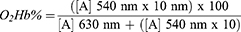Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 14
Technique for Darkening of Extracted Teeth Simulating Pulpal Necrosis Discoloration
Authors Paini TRD, Uchimura JYT, Sato F, Medina Neto A, Shimauti ELT, Baesso ML, Hidalgo MM, dos Santos MLA, Previdelli ITS, Pascotto RC
Received 9 February 2022
Accepted for publication 9 April 2022
Published 28 April 2022 Volume 2022:14 Pages 103—112
DOI https://doi.org/10.2147/CCIDE.S361230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Thais RD Paini,1 Joana YT Uchimura,1 Francielle Sato,2 Antonio Medina Neto,2 Eliana LT Shimauti,3 Mauro L Baesso,2 Mirian M Hidalgo,1 Marcia LA dos Santos,4 Isolde TS Previdelli,4 Renata C Pascotto1
1Department of Dentistry, State University of Maringa, Maringá, PR, Brazil; 2Department of Physics, State University of Maringa, Maringá, PR, Brazil; 3Department of Clinical Analysis and Biomedical Science, State University of Maringa, Maringá, PR, Brazil; 4Department of Statistics, State University of Maringa, Maringá, PR, Brazil
Correspondence: Renata C Pascotto, Department of Dentistry, State University of Maringa, Av. Mandacaru 1.550, Maringá, PR, 87080-000, Brazil, Tel +55 44 99820215, Fax +55 44 30319051, Email [email protected]
Background: The use of discolored teeth is required to test whitening products, and it is difficult to obtain them, given their scarcity.
Objective: To present a technique for in vitro darkening of extracted teeth simulating pulpal necrosis discoloration.
Materials and Methods: Hemolysates I and II from human blood were subjected or not to laser irradiation (442 nm) for 1 h. The concentration of oxyhemoglobin (O2Hb) was analyzed by ultraviolet/visible spectroscopy, and the conversion of O2Hb to methemoglobin (MetHb) by transmission spectroscopy was assessed immediately and after 3 and 40 days. For darkening evaluation, bovine incisors were divided into two groups (n = 25), and their pulp chambers were filled with hemolysate solution II (HSII) and hemolysate II solution + laser (HSII+L). After storage in artificial saliva for 40 days at 37°C, color changes were measured by a colorimeter and ΔE was compared with the NBS parameters. Data were analyzed using a mixed linear model (α=5%).
Results: HSII+L presented the lowest O2Hb and higher MetHb. The conversion of O2Hb to MetHb in HSII+L was 42% higher than in HSII. Both groups were effective in darkening the teeth, according to the NBS. Darkening stabilized from day 35. HSII promoted a marked color difference.
Conclusion: The proposed technique was effective in darkening the extracted teeth simulating necrosis discoloration for in vitro models.
Keywords: teeth, blood, colorimetry, hemoglobin, spectroscopy
Graphical Abstract:

Introduction
Extracted human teeth have been widely used in research around the world. In many in vitro experimental models, the use of discolored teeth is required to test whitening products, and it is difficult to obtain them, either from dental clinics or from tooth banks, given their scarcity.1,2 The alternative is to pigment extracted teeth and use alternative specimens.2
In the classic model for in vitro induction of tooth darkening proposed by Freccia and Peters (1982),3 the extracted teeth were immersed in blood containing a minimum of plasma, hemolyzed with distilled water, and centrifuged daily for 3 days, repeating this process for another 3 days.3 This methodology went through several modifications, such as total removal of plasma, saline solution, other hemolytic processes, variations in the centrifugation protocol, or different periods of tooth incubation in blood, which resulted in greater or less effective pigmentation time.4–7 To bring the in vitro protocol closer to clinical practice, once tooth discoloration in traumatized anterior teeth is commonly associated with necrotic pulp tissue and hemorrhage into the pulp cavity, Van Der Burgt, Mullaney and Plasschaert (1986)8 proposed the dental crown should be darkened from the inside out, ie, introducing the solution of blood components, consisting of a concentrated hemolysate with 10% hemoglobin, into the pulp chamber.8 They concluded that measurable color changes in the dental crowns were produced using this method, but they did not evaluate, more specifically, the conversion of oxyhemoglobin (O2Hb) to methemoglobin (MetHb) and its alteration during the oxidation process responsible for tooth discoloration.
There is a lack of studies demonstrating which hemolyzed solution is the most effective in producing MetHb and whether laser irradiation, which is capable of causing the direct conversion of O2Hb to MetHb, can accelerate tooth discoloration.
The objective of this study was to present a technique for in vitro darkening of extracted teeth simulating pulpal necrosis discoloration, evaluating the best hemolysate to be used in the technique. The first null hypothesis is that the proposed technique for darkening of teeth via application of hemolyzed blood inside the pulp chamber cannot darken the teeth. The second null hypothesis is that exposure of hemolyzed blood to laser irradiation does not promote more intense darkening of the tooth structure in a shorter period.
Materials and Methods
Blood Used for in vitro Tooth Darkening
Prior to starting the study, participant provided informed written consent to be part of the study, and then ethical approval has been obtained for all protocols from the Research Ethics Committee for research on humans from the State University of Maringá, Paraná, Brazil (CAAE 14382313.9.0000.0104).
Whole blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes upon venipuncture performed on a healthy adult female volunteer without hemoglobinopathies (HbAA) and without anemia (Hb total 12.9 g/dL). This solution was obtained from whole blood with EDTA, centrifugation, and removal of plasma, followed by the addition of double distilled water to promote hemolysis (hemolysate solution I, HSI) and additional centrifugation for 20 min to ensure that all red blood cells be ruptured (hemolysate solution II, HSII).
The electrophoretic profile of hemoglobin and hematological parameters were obtained using electrophoresis on cellulose acetate (Merck, Darmstadt, Germany) with Buffer tris (Merck, Darmstadt, Germany) at pH 8.59 and BC-300 PLUS-Mindray hematology analyzer (Mindray, Nanshan, Shenzhen), respectively. Whole blood was processed at 37°C to obtain HSI and HSII, and aliquots thereof (4 mL) were subjected to 70-watt He-Cd laser excitation (IK5651 R-G, Kimmon Koha CO. LTD, Japan) with a wavelength of 442 nm for 1 h, with homogenization every 15 minutes (Figure 1). Additional centrifugation in HSI allowed obtaining HSII without plasma, generating a more concentrated solution and making lysis of red blood cells easier. All samples, that is, whole blood, packed red blood cells, HSI and HSII, with and without laser, were analyzed immediately after collection, at 3 days, and at 40 days after collection and storage in a glacier at 4°C.
 |
Figure 1 Flowchart of laboratory processing of human blood, showing the samples to be used in the experiments. |
Oxyhemoglobin Concentration Analysis
Concentration of O2Hb relative to that of MetHb was determined in whole blood samples, packed red blood cells, HSI, and HSII, with and without laser application, stored at 4°C, at the three time points, using three replications. The proposed technique is based on the stabilization of hemoglobin with 60 mol L-1 phosphate buffer (Merck, Darmstadt, Germany), assessed by spectrophotometry at two wavelengths, one for MetHb (630 nm) and one for O2Hb (540 nm).10 Two tubes (labeled A and B) were employed for the analysis. An aliquot of 100 µL of the sample to be tested was placed in tube A, followed by 6 mL of 60 mol L-1 phosphate buffer. Tube B received 300 µL of the tube A solution, followed by 3 mL of 60 mol L-1 phosphate buffer. Spectrophotometric readings were performed at 630 nm in tube A, and at 540 nm in tube B, using SP-2000UV (Biosystems, São Paulo) spectrophotometer. The concentration of O2Hb (%) was obtained with the following formula:10
Note. [A]: Absorbance values; coefficient 10; dilution performed in tube B (300 µL from tube A and 3 mL of phosphate buffer) to obtain technical sensitivity in the spectrophotometric reading of oxyhemoglobin at 540 nm.10
The regression model was estimated using generalized least squares, allowing the residual variance of the observations in each treatment to differ. A graphical analysis showed randomness in the residuals and no discrepancy, indicating good fit/adaptation of the model.
Component Absorbent Analysis
Component absorbents (chromophores) were analyzed in samples from all stages of laboratory blood processing immediately after collection and 40 days after collection, using a Lambda 1050 UV/Vis/Nir Perkin Elmer spectrophotometer (Perkin Elmer, Walthan, MA, USA). An aliquot of blood samples (50 µL) was inserted between two quartz slides, reducing the optical path across the sample. This procedure was adopted because of the high sample concentration. All the samples were prepared likewise, at room temperature. The transmitted spectra were recorded from 200 to 800 nm, using two blank quartz slides as background.11
To analyze the laser action as a function of time, an aliquot of the newly collected HSII was also prepared by this same technique and subjected to blue laser radiation (442 nm) for 6 hours, with spectrophotometric analyses every hour.
The obtained spectra were analyzed using SciDavis software. The absorption bands of O2Hb (541 nm and 577 nm) and MetHb (635 nm) were identified and analyzed as a function of time.
Selection, Preparation, and in vitro Darkening of Teeth
Fifty upper central bovine incisors, donated by a regulated and certified slaughterhouse, were used. The animals used were from the same herd to ensure that they were of similar ages and had a similar stage of dental calcification. The teeth were cleaned with curettes and carefully examined using a magnifying glass (4x, Bio-Art, São Carlos, São Paulo, Brazil) to check for cracks and fractures which, if present, would lead to the exclusion of the specimens from the sample.
A cross-section was carried out at the cervical third of the root with a diamond disk at low speed adapted in a cutting machine (Isomet 1000, Buehler, Lake Bluff, Illinois) under water cooling, disregarding the middle and apical thirds, for insertion of the solution samples (HSII and HSII + laser, n = 25 for each group) until the pulp chamber was completely filled and the teeth were artificially darkened. Maxxion R® glass ionomer cement (FGM, Joinville, Santa Catarina, Brazil) was used for sealing at the cement enamel junction, and the teeth were immersed in artificial saliva12 in individual flasks and stored at 37°C for 40 days, with medium change every 24 hours.
Color Evaluation
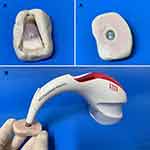
The VITA Easyshade® Advance spectrophotometer (VITA Zahnfabrik H. Rauter GmbH & Co. KG, Bad Sackingen, Baden-Wuerttemberg, Germany) was used for the color readings of teeth darkened in vitro, measured by the CIELab system – L * (luminosity), a * (red-green axis), and b * (yellow-blue axis), as recommended by the central bureau of the International Commission on Illumination (1986).13 The teeth were removed from artificial saliva, gently dried with absorbent paper, and positioned on an individual silicone guide (Figure 2). The guide had a hole built at the middle third of the crown to fit perfectly on the device, allowing the standardization of the reading area and preventing possible light reflection and interference with color reading. Color readings were done in triplicate, every 5 days up to 40 days, and the mean value was used. ΔE color variation was determined by Hunter’s formulation,14 and the resulting color change was analyzed according to the National Bureau of Standards (NBS) ΔE * ab distribution and categories, as follows:
1 0.0 to 0.5 – extremely slight change;
2 0.5 to 1.5 – slight change;
3 1.5 to 3.0 – noticeable change;
4 3.0 to 6.0 – marked change;
5 6.0 to 12.0 – extremely striking change; and
6 12.0 or higher – change to another color.15
Statistical Analysis of Color Evaluation
The sample size was estimated using a samples_mixed function in the sjstats library of the R software, which had a sample size of approximately n = 50, considering the test power of 85%, significance level of 5% and size of the effect given by the generalized eta square ges = 0.409.
Considering that the main goal of this study was to verify if there was any difference in the response variable between groups and time, mixed linear models were employed, incorporating the dependence between the observations, ie, the effect of the variability inherent to each observational unit (tooth),16 resulting in more robust and efficient models.
This methodology seeks to consider the fixed effect on the response variable we are used to finding in linear models and it also models the covariance structure by means of a random effect, in this case, the dependence of the inherent variability of each tooth on measurements over time. Therefore, this methodology considers the variability within/between experimental units.
All the models were adjusted in the R application using the lme4 command.
Results
Table 1 shows the concentration of O2Hb by UV/VIS spectroscopy at each stage of the laboratory processing of blood samples, with and without the use of laser, at different time points. The results indicated T3 (p = 0.001) and T40 (p = 0.002) differed from T0, as packed red blood cell and whole blood treatments differed from the control – HSII + laser (p = 0.001). The interactions between whole blood (p < 0.001) and packed red blood cell (p < 0.001) treatments over time were also significant, ie, changes in O2Hb concentration due to these treatments over time were different from other treatments. For example, at T40, O2Hb concentration showed a large increase in whole blood, whereas the concentration of packed red blood cells decreased, unlike other treatments (linear regression, generalized least squares).
 |
Table 1 UV/VIS Spectroscopy Evaluation of O2Hb Concentrations in Blood Samples from Different Laboratory Processing Stages with and without Laser Irradiation for 1 Hour, in Different Periods |
Figure 3A shows the absorption spectra obtained by transmission spectroscopy of the HSII solution during different periods of exposure to laser irradiation. In the representative spectrum of the sample without laser exposure, the centers of the bands were found at 577 nm, 541 nm, 415 nm, 348 nm, and 278 nm, representing, respectively, β-O2Hb, α-O2Hb, Soret band, globin-heme interaction, and amino acids/chromophores of blood.17 Soret band is a term commonly used in spectroscopy to refer to the absorption of the heme group of hemoglobin (Hb). As the time of exposure to the laser increased, there was displacement of the Soret band from ~415 nm to ~407 nm, a decrease in the intensity of O2Hb bands, and the presence of MetHb bands centered at ~498 nm and ~630 nm. Figure 3B shows the conversion of O2Hb to MetHb in the periods of exposure to the laser, and the highest conversion rate was observed after 2 hours. For longer periods of time, conversion tended to stabilize. Figure 3C shows the presence of MetHb in the HSII solution after 40 days with and without laser exposure. The area under the curve for the MetHb band in the laser-irradiated sample was 42% higher than that of the non-irradiated sample.
The means and standard deviations of the CIELab system for the teeth darkened with HSII with and without the use of laser exposure, in comparison with their initial color, are shown in Table 2. The resulting color differences (ΔEab) are also shown, compared to the natural color of the teeth, according to the NBS categorization.
The analysis of the variables “days” and “group” with the mixed linear model revealed no statistically significant difference in the darkening of the teeth between days 35 and 40, that is, the darkening seems to stabilize from day 35, independently of the group (Figure 4). However, when we analyzed the darkening using NBS as a reference, we observed that, from day 10 onwards, both groups reached an extremely marked color change (NBS 5) or color change (NBS 6) - Table 2.
 |
Figure 4 ΔEab representation of teeth darkened with hemolysate II solution with and without laser application. |
In the mixed linear model, the group*days interaction was not significant (p > 0.05), ie, the results demonstrated no significant difference in the darkening of teeth by the two tested techniques (HSII and HSII+L). However, there was a significant difference in darkening over the days (p < 0.001), ie, there was a temporal effect, but it did not differ between the HSII and HSII+L groups.
Discussion
The first null hypothesis was rejected, given that the technique proposed for the darkening of teeth by application of hemolyzed blood inside the pulp chamber promoted tooth darkening.
HSII was chosen for tooth darkening and proved to be superior to HSI, once the laboratory blood processing stage demonstrated that this hemolysate solution has a higher O2Hb concentration, which indicates that a larger amount of MetHb is being formed and converted to heme and Fe+++, increasing proportionally the concentration of O2Hb. Experimental data supported the choice of HSII and allowed suggesting a mechanism of action. A quantitative analysis using a spectrophotometer and color variation calculations proved the effectiveness of the color change obtained.
The O2Hb measured by UV/VIS spectrophotometry in the HSII+L group exhibited a statistically significant decrease in the percentage between this solution and non-lysed samples (Table 1). As there is an inversely proportional relationship between O2Hb and MetHb,10 there would be a higher concentration of MetHb in the HSII+L group. It is known that an increase in MetHb concentration makes the color of blood change from red to brown and eventually degrades MetHb inside red blood cells.18,19 The accumulation of MetHb can lead to the formation of hemichromes and these, when denatured, can release the heme portion and Fe+3 atoms. This metal can act as a catalyst for oxidation reactions of biomolecules in the Fenton and Haber-Weiss reactions, producing the highly reactive hydroxyl (OH−) radical, responsible for protein denaturation and lipid peroxidation.10,20
The experiment with time variation in laser exposure of up to 6 hours (Figure 3A) was performed to verify its direct effect on the sample of HSII without the tooth, considering an extreme condition, ie, minimum sample volume with maximum area of incidence, thus exposing most of the sample to incident radiation. The absorption spectrum obtained without laser exposure was similar to that which had been previously described.17,21 It was possible to observe the bands corresponding to the globin–heme interaction, blood amino acids/chromophores, and heme groups of O2Hb, α-O2Hb, and β-O2Hb. Laser irradiation caused direct conversion of O2Hb to MetHb, as described by Tomoda, Tsuji, Yoneyama,21 who cited intermediate processes of this conversion using oxidizing reagents and indicated some spectral changes different from those obtained in this study. Laser accelerates biological processes by breaking the molecules apart, facilitating oxidation and boosting the production of free radicals,22 and due to their influence on rheological factors,23 free radicals can interact with O2Hb and generate MetHb.20,21
In the presence of MetHb, the maximum conversion rate of O2Hb to MetHb occurred after 2 hours of laser incidence, tending to stabilize thereafter (Figure 3B). Therefore, laser irradiation was applied for one hour, considering that the process would have already started. This was confirmed by the analysis of the HSII spectra after 40 days with and without laser exposure (Figure 3C), which demonstrated a 42% larger area under the curve in the MetHb band after laser exposure than that of the non-irradiated sample. In this process, the formation of hemichromes was not observed, which would be characterized by the displacement of the bands centered at 540 nm and 575 nm, from α-O2Hb and β-O2Hb, respectively, to 535 nm and 565 nm.24
The use of HSII inside the pulp chamber, providing darkening from the inside out, is justified, as it is known that the demand for tooth darkening in the clinic occurs mainly due to trauma, in which the rupture of blood vessels causes hemorrhage in the pulp chamber with consequent diffusion of red blood cells, followed by hemolysis. The released Hb combines with the pulp tissue that becomes putrefied and with the hydrogen sulfates produced by the bacteria, giving rise to ferrous sulfide (FeS), a black compound responsible for pigmentation, which penetrates into the dentinal canaliculi.4,25
Contrary to what Van Der Burgt, Mullaney and Plasschaert did in their study, in the present study, the pulp was not extirpated through an access opening to follow a protocol as close as possible to the clinical reality. The presence of the pulp tissue possibly contributed to the further discoloration of teeth.
The second null hypothesis was accepted, as the exposure of hemolyzed blood to laser irradiation did not promote more intense darkening of the tooth structure in a shorter period.
The mixed linear model demonstrated no significant difference between the groups (HSII and HSII+L) regarding the darkening of the teeth or when comparing the variation over time (p < 0.001). However, a significant difference was observed in tooth darkening over the days (p < 0.001), ie, there was a temporal effect, but it did not differ between the HSII and HSII+L groups. Therefore, the mixed linear model evaluating only the variable “days” allowed us to observe no statistically significant difference in the darkening of the teeth between days 35 and 40, that is, darkening stabilized from day 35, regardless of the group. However, when darkening was analyzed using NBS as a reference, both groups reached an extremely marked color change (NBS 5) or color change (NBS 6) from day 10 onwards.
The classic model for in vitro tooth darkening suggests immersion of extracted teeth in blood containing a minimum of plasma, made by hemolysis with distilled water and daily centrifugations.3 This procedure only breaks down the red blood cells. In the present study, the hemolysate was injected into the pulp chamber, as proposed by Van Der Burgt Mullaney and Plasschaert.8 Other studies suggest modifications, including total removal of plasma, addition of iron sulfide to the tooth before immersing it in blood, other hemolytic processes with the addition of other solutions, variations in the centrifugation protocol in relation to the number of days, or different immersion periods of teeth in the blood,4–7 but none of these methodologies are close to the clinical reality (necrosis). There are no studies in the literature describing a tooth darkening method that comes close to the discoloration caused by necrosis, which is the innovative element of the methodology proposed in the present study.
The proposed technique for darkening extracted teeth consists in placing HSII in the pulp chamber. This solution is obtained from whole blood with EDTA, centrifugation, and removal of plasma, followed by the addition of double distilled water to promote hemolysis, and additional centrifugation for 20 minutes to ensure that all red blood cells have ruptured. The access to the pulp chamber is sealed with glass ionomer cement, and the tooth should be immersed in artificial saliva for 35 days, at 37°C, with medium change every 24 hours.
However, in this study, HSII exposed to laser irradiation for one hour had the lowest O2Hb concentration and, consequently, the highest MetHb concentration, it did not promote a significant change in the absorption spectrum, indicating that HSII properties were maintained. The conversion of O2Hb to MetHb started within one hour after laser application, reaching the maximum rate at 2 hours, and tending to stabilize thereafter. Laser application, however, did not promote faster darkening of the tooth structure, considering that both hemolysates were effective, as evidenced by the change to another color, according to the NBS parameters.
A limitation of this study was the use of bovine teeth to test the darkening technique. Obtaining sound extracted human incisors is very difficult, whether in dental clinics or in tooth banks, given their low occurrence.2
Although a recent study of Wang et al 202126 has observed that bovine teeth can be used as an excellent alternative to human teeth in dental research given the proximity of the enamel microstructure, these teeth have dimensions much larger than that of human incisors, and consequently, they needed a greater amount of hemolyzed blood to fill the pulp chamber, which could influence the tooth darkening time.
Because the tooth pigmentation with the proposed method has the potential of naturally resembling tooth necrosis, the method can be used in future studies for comparisons with in vitro bleaching treatments.
Conclusion
Laser application of the hemolysate blood did not promote faster darkening of the tooth structure. Intracoronal tooth darkening with or without laser application proved to be effective for in vitro darkening of teeth, simulating discoloration by pulp necrosis from day 35.
Acknowledgments
The authors thank the Brazilian funding agencies FINEP (Financiadora de Estudos e Projetos) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for their financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nassif ACS, Tieri F, Ana PA, et al. Structuralization of a human teeth bank. Braz Oral Res. 2003;17 Suppl 1:70–74. doi:10.1590/s1517-74912003000500012
2. Teruel JD, Alcolea A, Hernández A, et al. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol. 2015;60(5):768–775. doi:10.1016/j.archoralbio.2015.01.014
3. Freccia WF, Peters DD. A technique for staining extracted teeth: a research and teaching aid for bleaching. J End. 1982;8(2):67–69. doi:10.1016/S0099-2399(82)80260-4
4. Marin PD, Bartold PM, Heithersay GS. Tooth discoloration by blood: an in vitro histochemical study. Dent Traumatol. 1997;13(3):132–138. doi:10.1111/j.1600-9657.1997.tb00026.x
5. Kaneko J, Inoue S, Kawakami S, et al. Bleaching effect of sodium percarbonate on discolored pulpless teeth in vitro. J End. 2000;26(1):25–28. doi:10.1097/00004770-200001000-00006
6. Yui KCK, Rodrigues JR, Mancini MNG, et al. Ex vivo evaluation of the effectiveness of bleaching agents on the shade alteration of blood stained teeth. Int J Endod. 2008;41(6):485–492. doi:10.1111/j.1365-2591.2008.01379.x
7. Cardoso M, Martinelli CSM, Carvalho CAT, et al. Ultrasonic activation of internal bleaching agents. Int Endod J. 2013;46(1):40–46. doi:10.1111/j.1365-2591.2012.02090.x
8. Van Der Burgt TP, Mullaney TP, Plasschaert AJM. Method for inducing reproducible intrinsic discoloration in extracted human teeth. Int J Endod. 1986;19(1):19–35. doi:10.1111/j.1365-2591.1986.tb00887.x
9. Marengo-Rowe AJ. Rapid electrophoresis and quantitation of haemoglobin on cellulose acetate. J Clin Pathol. 1965;18(6):790–792. doi:10.1136/jcp.18.6.790
10. Naoum PC, Radispiel J, Moraes MS. Dosagem espectrométrica de metahemoglobina sem interferentes químicos ou enzimáticos. Rev Bras Hematol Hemoter. 2004;26(1):19–22. doi:10.1590/S1516-84842004000100004
11. Wang Y, Qian W, Tan Y, Ding S. A label-free biosensor based on gold nanoshell monolayers for monitoring biomolecular interactions in diluted whole blood. Biosens Bioelectron. 2008;23(7):1166–1170. PMID: 18078744. doi:10.1016/j.bios.2007.10.020
12. Hashimoto M. A review-micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J Biomed Mater Res A. 2010;92(1):268–280. doi:10.1002/jbm.b.31535
13. Central Bureau of the International Commission on Illumination. Colorimetry. Vienna: CIE Publication 30; 1986.
14. Browning WD, Contreras-Bulnes R, Brackett MG, et al. Color differences: polymerized composite and corresponding Vitapan Classical shade tab. Int J Dent. 2009;37(Suppl1):e34–9. doi:10.1016/j.jdent.2009.05.008
15. Lee YK. Comparison of CIELAB Delta E(*) and CIEDE 2000 color-differences after polymerization and thermo cycling of resin composites. Dent Mater. 2004;21(7):678–682. doi:10.1016/j.dental.2004.09.005
16. Diggle PJ, Heagerty P, Liang KY, et al. Analysis of longitudinal data. Oxford: Oxford University Press; 2002: 2.
17. Maciel C, Fujita A, Gueroni DI, et al. Evans blue as simple method to discriminate mosquitoes’ feeding choice on small laboratory animals. PLoS One. 2014;9(10):e110551. doi:10.1371/journal.pone.0110551
18. Dacie JV, Lewis SM. Practical Haematology. Churchill Livingstone, Edinburgh-UK; 1995.
19. Hegesh F, Hegesh J, Raftary A. Congenital methemoglobinemia with a deficiency of cytochrome 65. N Engl J Med. 1986;314(12):757–761. doi:10.1056/nejm198603203141206
20. Kanias T, Acker JP. Biopreservation of red blood cells – the struggle with hemoglobin oxidation. FEBS J. 2010;277(2):343–356. doi:10.1111/j.1742-4658.2009.07472.x
21. Tomoda A, Tsuji A, Yoneyama Y. Mechanism of hemoglobin oxidation by ferricytochrome c under aerobic and anaerobic conditions. J Biol Chem. 1980;255(16):7978–7983. doi:10.1016/S0021-9258(19)43929-X
22. Wagner VP, Meurer L, Martins MAT, et al. Influence of different energy densities of laser phototherapy on oral wound healing. J Biomed Opt. 2013;18(12):1–7. doi:10.1117/1.JBO.18.12.128002
23. Mi XQ, Chen JY, Cen Y, et al. A comparative study of 632.8 and 532 nm laser irradiation on some rheological factors in human blood in vitro. J Photochem Photobiol B. 2004;19(74):7–12. doi:10.1016/j.jphotobiol.2004.01.003
24. Molchanova T. Alpha and Beta human hemoglobins. Hemichromes and their stability according to the proteolysis in vitro. Fragments from Doctoral thesis; 1981. Available from: http://www.tatianamolchanova.com/files/Hemichrome_alphaHb_AHSP_Molchanova.pdf.
25. Plotino G, Buono L, Grande NM, Pameijer CH, Somma F. Non Vital tooth bleaching: a review of the literature and clinical procedures. J Endod. 2008;8(4):394–407. doi:10.1111/j.1600-9657.1997.tb00026.x
26. Wang C, Fang Y, Zhang L, Su Z, Xu J, Fu B. Enamel microstructural features of bovine and human incisors: a comparative study. Ann Anat. 2021;235:151700. PMID: 33588042. doi:10.1016/j.aanat.2021.151700
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.