Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 7
Targeting of interleukin-17 in the treatment of psoriasis
Authors Lønnberg A, Zachariae C , Skov L
Received 10 May 2014
Accepted for publication 1 July 2014
Published 15 September 2014 Volume 2014:7 Pages 251—259
DOI https://doi.org/10.2147/CCID.S67534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Ann Sophie Lønnberg, Claus Zachariae, Lone Skov
Department of Dermato-Allergology, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark
Abstract: “Psoriasis” is a chronic immune-mediated inflammatory disorder with epidermal hyperplasia. There is some evidence that the cytokine interleukin-17A (often known as IL-17), which is mainly produced by Th17 cells, has a role in the pathogenesis of psoriasis. “IL-17” is a pro-inflammatory cytokine mainly important in the host's defense against extracellular bacteria and fungi. The three new therapies with biologic drugs – brodalumab, secukinumab, and ixekizumab – all target the IL-17 signaling pathway. Secukinumab and ixekizumab neutralize IL-17A, while brodalumab blocks its receptor. Results from clinical trials have shown marked improvements in disease severity in patients with moderate-to-severe plaque psoriasis, using any of these three drugs. The biologic agents were generally well tolerated, but the duration of the trials was relatively short. In this review, we focus on the role of the IL-17 cytokine family in the pathogenesis of psoriasis; the efficacy, safety, and tolerability of brodalumab, secukinumab, and ixekizumab in clinical trials; and possible differences between targeting of the IL-17A receptor and targeting of the IL-17A ligand.
Keywords: anti-IL-17 agents, IL-17, brodalumab, secukinumab, ixekizumab, psoriasis
Introduction
“Psoriasis” is a chronic immune-mediated inflammatory disorder characterized by uncontrolled proliferation of keratinocytes, activated dendritic cells, release of pro-inflammatory cytokines, and recruitment of T-cells to the skin.1,2 The disease affects approximately 2%–3% of the population in Western countries.3,4 Psoriasis arthritis affects up to 24% of patients with psoriasis.5 Diagnosis of psoriatic arthritis is important for the therapeutic strategy; however, it is often delayed or overlooked.6 Psoriasis is associated with several comorbidities, including cardiovascular disease, and several cardiovascular risk factors: hypertension, obesity, dyslipidemia, metabolic syndrome, diabetes mellitus, and smoking. Furthermore, psoriasis is associated with several psychiatric disorders, including depression, anxiety, and suicide tendency.7,8 This is probably related to the negative effect that the disease has on quality of life. Even patients with limited involvement often find the disease to be a great problem in everyday life.9,10
The pathogenesis of psoriasis is not fully understood. From family and twin-based studies, it is known that there is a genetic contribution, and several psoriasis risk loci have been identified. Twin studies have shown that concordance rates are much higher in monozygotic twins than in dizygotic twins. Genetic factors explain 68% of the variation in the susceptibility to psoriasis.11 Several genome-wide association studies on psoriasis have been performed and have primarily identified the psoriasis susceptibility genes one through ten (PSORS1 through PSORS10). PSORS1, the most important gene, is present on chromosome 6 and resides within the HLA (human leukocyte antigen) region. PSORS1 includes the HLA-Cw6 allele, which has the greatest genetic effect in Caucasians. Genome-wide association studies of psoriasis have identified 36 gene loci associated with psoriasis.12 These genes are involved in skin-barrier function (LCE3A, LCE3C, and LCE3D), T-cell signaling (IL2, IL21, IL12B IL13, IL23A, IL23R, RNF114 [previously ZNF313], NFKB, NFKBIA, TYK2, TNFAIP3, TNIP1, and TRAF3IP2), and antigen presentation (ERAP1 and HLA-C).13–15
Most patients diagnosed with psoriasis suffer from mild disease, for which topical medications and phototherapy are sufficient treatments. However, 20%–30% of patients suffer from moderate-to-severe disease and usually need systemic therapy. With conventional treatment, some patients experience loss of efficacy, side effects, or insufficient response. It is therefore important to investigate new possibilities for therapy.
The Psoriasis Area and Severity Index (PASI) is useful for selection of a treatment strategy.16 A “PASI 75” response refers to a 75% or greater reduction in baseline PASI. It is the most commonly used measure of efficacy in recent clinical trials, and in the studies described in this article. It is widely accepted as a measure of a clinically meaningful improvement.17–19
Methods
We performed a systematic review of all studies investigating the efficacy and safety of brodalumab, secukinumab, and ixekizumab for the treatment of psoriasis. The PubMed database was systematically searched using individual search terms or combinations of the search terms “psoriasis”, “IL-17”, “anti-IL-17”, “interleukin-17”, “anti-interleukin-17”, “brodalumab”, “secukinumab”, “ixekizumab”, “AMG 827”, “AIN 457”, “LY2439821”, “adverse effect”, and “safety”. Based on their titles and abstracts, relevant articles were selected for this review. Additional publications were collected from the reference lists. Abstracts presented at the American Academy of Dermatology meetings in 2013 and 2014 were reviewed for new results. Ongoing and completed trials were identified from the US National Institutes of Health ongoing trials register, ClinicalTrials.gov.
The IL-17 cytokine family
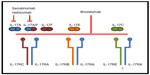
“Interleukin (IL)-17” (IL-17A) is a pro-inflammatory cytokine belonging to a family of six ligand members (IL-17A to IL-17F); these interleukins bind to five receptors (IL-17RA to IL-17RE). IL-17A is the prototype of the family. It is a dimeric glycoprotein, and in the circulation it exists as a homodimer of two IL-17A chains or as a heterodimer of IL-17A with IL-17F. A homodimer of IL-17A or one of IL-17F, and also the heterodimer of IL-17A and IL-17F, can bind to a heteromeric receptor consisting of IL-17RA and IL-17RC. All three cytokines require both receptors for their biological activity (Figure 1).20–23
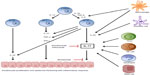
Cytokines of the IL-17 family are mainly produced by Th17 cells. These cells differentiate from naïve CD4+ cells in the presence of transforming growth factor-beta (TGF-β), IL-6, and IL-1β, whereas their survival and activation are controlled mainly by IL-23 (Figure 2). Thus, the production of IL-17 is closely related to and dependent on the presence of IL-23. Other cells involved in the production of IL-17 are as shown in Figure 2: Tc17 cells, mast cells, neutrophils, and natural killer (NK) cells.24–29
Th17 cells provide protection against extracellular bacterial and fungal pathogens such as Klebsiella pneumoniae, Citrobacter rodentium, Candida albicans, and, to a lesser extent, Staphylococcus aureus. Although less directly, Th17 cells also have a role in protection against intracellular bacteria such as Listeria monocytogenes, Salmonella enterica, and Mycobacterium tuberculosis.30–34
IL-17 has an important role in several immune-mediated disorders, including psoriasis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, uveitis, Crohn’s disease, multiple sclerosis, and asthma.23,35–37
Review of IL-17 in the pathogenesis of psoriasis
Both the innate and adaptive immune systems are involved in psoriasis. The hyper-proliferation of keratinocytes is driven by cytokines from T-cells, and the keratinocytes sustain and amplify the inflammatory response by producing further pro-inflammatory cytokines and chemokines.38 Psoriatic plaque shows infiltration of activated T-cells, especially Th1, Th17, and Th22 cells.2,39 These cells produce a large amount of interferon-gamma (IFN-γ), IL-17, IL-22, and tumor necrosis factor-alpha (TNF-α). There is some evidence that Th17 cells play an essential role in the pathogenesis of psoriasis.40
IL-17A and IL-17F act on a range of cells, including endothelial cells, fibroblasts, chondrocytes, osteoblasts, synovial cells, monocytes, and keratinocytes.23,40,41 This, together with the fact that several IL-17-related genes have been linked to psoriasis, and the remarkable results from clinical trials demonstrating the effect of the direct inhibition of both IL-17A and IL-17RA, is evidence of the central role of IL-17 in psoriasis.24,40,42 IL-17A and IL-17F are by far the most studied cytokines of the IL-17 family, but expression of IL-17C has also been found to be elevated in psoriatic skin. This cytokine also binds to a heterodimeric receptor complex, possibly including IL-17RA.40,43
Efficacy in trials of brodalumab, secukinumab, and ixekizumab
Three biologic drugs that target IL-17 signaling are currently being tested for the treatment of psoriasis in clinical trials: one monoclonal antibody directed against IL-17RA (brodalumab) and two monoclonal antibodies that directly neutralize IL-17A (ixekizumab, a humanized anti-IL-17A antibody, and secukinumab, a fully human anti-IL-17A antibody).
Brodalumab (AMG 827)
Brodalumab blocks the receptor subunit IL-17RA shared by IL-17A, IL-17C, IL17E, IL-17F, viral IL-17, and IL-17A/F heterodimer ligands.21,26,34
The efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis was investigated in a Phase II trial.44 In that double-blind study, 198 patients were randomized to receive either placebo or subcutaneously administered brodalumab (70, 140, or 210 mg) on day 1 and after 1, 2, 4, 6, 8, and 10 weeks, or 280 mg monthly. After 12 weeks of treatment, improvements in PASI score were significantly greater in patients who received 140, 210, or 280 mg than in the patients who received 70 mg or placebo. The highest improvement was seen in the group receiving 210 mg; in this group, PASI 75 was achieved by 82% of the patients, as compared with 0% in the placebo group. The Dermatology Life Quality Index (DLQI) was significantly lower in the brodalumab groups than in the placebo group. The patients of this Phase II study were selected on the basis of not having a serious concurrent medical illness, and they may therefore have been healthier than the general population of patients with psoriasis. From this study, 181 patients continued on to an open-label extension study and received 210 mg – or, if they weighed 100 kg or less, 140 mg – every second week. The data that are currently available (up to 96 weeks) show that the efficacy and the safety profile are generally consistent with those seen in the Phase II parent study.45 Clinical trials performed on a small sample with a single dose of brodalumab of up to 700 mg, which was given intravenously (iv), have shown lower effect, as illustrated in Table 1.46 According to ClinicalTrials.gov, current Phase III trials are investigating the safety and efficacy of brodalumab, including comparison with ustekinumab. Drug withdrawal and retreatment with brodalumab are also being investigated, as are drug–drug interactions and intensive pharmacodynamics.
Secukinumab (AIN 457)
“Secukinumab” is an antibody to the IL-17A ligand that selectively neutralizes IL-17A.25 The first results with this biologic agent in patients with psoriasis came from a randomized Phase I proof-of-concept clinical trial involving 36 patients diagnosed as having chronic plaque-type psoriasis.47 The patients were given a single dose of secukinumab at 3 mg/kg iv and the results showed a significant reduction in PASI of 63% at week 12, as opposed to 9% with placebo. Skin samples from typical psoriasis plaques showed decreased production of inflammatory cytokines and chemokines, and a reduction in T-cell infiltration.
In a randomized, double-blind, placebo-controlled Phase II dose-range study, secukinumab was tested in 125 patients diagnosed with psoriasis.48 They were randomized to receive either secukinumab (25, 75, or 150 mg) subcutaneously or placebo at weeks 0, 4, and 8, or a single dose of 25 mg secukinumab at week 0 followed by placebo. After 12 weeks, secukinumab at the two highest doses (75 and 150 mg) had significantly improved the PASI 75 score compared with placebo (57% and 82% of patients vs 9% for placebo). After the 12 weeks of treatment, the patients entered a 24-week follow-up period, during which the PASI 75 response rates for the two highest doses remained. When body weight was analyzed, the results clearly showed that patients weighing less than 90 kg had better PASI 75 responses than patients weighing more than 90 kg. Results from the same study suggested that there was an early and beneficial effect of secukinumab on psoriasis affecting the hands and/or feet.49
In a separate Phase II, randomized, double-blind, placebo-controlled, regimen-finding study, secukinumab was tested in 404 patients diagnosed with psoriasis.50 The patients were randomized to receive placebo or 150 mg secukinumab in three different regimes: single (week 0; n=66), early (weeks 0, 1, 2, and 4; n=133), or monthly (weeks 0, 4, and 8; n=138). After 12 weeks, PASI 75 responders from active treatment groups were re-randomized to either a fixed-interval regimen of 150 mg secukinumab at weeks 12 and 24 or a treatment-at-start-of-relapse regimen, also at a dose of 150 mg. The results after 12 weeks showed higher PASI 75 response rates with the early and monthly regimens than with placebo (Table 1). The highest improvement in PASI was seen with the early regimen. Results in patients entering the maintenance period showed that the fixed-interval regimen was superior to the treatment-at-start-of-relapse regimen. However, both were very effective: less than 10% of patients on either regimen experienced relapse 15 weeks after the last study drug was administered. The effect of body weight on treatment response rates showed slightly higher PASI 75 responses in patients weighing less than 90 kg than in those weighing more than 90 kg. Patients from that study who had involvement of hands, feet, or nails were analyzed in a post-hoc study.51 Altogether, 131 patients were included because of involvement of hands and/or feet, and 304 patients were included because of psoriasis of the fingernails. There were clinically relevant improvements in hand and foot involvement after 12 weeks of treatment with secukinumab using the early regimen. The early and monthly regimens were associated with substantial reductions in affection of the fingernail.
Results from abstracts presented at the 72nd Annual Meeting of the American Academy of Dermatology (2014) of Phase III studies comparing secukinumab with placebo and etanercept over a period of up to 52 weeks showed that subjects in each secukinumab-dose group (150 or 300 mg every 4 weeks) were significantly less likely than subjects in the placebo group or the etanercept group (50 mg every week) to experience loss of response; the benefit was greater in the 300 mg group.52,53 Also, patients receiving secukinumab reported faster meaningful improvement of symptoms of itching, pain, and scaling than patients who received etanercept or placebo.54
Many ongoing and completed trials can be found at ClinicalTrials.gov, but they are still not available through PubMed – including Phase III studies investigating the efficacy, safety, and tolerability of secukinumab in subjects with moderate-to-severe plaque psoriasis, palmoplantar psoriasis, or nail psoriasis.
Ixekizumab (LY2439821)
“Ixekizumab” is a humanized anti-IL-17 monoclonal antibody. It has been tested in 40 patients diagnosed with psoriasis in a 20-week-long, randomized, double-blind, placebo-controlled, Phase I trial.55 Patients were randomized to receive 5, 15, 50, or 150 mg of subcutaneous ixekizumab or placebo at weeks 0, 2, and 4. Punch biopsies were taken from the same psoriasis lesion at weeks 0, 2, and 6. The purpose of the study was to determine the effect that the neutralization of IL-17 has on the clinical features of psoriasis and to identify roles of IL-17 in inflammatory pathways underlying psoriasis. After 2 weeks, there were significant dose-dependent reductions in keratinocyte proliferation, hyperplasia, epidermal thickness, infiltration into the dermis and epidermis by T-cells and dendritic cells, and keratinocyte expression of innate defense peptides compared with at week 0. At 6 weeks, biopsy showed the near normalization of skin in patients treated with the two highest doses of ixekizumab (50 and 150 mg). Clinical efficacy was measured with PASI 75 and PASI 90 after 2, 6, and 20 weeks. The results were significant at weeks 6 and 20 for subjects who received 15, 50, and 150 mg of ixekizumab relative to placebo and relative to subjects who received 5 mg of ixekizumab (Table 1).
In a randomized, double-blind, placebo-controlled, Phase II trial, ixekizumab was evaluated in 142 patients.56 They received placebo or 10, 25, 75, or 150 mg of ixekizumab at weeks 0, 2, 4, 8, 12, and 16. At week 12, the proportion of patients who achieved PASI 75 was significantly greater in the groups that received ixekizumab (except for the lowest dose, of 10 mg) than in the placebo group (77%, 83%, and 82% of patients as opposed to 8% of patients who received placebo). For patients suffering from scalp psoriasis, nail psoriasis, and psoriatic arthritis, significant improvements were observed with the higher doses. Significant reductions in the mean DLQI scores were detected at 8 weeks, and this was sustained through 16 weeks with the three highest doses relative to placebo. In a post-hoc analysis derived from the same study, the early clinical improvements in disease symptoms at weeks 4 and 6 were predictive of improvement in PASI 75 at week 12 in more than 90% of the cases.57 An open-label treatment study showed that clinical responses to ixekizumab were maintained after 52 weeks.34 According to ClinicalTrials.gov, Phase III clinical trials are underway; these are examining the efficacy of ixekizumab compared with that of placebo and to that of etanercept.
Safety and tolerability
The main physiological function of IL-17 is protection from infectious diseases, by recruiting inflammatory cells to local sites of infection. One could therefore fear that infections might arise as possible side effects when neutralizing IL-17A or inhibiting IL-17RA.
IL-17A has been associated with the pathogenesis of several malignancies, including human cervical carcinoma; however, the pro-inflammatory action of IL-17A has also been considered to be a protective factor in some cancers.23
Brodalumab, secukinumab, and ixekizumab were generally well tolerated in the trials reported; however, one must take into account the relatively short duration of these trials. A few incidents of neutropenia were observed in subjects receiving all three biologic therapies, but the underlying mechanism has not been studied to determine the contribution to this made by IL-17 inhibition.44,48,50,56,58 Two patients who received ixekizumab had elevation of creatine kinase and aspartate aminotransferase levels.56 One patient who received secukinumab had abnormal transaminase levels.48 Generally, the most common adverse events in the trials (including both the group receiving active drug and the placebo group) were worsening of disease, nasopharyngitis, upper-respiratory-tract infection, arthralgia, injection-site erythema, pain in the extremities, nausea, headache, and pruritus.44–56,59
Papp et al reported discontinuation of brodalumab in one patient due to mild urticaria.44 In the open-label extension study, one serious adverse event of esophageal adenocarcinoma and one fatal aortic aneurysm rupture in patients receiving brodalumab were reported.45 In the study on secukinumab by Papp et al, adverse events led to discontinuation of the study drug in two patients due to exacerbation of psoriatic arthropathy and abnormal liver-function test results.48 Rich et al reported that five patients discontinued treatment with secukinumab within the first 12 weeks because of adverse events; these consisted of worsening of psoriasis, pharyngitis (placebo group), bacterial pneumonia, and two patients experienced erythrodermic psoriasis. In the maintenance period, ten patients discontinued treatment with secukinumab due to adverse events (worsening of psoriasis, erythrodermic psoriasis, diarrhea, appendicitis, ear infection, colon cancer, testicular cancer, and rhabdomyolysis that was probably due to viral infection).50 In the ixekizumab trial by Leonardi et al, a total of four patients from the placebo group and from the two groups with the mildest dose of ixekizumab discontinued due to adverse events. These events were hypertriglycemia, peripheral edema, hypersensitivity, and urticaria.56 In the study by Krueger et al, three subjects (one from the placebo group) experienced adverse events leading to discontinuation.55
Most of the adverse events that occur in the trials with brodalumab, secukinumab, and ixekizumab are probably not due to the biologic therapy itself but instead to chance. Some authors have even reported the same number of adverse events in the groups receiving the biologic treatment and in the placebo group.46,50 However, further longer-term studies are needed to investigate the safety and tolerability of brodalumab, secukinumab, and ixekizumab.
Possible differences in efficacy or safety in targeting of the IL-17 receptor versus targeting of the IL-17 ligand
Brodalumab targets the IL-17 receptor subunit IL-17RA, while secukinumab and ixekizumab target the IL-17A ligand. The receptor subunit IL-17RA is probably shared by IL-17A, IL-17C, IL-17E, IL-17F, viral IL-17, and IL-17A/F heterodimer ligands.21,26,35 Thus, brodalumab targets much more broadly than secukinumab and ixekizumab, with the potential for better efficiency but also with an increased risk of adverse effects. IL-17A, IL-17C, and IL-17F expression has been found to be elevated in psoriatic skin; therefore, targeting of the receptor for all these ligands and not only IL-17A could be more effective in controlling psoriasis, but it might also be associated with an increased risk of infection.35,40,43 IL-17E has been shown to have a role in dealing with parasitic infections and allergic reactions. Blocking of its receptor with brodalumab could therefore interfere with these mechanisms. The blocking of IL-17E results in a broader anti-inflammatory effect than the blocking of IL-17A and IL-17F alone.20,23,35
Both IL-17A and IL-17F are needed for mucocutaneous immunity against C. albicans, and the blocking of only one of these cytokines with secukinumab or ixekizumab may have a smaller effect on infection risk. It has been shown that genetic deficiency in IL-17RA, in humans, is associated with recurrent or persistent mucocutaneous infections caused by C. albicans and infections with S. aureus. IL-17A deficiency is also associated with chronic mucocutaneous candidiasis.20,31–33 No invasive fungal infections have been reported in trials with brodalumab, secukinumab, or ixekizumab. Two oral and two vaginal cases of candida have been reported with ixekizumab during a treatment period of 52 weeks.34
In conclusion, IL-17A may offer a more specific target; however, the targeting of IL-17RA may be more efficient. Further studies are needed to clarify the pros and cons of targeting IL-17A rather than IL-17RA.
Conclusion: significance in therapy
Psoriasis is a disease of great importance because it affects 2%–3% of the Western population, is associated with several severe comorbidities, and is associated with impaired quality of life and a shortened lifespan in patients with severe disease. Brodalumab, secukinumab, and ixekizumab represent a new frontier in our therapeutic strategy for the treatment of psoriasis. The significant clinical responses seen in patients diagnosed with psoriasis following blockade of either the ligand IL-17A or its receptor confirm the hypothesis that IL-17 signaling has a critical role in this immune-mediated disease. So far, the results of clinical trials on brodalumab, secukinumab, and ixekizumab indicate that the three drugs are more or less equally efficient when the therapeutic agent is given at the right dose and in the right regimen. PASI 75 is the most frequently used measurement to evaluate drug efficiency in clinical trials on plaque psoriasis; however, since the therapeutic agents have very high efficiency, the PASI 90 response could be the primary endpoint in future. The goal for patients is usually complete remission of their skin disease; it is therefore beneficial – both for the patients and for evaluation of the therapeutic agent – to use PASI 90.
Th17 cells contribute to the pathogenesis of several inflammatory diseases, but their main function, a critical one, is their participation in the adaptive immune response against bacterial and fungal infections. To date, short-term clinical trials have not found that such infections are a problem. Brodalumab, secukinumab, and ixekizumab were generally well tolerated in the trials reported; however, larger and longer-term trials are needed to fully determine the safety and tolerability of these three biologic agents.
Although all three of the mentioned agents target IL-17A either directly or indirectly, there are pharmacological differences between them. Future studies will tell whether one agent is superior to the others based on efficacy and side effects. Large-scale Phase III clinical trials are underway and they will provide more data about efficacy. However, long-term follow-up will be needed to determine the long-term efficacy and safety of brodalumab, secukinumab, and ixekizumab.
Disclosure
Lone Skov has received consultancy and/or speaker honoraria from Abbott, Pfizer, Janssen-Cilag, MSD, and Leo Pharma, and is a member of the advisory boards of MSD, Novartis, Eli Lilly, Abbvie, and Janssen-Cilag. Claus Zachariae has received consultancy and/or speaker honoraria from Abbott, Pfizer, and Takeda, and is a member of the Advisory Boards of AbbVie, Novartis, Eli Lilly, MSD, and Janssen-Cilag. The authors declare no other conflicts of interest in this work.
References
Peters BP, Weissman FG, Gill MA. Pathophysiology and treatment of psoriasis. Am J Health Sys Pharm. 2000;57(7):645–659. | |
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. | |
Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol. 2001;15(1):16–17. | |
Schäfer T. Epidemiology of psoriasis. Review and the German perspective. Dermatology. 2006;212(4):327–337. | |
Prey S, Paul C, Bronsard V, et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: a systematic review of the literature. J Eur Acad Dermatol Venereol. 2012;24 Suppl 2:31–35. | |
Reich K, Krüger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. 2009;160(5):1040–1047. | |
Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):23–30. | |
Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. | |
Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139. | |
Schmid-Ott G, Schallmayer S, Calliess IT. Quality of life in patients with psoriasis and psoriasis arthritis with a special focus on stigmatization experience. Clin Dermatol. 2007;25(6):547–554. | |
Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Heritability of psoriasis in a large twin sample. Br J Dermatol. 2013;169(2):412–416. | |
Tsoi LC, Spain SL, Knight J, et al; Collaborative Association Study of Psoriasis (CASP); Genetic Analysis of Psoriasis Consortium; Psoriasis Association Genetics Extension; Wellcome Trust Case Control Consortium 2, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44(12):1341–1348. | |
Bowcock AM, Cookson WO. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Hum Mol Genet. 2004;13 Spec No 1:R43–R55. | |
Carpon F, Burden AD, Trembath RC, Barker JN. Psoriasis and other complex trait dermatoses: from Loci to functional pathways. J Invest Dermatol. 2012;132(3 Pt 2):915–922. | |
Chen H, Poon A, Yeung C, et al. A genetic risk score combining ten psoriasis risk loci improves disease prediction. PLoS One. 2011;6:e19454. | |
Savolainen L, Kontinen J, Alatalo E, Röning J, Oikarinen A. Comparison of actual psoriasis surface area and the psoriasis area and severity index by the human eye and machine vision methods in following the treatment of psoriasis. Acta Derm Venereol. 1998;78(6):466–467. | |
Ashcroft DM, Wan Po AL, Williams HC, Griffiths CE. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141(2):185–191. | |
Reich K, Mrowietz U. Treatment goals in psoriasis. J Dtsch Dermatol Ges. 2007;5(7):566–574. English, German. | |
Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23 Suppl 2:1–70. | |
Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23(5):613–619. | |
Gaffen SL. Structure and signaling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. | |
Wright JF, Bennett F, Li B, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181(4):2799–2805. | |
Gaffen S, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. Vitam Horm. 2006;74:255–282. | |
Girolomoni G, Mrowietz U, Paul C. Psoriasis: rationale for targeting interleukin-17. Br J Dermatol. 2012;167(4):717–724. | |
van den Berg WB, McInnes IB. Th17 cells and IL-17 a – focus on immunopathogenesis and immunotherapeutics. Semin Arthritis Rheum. 2013;43:158–170. | |
Chiricozzi A, Krueger JG. IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs. 2013;22(8):993–1005. | |
Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. | |
Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–1057. | |
Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–352. | |
Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126(2):177–185. | |
Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. | |
Huppler AR, Bishu S, Gaffen SL. Mucocutaneous candidiasis: the IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther. 2012;14(4):217. | |
Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11(5):425–435. | |
Gordon K, Leonardi C, Braun D, et al. Results after at least 52 weeks of open label treatment with ixekizumab, an anti-IL-17A monoclonal antibody, in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2014;70(5 Supplement 1):AB183. | |
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. | |
Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. | |
Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013; 72 Suppl 2:ii116–ii123. | |
Monteleone G, Pallone F, MacDonald TT, Chimenti S, Costanzo A. Psoriasis: from pathogenesis to novel therapeutic approaches. Clin Sci (Lond). 2011;120(1):1–11. | |
Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–691. | |
Martin DA, Towne JE, Kricorian G, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133(1):17–26. | |
Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–2183. | |
Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs. 2013;27(4):359–373. | |
Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134(1):8–16. | |
Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13); 1181–1189. | |
Papp K, Milmont C, Leonardi C, Ortonne JP, Klekotla P. Maintenance of clinical response with long-term brodalumab (AMG 827) therapy for psoriasis: Week 96 results from an open-label extension study. J Am Acad Dermatol. 2014;70(5 Suppl 1):AB174. | |
Papp KA, Reid C, Foley P, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132(10):2466–2469. | |
Hueber W, Patel DD, Dryja T, et al. Psoriasis Study Group, et al; Rheumatoid Arthritis Study Group, et al; Uveitis Study Group, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52–72. | |
Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168(2):412–421. | |
Sigurgeirsson B1, Kircik L, Nemoto O, et al. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. J Eur Acad Dermatol Venereol. Epub December 13, 2013. | |
Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2012;168(2):402–411. | |
Paul C, Reich K, Gottlieb AB, et al; the CAIN457A2211 study group. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. Epub January 7, 2014. | |
Reich K, Puig L, Draelos Z, Notter M, Papavassilis C. Sustainability of response with secukinumab to 52 weeks in moderate-to-severe plaque psoriasis: data from the full year investigative examination of secukinumab vs etanercept using 2 dosing regimens to determine efficacy in psoriasis (FIXTURE) study. Abstract/Program number 8101. Presented at the American Academy of Dermatology, March 21–25, 2014, Denver, CO, USA. | |
Warren R, Guettner A, Morita A, Gisondi P, Cooper S. Secukinumab efficacy in subjects with moderate to severe plaque psoriasis: Pooled subgroup analyses by patient age of 4 phase 3 clinical studies. J Am Acad Dermatol. 2014;70(5 Supplement 1):AB186. | |
Elewski B, McLeod L, Lebwohl M, Mallya UG, Zhao Y. Secukinumab versus placebo or etanercept on time to response on patient-reported psoriasis symptoms of pain, itching, and scaling (FIXTURE study). J Am Acad Dermatol. 2014;70(5 Supplement 1):AB189. | |
Krueger JG, Fretzin S, Suárez-Fariñas M, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145–154. | |
Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. | |
Zhu B, Edson-Heredia E, Cameron GS, et al. Early clinical response as a predictor of subsequent response to ixekizumab treatment: results from a phase II study of patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2013;169(6):1337–1341. | |
Griffiths CE, Prinz J, Güttner A, Cooper S. Behavior of neutrophil count during 52 weeks of treatment with secukinumab: an analysis of pooled data from the phase 2 and phase 3 clinical study programs for secukinumab in moderate-to-severe plaque psoriasis. Abstract/Program number 8266. Presented at the 72nd Annual Meeting of the American Academy of Dermatology, March 21–25, 2014, Denver, CO, USA. | |
Papp K, Nakagawa H, Melendez E, Karpov A, Papavassilis C; ERASURE Study Group. Efficacy in relationship with response to previous biologic psoriasis therapy: a subanalysis from the ERASURE phase 3 study in psoriasis. Abstract/Program number 8011. Presented at the American Academy of Dermatology, March 21–25, 2014, Denver, CO, USA. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.