome.8 We understand that, in a critical care setting, the priority is to keep the patient alive and stable, whilst other complications, such as visual threatening conditions are rendered secondary. Moreover, the ability of these patients to complain about their visual deficits is frequently affected by their depressed conscious level and other neurological disturbances. However, early diagnosis allows for the identification of patients who are more likely to require surgery, serial evaluations to assess the evolution of the haemorrhages and the implementation of conservative measures, such as limiting physical activity and adopting a semi-recumbent position to allow for settling of the blood, improved vision and more thorough fundoscopic examination.20 A longer period of haemorrhage increases the risk of iron toxicity, the formation of pre-retinal membranes, and the development of retinal atrophy in subretinal bleeding.21–23
Intraocular haemorrhage resolved in 40% of the eyes with conservative measures. The remaining 9 eyes were submitted to surgery. Shaw et al reported that as many as 44% of vitreous haemorrhage cases in Terson syndrome do not resolve within 19 months.24
Only one eye developed important postoperative complications – rebleeding and a secondary retinal detachment. However, it must be highlighted that the vitreous haemorrhage in this patient was diagnosed 4 months after the neurological episode, which may have affected the surgical result.
One patient displayed a bilateral yellow subretinal haemorrhage, which was obscured by combined vitreous and preretinal haemorrhage. Despite being submitted to PPV, bilateral outer retina atrophy developed with marked vision loss. An earlier diagnosis and intervention with combined tPA injection could have in theory partially prevented the development of atrophy.
Two eyes submitted to PPV developed a cataract in the follow-up period, which was an expected complication.19,25,26
The final BCVA for our sample was 0.32 logMAR (equivalent to 20/42 Snellen), which is a satisfactory improvement when compared to the low baseline BCVA registered. However, if we take into account only the eyes in which the ophthalmological diagnosis was made within 1 month after the neurological event, the final BCVA was 0.17 logMAR (20/29 Snellen). In fact, longer periods between the neurological and the ophthalmological diagnosis were correlated with worse final BCVA in this sample.
Because of the lower baseline BCVA, improvement in BCVA was higher in the surgical group and no statistical differences were found between the surgical and the conservative managed groups concerning final BCVA, asserting the importance of PPV in restoring visual function when there is no spontaneous resolution of the intraocular haemorrhage. Due to the small sample size, no comparisons were made within the surgical group, regarding the timing of PPV. Some studies report better visual outcomes when PPV is performed within 90 days of vitreous haemorrhage, while others do not find differences.13,27
There are some limitations to this study. Firstly, the sample size is small. However, this is a rare and underdiagnosed syndrome, limiting the capacity to gather relevant data. Second, the retrospective nature of the study has its intrinsic disadvantages, including the need to rely on the accuracy of recordkeeping. Lastly, there was no well-defined internal protocol with criteria for surgery and each case was managed according to the surgeons’ expertise.
Conclusion
In conclusion, Terson syndrome is a potential cause of irreversible visual loss. Evaluation by an ophthalmologist is essential in the initial stages of traumatic brain injury or subarachnoid/intracerebral haemorrhage, particularly if presenting ophthalmologic complaints or depressed neurological status. Early diagnosis permits the adoption of conservative measures and the identification of patients who are more likely to require surgery. PPV is indicated when intraocular haemorrhage is dense and does not resolve spontaneously or when visual acuity at presentation is low, allowing for good visual outcomes with minimal complications. More awareness about Terson syndrome is essential to minimize the diagnosis delay and improve visual outcomes.
Acknowledgments
The authors would like to thank the patients involved in this case series. An earlier version of this work was presented at the EURETINA Congress 2022 as an audio-narrated free paper presentation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Litten M. Ueber einige vom allgemein-klinischen Standpunkt aus interessante Augenveränderungen. Berl Klin Wochenschr. 1881;18:23–27.
2. Terson A. De l’hémorrhagie dans le corps vitre au cours de l’hémorrhagie cerebrale. Clin Ophthalmol. 1900;6:309–312.
3. Kumaria A, Gruener AM, Dow GR, Smith SJ, Macarthur DC, Ingale HA. An explanation for Terson syndrome at last: the glymphatic reflux theory. J Neurol. 2022;269(3):1264–1271. doi:10.1007/s00415-021-10686-4
4. Raevis J, Elmalem VI. Pseudotumor cerebri syndrome causing a terson like syndrome. Am J Ophthalmol Case Rep. 2020;20:100993. doi:10.1016/j.ajoc.2020.100993
5. Ko F, Knox DL. The ocular pathology of Terson’s syndrome. Ophthalmology. 2010;117(7):1423–9.e2. doi:10.1016/j.ophtha.2009.11.028
6. Czorlich P, Skevas C, Knospe V, Vettorazzi E, Westphal M, Regelsberger J. Terson’s syndrome - Pathophysiologic considerations of an underestimated concomitant disease in aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2016;33:182–186. doi:10.1016/j.jocn.2016.04.015
7. Fountas KN, Kapsalaki EZ, Lee GP, et al. Terson hemorrhage in patients suffering aneurysmal subarachnoid hemorrhage: predisposing factors and prognostic significance. J Neurosurg. 2008;109(3):439–444. doi:10.3171/JNS/2008/109/9/0439
8. Stewart MW, Hasan SA, Collins C, et al. Can baseline computed tomography scans be used to identify patients at high risk of vision loss due to terson syndrome? Am J Ophthalmol. 2020;211:217–228. doi:10.1016/j.ajo.2019.09.016
9. Ballantyne AJ. THE OCULAR MANIFESTATIONS OF SPONTANEOUS SUBARACHNOID HAEMORRHAGE. Br J Ophthalmol. 1943;27(9):383–414. doi:10.1136/bjo.27.9.383
10. Manschot WA. Subarachnoid hemorrhage; intraocular symptoms and their pathogenesis. Am J Ophthalmol. 1954;38(4):501–505. doi:10.1016/0002-9394(54)90700-X
11. Cordes FC. Subhyaloid Hemorrhage Following Subarachnoid Hemorrhage*: report of Two Cases. Am J Ophthalmol. 1953;36(9):1192–8b. doi:10.1016/0002-9394(53)92282-X
12. Walsh FB, Hedges TR. Optic nerve sheath hemorrhage. Am J Ophthalmol. 1951;34(4):509–527. doi:10.1016/0002-9394(51)90294-2
13. Nazarali S, Kherani I, Hurley B, et al. OUTCOMES OF VITRECTOMY IN TERSON SYNDROME: a Multicenter Canadian Perspective. Retina. 2020;40(7):1325–1330. doi:10.1097/IAE.0000000000002570
14. Clarkson JG, Flynn HW
15. Narayanan R, Taylor SC, Nayaka A, et al. Visual outcomes after vitrectomy for terson syndrome secondary to traumatic brain injury. Ophthalmology. 2017;124(1):118–122. doi:10.1016/j.ophtha.2016.09.009
16. Liu X, Yang L, Cai W, Gao L, Li Y. Clinical features and visual prognostic indicators after vitrectomy for Terson syndrome. Eye. 2020;34(4):650–656. doi:10.1038/s41433-019-0547-3
17. Hong EH, Seong M, Yeom H, et al. Incidence of Terson Syndrome in Treated Subarachnoid Hemorrhage in South Korea: a National Health Insurance Database Study. Sci Rep. 2019;9(1):19048. doi:10.1038/s41598-019-55566-0
18. Kang HM, Cho JM, Kim SY, Choi JH. Clinical characteristics of asymptomatic Terson syndrome in the patients with aneurysmal subarachnoid hemorrhage. Int J Ophthalmol. 2020;13(2):292–300. doi:10.18240/ijo.2020.02.14
19. Gnanaraj L, Tyagi AK, Cottrell DG, et al. Referral delay and ocular surgical outcome in Terson syndrome. Retina. 2000;20(4):374–377. doi:10.1097/00006982-200007000-00009
20. Aboulhosn R, Raju B, Jumah F, et al. Terson’s syndrome, the current concepts and management strategies: a review of literature. Clin Neurol Neurosurg. 2021;210:107008. doi:10.1016/j.clineuro.2021.107008
21. Winter FC. Ocular hemosiderosis. Trans Am Acad Ophthalmol Otolaryngol. 1967;71(5):813–819.
22. Wise JB. Treatment of experimental siderosis bulbi, vitreous hemorrhage, and corneal blood staining with deferoxamine. Arch Ophthalmol. 1966;75(5):698–707. doi:10.1001/archopht.1966.00970050700023
23. Spraul CW, Grossniklaus HE. Vitreous Hemorrhage. Surv Ophthalmol. 1997;42(1):3–39. doi:10.1016/s0039-6257(97)84041-6
24. Shaw HE
25. Ritland JS, Syrdalen P, Eide N, Vatne HO, Øvergaard R. Outcome of vitrectomy in patients with Terson syndrome. Acta Ophthalmol Scand. 2002;80(2):172–175. doi:10.1034/j.1600-0420.2002.800210.x
26. Kuhn F, Morris R, Witherspoon CD, Mester V. Terson syndrome: Results of vitrectomy and the significance of vitreous hemorrhage in patients with subarachnoid hemorrhage. Ophthalmology. 1998;105(3):472–477.
27. Garweg JG, Koerner F. Outcome indicators for vitrectomy in Terson syndrome. Acta Ophthalmol. 2009;87(2):222–226. doi:10.1111/j.1755-3768.2008.01200.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
