Back to Journals » Drug Design, Development and Therapy » Volume 12
Tanshinol ameliorates CCl4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway
Authors Wang R, Wang J, Song F, Li S , Yuan Y
Received 11 December 2017
Accepted for publication 15 March 2018
Published 17 May 2018 Volume 2018:12 Pages 1281—1292
DOI https://doi.org/10.2147/DDDT.S159546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Rong Wang,* Jing Wang,* Fuxing Song, Shengnan Li, Yongfang Yuan
Department of Pharmacy, Shanghai 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
*These authors contributed equally to this work
Abstract: Tanshinol, a water-soluble component isolated from Salvia miltiorrhiza Bunge, has a variety of biological activities involving anti-fibrotic effect. However, the exact role and the underlying mechanisms remain largely unclear. This study mainly focused on the anti-hepatic fibrotic activities and mechanisms of tanshinol on carbon tetrachloride (CCl4)-induced liver fibrosis in rats via anti-oxidative and anti-inflammation pathways. The rats were divided into 4 groups as follows: control, model, tanshinol 20 mg/kg, and tanshinol 40 mg/kg. Except for the control group, CCl4 was used to induce liver fibrosis processing for 8 weeks, meanwhile rats in tanshinol groups were intraperitoneally injected with additional tanshinol. Control group simultaneously received the same volumes of olive oil and saline. The potentially protective effect and mechanisms of tanshinol on liver fibrosis in rats were evaluated. The serum levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin were obviously lower in the tanshinol treatment groups related to model group. Compared with the model group, the levels of hyaluronic acid, type IV collagen, Laminin (LN), and procollagen III peptide (PIIIP) in serum were significantly decreased after tanshinol treatment. Furthermore, tanshinol could regulate Nrf2/HO-1 signaling pathway and increase the level of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and also decrease the level of malondialdehyde (MDA) to against damage induced by oxidative stress. Simultaneously tanshinol could regulate nuclear factor kappa B signaling pathway to inhibit expression of inflammation factors, including transforming growth factor-β, tumor necrosis factor-α, Cox-2, interleukin-1β, and interleukin-6. In summary, our research demonstrated that tanshinol has protective effect on CCl4-induced liver fibrosis via inhibiting oxidative stress and inflammation, which may be associated with the regulation of nuclear factor erythroid2-related factor 2/hemeoxygenase-a and nuclear factor kappa B/inhibitor of kappa B alpha signaling pathways.
Keywords: liver fibrosis, tanshinol, oxidative stress, inflammation, Nrf2, NF-κB
Introduction
Liver fibrosis refers to excessive accumulation of fibrotic collagen in liver tissue during the process of wound healing, leading to liver failure, cirrhosis, and even cancer.1 Additionally, liver fibrosis is a consequence of imbalance between extracellular matrix (ECM) overexpression and deficiency of ECM decomposition, and in the development of liver fibrosis, hepatic stellate cells (HSCs) recognized as a major player.2,3 Oxidative stress has been implicated in the pathogenesis and development of liver fibrosis. Reactive oxygen species (ROS) are considered an inducement to HSCs’ proliferation, migration, and collagen accumulation.4 It was reported that cytochrome P450 2E1 (CYP2E1) in liver activates the production of trichloromethyl free radical and ROS, which injure the liver.5,6 Nuclear factor erythroid2-related factor 2 (Nrf2) was regarded as a significant transcription factor that induces the transcription of Phase II detoxifying anti-oxidant genes, including hemeoxygenase-a (HO-1), glutamate-cysteine ligase (GCLC), and quinine oxidoreductase-1 (NQO-1) through binding to antioxidant response elements (AREs).7,8 Therefore, target Nrf2/HO-1 signaling may be a treatment strategy to inhibit liver fibrosis by against oxidative damage.
Recent investigations have unraveled many mechanisms underlying liver fibrosis. Increasing evidence showed that nuclear factor kappa B (NF-κB), as a transcriptional regulator, is a key mediator of liver fibrosis.9 NF-κB is a family of dimeric transcription factors and mediates the expression of genes associated with inflammation in response to stimulation.10 Except that, NF-κB is constitutively active in pro-fibrogenic liver myofibroblasts, which result from an excess of Rel factors through an epigenetic down-regulation of the inhibitory IκB protein.11 NF-κB regulates inflammatory signals elicited in macrophages and other inflammatory cells in the liver, and at the same time, promotes the expression of inflammatory factors, such as Cyclooxygenase-2 (Cox-2), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), which play critical roles in the development of liver fibrosis.10,12 Therefore, inhibiting NF-κB/inhibitor of kappa B alpha (IκBα) signaling pathway may be an optimal therapeutic approach in liver fibrosis and inflammation.
Tanshinol (3-(3,4-dihydroxyphenyl) lactic acid), a water-soluble component isolated from Salvia miltiorrhiza Bunge, exhibits various pharmacological activities, including anti-coagulative,13 anti-cardiovascular14,15 and antitumor effects,16 antioxidant effects,17 and neuroprotection.18 Our previously published research has declared that tanshinol can prevent liver fibrosis by regulating PI3K/AKT/mTOR/p70S6K1 signaling pathways, which played crucial roles in the development of liver fibrosis by regulating the synthesis and degradation of ECM, activation and apoptosis of HSCs, and hepatic sinusoid capillarization.19 As we know, oxidative stress and inflammation are implicated in the development of liver fibrosis. So far, the exact molecular mechanisms of tanshinol on liver fibrosis remain limited. Therefore, in summary, this study aimed to explore the effect of tanshinol on carbon tetrachloride (CCl4)-induced liver fibrosis in rats and to illustrate its anti-hepatofibrotic mechanisms from points of oxidative stress and inflammation.
Materials and methods
Materials
Tanshinol (purity>99%) was purchased from Shanghai Nature Standard R&D and Biotech Co., Ltd (Shanghai, China). CCl4 was purchased from Shanghai Lingfeng Chemical Regent Co., Ltd (Shanghai, China). NE-PER Nuclear and Cytoplasmic Extraction Reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Monoclonal NF-κB (p65), IκBα, Nrf2, HO-1, NQO-1, GCLC, β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and Histone H3 rabbit antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). The horseradish peroxidase (HRP)-labeled GAPDH antibody was obtained from Bioworld Technology Inc (St Louis Park, MN, USA). The secondary antibody, HRP-labeled goat anti-rabbit IgG was purchased from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein-loading buffer (5×) was purchased from Beyotime Biotechnology Corporation (Shanghai, China). TRIzol®reagent was obtained from Thermo Fisher Scientific.
Animal models
Forty-eight Sprague-Dawley rats (6–8 weeks; 180–220 g) were obtained from the B&K Universal Group Ltd. (Shanghai, China). All rats had free access to food and drinking water and were maintained under a controlled environment in an experimental animal unit of Shanghai 9th People’s Hospital and acclimatized for 1 week before experiment. Rats were randomly divided into 4 groups (n=12 for each group): control group; model group; tanshinol 20 mg/kg group; and tanshinol 40 mg/kg group. Except for the control group, all rats received 1 mL/kg of CCl4 orally (diluted in 50% olive oil) twice a week for 8 weeks to induce liver fibrosis. Rats in tanshinol 20 or 40 mg/kg groups were intraperitoneally injected with tanshinol (20 or 40 mg/kg) once a day for 8 consecutive weeks. Control group simultaneously received the same volumes of olive oil and saline without tanshinol. At the end of 8 weeks, surviving rats were weighed and anesthetized with pentobarbital sodium (Shanghai Beizhuo Biochemical & Technological Co., Ltd., Shanghai, China). Blood and liver samples were collected. The blood samples were centrifuged at 1,000×g, 30 min at 4°C. The excised liver in each group was fixed with 4% paraformaldehyde for histopathological examination, and the remnants of liver were then stored at −80°C for later analysis. All the experimental procedures were approved by the Animal Ethics Committee of Shanghai 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine (Approval ID: 201773), complied with the Experimental Animal Regulation by the National Science and Technology Commission, China.
Calculation of the liver index
The liver index was calculated using the following formula: liver index = (liver weight/body weight)×100.
Determination of liver biological activities
Serum total bilirubin (TBIL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured by an automatic biochemical analytic instrument (Beckman Coulter, Lx-20, Brea, CA, USA) at the department of clinical laboratory. Plasma liver fibrosis indices, including hyaluronic acid (HA), type IV collagen (IV-C), laminin (LN), and precollagen III peptide (PIIIP), and their concentrations were measured by radioimmunoassay using commercial kits (Beifang Institute of Biotechnology, Beijing, China).
Histopathological and immunohistochemical examination
Histopathological examination
Liver tissues were dehydrated in alcohol and embedded in paraffin and cut into 5 μm thick slices. Specimens were stained with H&E and Masson’s trichrome staining according to standard instructions. All histological examinations were undertaken by an experienced operator who was blinded to the study protocol. All liver sections were randomly selected for examination through using a light microscope (Ti-S Inverted Fluorescence Microscope, Type 108, Nikon Corporation, Tokyo, Japan). The quantitative assays of collagen deposition were determined via the image software according to the procedure. The scores of liver fibrosis degree were evaluated following the criteria:
0: | no obvious fibrosis; |
1: | fibrosis present: collagen fibers that extend from the portal triad or central vein to peripheral regions; |
2: | mild fibrosis: few collagen fibers extending without formation of compartments; |
3: | moderate fibrosis: collagen fibers with formation of “pseudo leaves”; |
4: | severe fibrosis: many collagen fibers with thickening of partial compartments and formation of “pseudo lobes”. |
Immunohistochemical examination
The slices were used for immunohistochemical examination. Briefly, these sections were dewaxed and dehydrated, and antigen retrieval was performed, followed by incubation with 0.1% bovine serum albumin in PBS for 30 min; after that, the sections were incubated with HO-1, NQO-1, and GCLC primary antibodies at 4°C overnight. The goat anti-rabbit IgG was used as the secondary antibody. Subsequently, positive areas within the fibrotic region were then observed under a light microscope.
Western blot analyses
Total, nuclear and cytosolic proteins of livers tissues were extracted as described in a previous study.20 Proteins concentration was determined by using a bicinchoninic acid (BCA) protein assay kit. Proteins were separated by 12% or 15% sodium dodecylsulfate-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes. Then, the membranes were incubated overnight at 4°C with various primary antibodies, including: Nrf2, HO-1, NQO-1, GCLC, NF-κB (p65), IκBα, Histone H3, β-actin, and GAPDH. After washing 3 times with tris buffered saline, the next day secondary antibodies were added respectively, and developed with the enhanced chemiluminescence plus detection system (Fusion FX7 Spectra; VilberLourmat, Eberhardzell, Germany).
Real-time polymerase chain reaction (PCR) analyses
Liver tissue was dissected and homogenized using a TL2020 grinding instrument (DHS Life Science & Technology, Beijing, China). Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and evaluated for concentration and purity through Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Gene Company Limited, Shanghai, China). Then, the total RNA was reverse transcribed by the PrimeScript™ RT Master Mix reagent kit (Takara, Shiga, Japan). Afterward, the level of HO-1, NQO-1, GCLC, TGF-β, TNF-α, Cox-2, IL-1β, IL-6, and GAPDH genes were detected using Sybr® Premix Ex Taq™ II (TliRNaseH Plus) reagent in a Light Cycler 480 instrument (Hoffman-La Roche Ltd., Basel, Switzerland). The sequences of PCR primers were designed and synthesized by Sangon Bio-tech Co. Ltd. (Shanghai, China) and the primers in Table S1 were used for the experiment.
Antioxidative stress activity analyses
Liver tissue was homogenized with saline to get 10% liver tissue homogenate and centrifuged at 4°C, 3,000 rpm for 10 min, then the supernatant was collected. The supernatant protein concentrations were determined by the BCA protein assay kit. After that, the supernatant was analyzed for the levels of superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) according to the protocols provided by the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analyses
All data were expressed as means ± SD. The differences between multiple comparisons were evaluated using one-way analysis of variance test, followed by Student–Newman–Keuls post hoc test. Nonparametric comparison was evaluated using Kruskal–Wallis test, followed by Dunn–Bonferroni post hoc test. All statistics were analyzed by SPSS 21.0 (Chicago, IL, USA). Differences were considered as the statistical significance when p-value was <0.05.
Results
Effects of tanshinol on body weight, liver weight, and the liver index of rats
Effects of tanshinol on body weight, liver weight, and liver index of rats were showed in Table 1. It shows that no deaths occurred in control group, but 2 rats died in the model group and the tanshinol 20 mg/kg group, and 3 died in the tanshinol 40 mg/kg group. Table 1 shows that compared with the control group, the body weight obviously decreased, while liver weight and liver index increased in the model group (p<0.05). However, in relation to the model group, the body weight of the rats significantly increased in both the tanshinol 20 mg/kg and 40 mg/kg group (p<0.05). At the same time, liver weight and liver index markedly decreased in tanshinol 40 mg/kg group compared with model group (p<0.05).
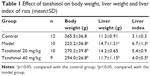  | Table 1 Effect of tanshinol on body weight, liver weight and liver index of rats (mean±SD) |
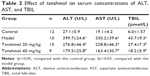
Effects of tanshinol on serum concentrations of ALT, AST, and TBIL
To investigate the effects of tanshinol on liver function, the serum levels of liver function markers were detected. As shown in Table 2, the model group significantly increased the concentrations of TBIL, ALT, and AST in serum compared with the control group (p<0.05). In contrast, treatment with tanshinol (both 20 and 40 mg/kg groups) obviously reduced the serum level of ALT, AST, and TBIL compared with that of the model group, especially in tanshinol 40 mg/kg group (p<0.05).
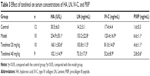
Effects of tanshinol on serum concentrations of HA, LN, IV-C, and PIIIP
To assess the effects of tanshinol on liver fibrosis markers, the serum concentrations of HA, LN, IV-C, and PIIIP were tested. As shown in Table 3, compared with the control group, the model group had significantly increased the serum concentrations of HA, LN, IV-C, and PIIIP (p<0.05). In both the 20 and 40 mg/kg tanshinol treatment groups (especially in 40 mg/kg group) the concentrations of HA, LN, IV-C, and PIIIP (p<0.05) were attenuated.
Effects of tanshinal on liver pathology
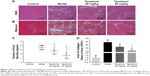
To further study the anti-fibrosis effects of tanshinol on rat liver, the degree of rat liver fibrosis was determined by H&E and Masson staining. As indicated in Figure 1, H&E staining (Figure 1A and C) and Masson staining (Figure 1B and D) of the liver tissues showed liver tissues in the control group with integrated lobular structure with clear central veins and radiating hepatic cords. No sign of necrosis, inflammation, or fibrosis development and a few collagen fibers were observed around the central vein. In the model group, CCl4 significantly induced prominent hepatic steatosis, necrosis, and formation of regenerative nodules in liver tissues, which was obviously improved by tanshinol treatment (p<0.05). Notably, tanshinol 40 mg/kg treatment markedly ameliorated the degree of liver fibrosis and alleviated collagen deposition in relation to the model group and tanshinol 20 mg/kg group (p<0.05).
Effects of tanshinol on CCl4-induced hepatic oxidative stress
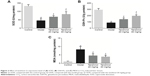
It is known that the development of liver fibrosis is accompanied with oxidative damage. We hypothesized that the antioxidant property of tanshinol might play a major role in the inhibitory effects of liver fibrosis. The levels of SOD, GSH-Px, and MDA in liver tissues were all tested through manufacturer procedures. Figure 2 indicated that in the model group, CCl4 obviously decreased the level of SOD and GSH-Px (p<0.05) and increased the level of MDA in liver tissues (p<0.05), compared with the control group. Surprisingly, not only the levels of SOD and GSH-Px in the liver tissues were increased (p<0.05, p<0.05), but also the level of MDA was decreased (p<0.05) after both 20 and 40 mg/kg tanshinol treatment, especially 40 mg/kg (p<0.05).
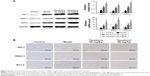
The effects of tanshinol on inflammatory factors (TNF-α, TGF-β, Cox-2, IL-1β, and IL-6)
The mRNA level of TNF-α, TGF-β, Cox-2, IL-1β, and IL-6 were detected in the livers of rats of different groups. As shown in Figure 3, the expression levels of TNF-α, TGF-β, Cox-2, IL-1β, and IL-6 in the model group were markedly increased compared to control group (p<0.05), however, compared with model group, these factors were significantly down-regulated by tanshinol 20 and 40 mg/kg treatment.
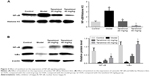
Effects of tanshinol on NF-κB/IκBα pathway
As shown in Figure 4A, the expression of nuclear NF-κB in model group was markedly increased compared with the control group (p<0.05). Treatment with tanshinol at 20 and 40 mg/kg decreased the expression of nuclear NF-κB in a dose-dependent manner compared with the model group (p<0.05, p<0.05). In contrast, in Figure 4B, the expression of cytosolic NF-κB and IκBα in the model group obviously decreased in relation to the control group (p<0.05). Treatment with tanshinol at 20 and 40 mg/kg increased the expression of cytosolic NF-κB and IκBα in a dose-dependent manner compared with the model group (p<0.05, p<0.05).
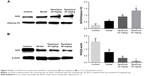
Effects of tanshinol on Nrf2/HO-1 pathway
Tanshinol has been confirmed to inhibit liver fibrosis and increase the levels of SOD and GSH-Px activities in liver. Nrf2 is a major transcription factor in the process of antioxidant response. Nrf2 is constitutively sequestered in the cytoplasm in basal conditions. In contrast, under oxidative stress conditions, Nrf2 moves into the nucleus and serves as a transcriptional factor to regulate the expression of corresponding downstream genes, thereby reducing the oxidative stress.21 To further investigate whether tanshinol induces translocation of Nrf2, Western blot and immunohistochemical staining were used. We observed that CCl4 can induce Nrf2 from cytoplasm to move into the nucleus compared with the control group, meanwhile 20 and 40 mg/kg tanshinol treatment obviously dose-dependently increased the nuclear accumulation of Nrf2 and decreased Nrf2 in the cytoplasm (Figure 5). Next, we examined the expression levels of HO-1, NQO-1, GCLC in the liver tissues by Western blot, real-time PCR, and immunohistochemical staining. The expression levels of HO-1, NQO-1, GCLC were significantly increased by tanshinol 20 and 40 mg/kg treatment in a dose-dependent manner compared with the model group (Figure 6).
Discussion
Liver fibrosis is a pathological process involving a fibrogenic response, which is characterized by scar formation due to increased production and deposition of ECM that culminates in major changes in the liver architecture.22 If untreated, liver fibrosis may progress to cirrhosis and liver failure or malignancy. Liver fibrosis is a reversible process, and presents as the critical pre-stage of liver cirrhosis.23 Currently, the most effective treatment for liver fibrosis is liver transplantation; however, transplantation is limited by shortage of donor organs, surgical complications, immunological rejection, and high costs.24 In addition, there are few therapeutic drugs for patients with liver fibrosis. In recent years, Chinese herbal medicine has attracted more and more attention for anti-fibrotic treatment due to its low toxicity and few side effects. Thus, it is necessary and of considerable interest to find new medicines or chemical compounds from Chinese herbs to treat liver disease.25 Our previously published research demonstrated that tanshinol, a biologically active monomer derived from the Chinese herb Salvia miltiorrhiza Bunge, has a therapeutic effect on CCl4-induced liver fibrosis in rats.
Usually, the increased levels of cytoplasmic enzymes (involving TBIL, ALT, and AST) are used as specific markers of liver damage, because these markers in the cytoplasm of liver cells are released into blood serum when degeneration, hyperpermeability, and necrosis of liver cells occur.26 Our study indicated that tanshinol can effectively lower the serum levels of ALT, AST, and TBIL compared with model group, showing that tanshinol can ameliorate liver function. HA, LN, IV-C, and PIIIP exist in liver tissue and are major indicators for diagnosing the degree of liver fibrosis.20 In this study, the levels of HA, LN, IV-C, and PIIIP in liver tissue were significantly decreased after tanshinol treatment in relation to the CCl4 model group, which demonstrated tanshinol could attenuate liver fibrosis. Furthermore, the H&E and Masson staining also showed that tanshinol can obviously improve the hepatic morphology and architecture and markedly ameliorate the degree of liver fibrosis. These results together indicated that tanshinol exerts anti-fibrotic effects, which is compatible with our previous report.19
Liver fibrosis is a pathological response to hepatocyte damage resulting from viral infection, inflammation, as well as other factors, including oxidative stress, which is primary mechanism of liver injury.27 Oxidative stress can induce HSCs activation via paracrine and autocrine mechanism, which result in formation of liver fibrosis.28 Antioxidants play important roles in the development of liver fibrosis. The enzymatic antioxidant defense system is the natural protector against free radicals accumulation. SOD and GSH-Px, scavengers of superoxide, protect the cells from oxidative damage.29 It is reported that CCl4 can inhibit the activities of antioxidant enzymes.30 In this study, we observed that the levels of SOD and GSH-Px in liver tissue are significantly lower in the model group compared with the control group, while the levels obviously increased after tanshinol treatment. The level of MDA indicates an enhanced lipid peroxidation leading to liver tissue injury and failure of antioxidant defense.8 A significant decrease in the MDA level was observed in the tanshinol treatment group in relation to the CCl4 model group. The changes of SOD, GSH-Px, and MDA level suggested that tanshinol can inhibit the oxidative stress in CCl4-induced liver fibrosis rats. SOD, GSH-Px, and MDA are recognized enzymes that are closely related to Nrf2.31,32 Nrf2 is an important transcription factor regulating the expression of antioxidative stress factors. Nrf2 binds to an ARE, induces the expression of anti-oxidant genes, such as HO-1, GCLC, and NQO1, and protects against oxidative damage.32 Nrf2 activation is observed in nonparenchymal cells, including HSCs and Kupffer cells as well as in parenchymal hepatocytes. Nrf2 plays complex roles in liver inflammation, fibrosis, cancer, and regeneration.33 Our results illustrated that tanshinol can significantly induce nuclear translocation of Nrf2 and increase the expression of HO-1, NQO-1, and GCLC in mRNA and protein levels. These results showed that tanshinol promotes the synthesis and activation of Nrf2 in liver tissues against oxidative damage.
NF-κB pathway has emerged as one of the best-characterized signaling pathways in the pathogenesis of a wide variety of diseases, including liver diseases, inflammatory disorders, and tumor development.31 More and more reports have demonstrated that NF-κB is considered to play a major role in HSCs activation and fibrogenesis.34 NF-κB is a family of dimeric transcription factors that regulate the expression of genes related to inflammation in response to a range of stimuli.35 In a resting cell, NF-κB dimers interact with inhibitor of NF-κB (IκBα) to form inactive complexes that are localized to the cytoplasm. Once cell accepts stimulation, IκBα is rapidly phosphorylaed, followed ubiquitinylation, and ultimately proteolytic degradation. Stabilized NF-κB then translocates into the nuclei, where it regulates gene expression by binding κB sites, including cytokines, growth factors, or chemokines.36 Interestingly, it is reported that Nrf2 knockout mice displayed enhanced DNA binding of NF-κB after partial hepatectomy. This consequence most likely resulted from the oxidative stress due to ROS that has been shown to activate NF-κB.37,38 In this study, we found the increased level of cytokines, including TNF-α, Cox-2, IL-1 β, IL-6, TGF-β, and activated NF-κB in model group compared with control group. However, tanshinol treatment had effective ability to decrease the level of cytokines and inhibit the activation of NF-κB. These results indicated that tanshinol can inhibit the inflammation through regulating the NF-κB/IκBα signaling pathway in CCl4-induced liver fibrosis.
In summary, these experiments showed that tanshinol has therapeutic effect on CCl4-induced liver fibrosis in rats. The anti-fibrotic effects of tanshinol are associated with its ability to up-regulate the Nrf2/HO-1 signaling pathway against oxidative damage and down-regulate the NF-κB/IκBα signaling pathway to inhibit the inflammation. This study suggested that tanshinol is a promising candidate in liver fibrosis treatment.
Acknowledgments
This project was financially supported by the Fund of Shanghai Jiao Tong University School of Medicine (TM201714, JDYX2016QN012, JDYX2017QN008).
Disclosure
The authors report no conflicts of interest in this work.
References
Oakley F, Meso M, Iredale JP, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128(1):108–120. | ||
Dong S, Chen QL, Song YN, et al. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J Toxicol Sci. 2016;41(4):561–572. | ||
Li JP, Gao Y, Chu SF, et al. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis. Acta Pharmacol Sin. 2014;35(8):1031–1044. | ||
Sánchezvalle V, Cháveztapia NC, Uribe M, Méndezsánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19(28):4850–4860. | ||
Zhu W, Fung PC. The roles played by crucial free radicals like lipid free radicals, nitric oxide, and enzymes NOS and NADPH in CCl(4)-induced acute liver injury of mice. Free Radic Biol Med. 2000;29(9):870–880. | ||
Chen S, Zou L, Li L, Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. 2013;8(1):e53662. | ||
Gu L, Tao X, Xu Y, et al. Dioscin alleviates BDL- and DMN-induced hepatic fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway. Toxicol Appl Pharmacol. 2016;292:19–29. | ||
Cai Z, Lou Q, Wang F, et al. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway. Int J Clin Exp Pathol. 2014;8(7):8655–8662. | ||
Liu M, Wu Q, Chen P, et al. A boswellic acid-containing extract ameliorates schistosomiasis liver granuloma and fibrosis through regulating NF-κB signaling in mice. PLoS One. 2014;9(6):e100129. | ||
Luedde T, Schwabe RF. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2):108–118. | ||
Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14(2):275–285. | ||
Kong D, Zhang F, Wei D, et al. Paeonol inhibits hepatic fibrogenesis via disrupting nuclear factor-κB pathway in activated stellate cells: in vivo and in vitro studies. J Gastroenterol Hepatol. 2013;28(7):1223–1233. | ||
Yu C, Qi D, Lian W, Li QZ, Li HJ, Fan HY. Effects of danshensu on platelet aggregation and thrombosis: in vivo arteriovenous shunt and venous thrombosis models in rats. PLoS One. 2014;9(11):e110124. | ||
Hung M. A novel Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways. Int J Cardiol. 2013;168(2):1349–1359. | ||
Fan G, Yu J, Fordjour AP, et al. Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. J Cell Mol Med. 2016;20(10):1908–1919. | ||
Zhang LJ, Chen L, Lu Y, et al. Danshensu has anti-tumor activity in B16F10 melanoma by inhibiting angiogenesis and tumor cell invasion. Eur J Pharmacol. 2010;643(2–3):195–201. | ||
Hua L, Fan S, Duan LR, et al. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep. 2016;6:23693. | ||
Guo C, Yin Y, Duan J, et al. Neuroprotective effect and underlying mechanism of sodium danshensu [3-(3,4-dihydroxyphenyl) lactic acid from Radix and Rhizoma Salviae miltiorrhizae=Danshen] against cerebral ischemia and reperfusion injury in rats. Phytomedicine. 2015;22(2):283–289. | ||
Peng R, Wang S, Wang R, Wang YY, Wu Y, Yuan YF. Antifibrotic effects of tanshinol in experimental hepatic fibrosis by targeting PI3K/AKT/mTOR/p70S6K1 signaling pathways. Discov Med. 2017;23(125):81–94. | ||
Wang R, Zhang H, Wang YJ, Song FX, Yuan YF. Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-κB/IκBα, p38 MAPK, and Bcl-2/Bax signaling. Int Immunopharmacol. 2017;47:126–133. | ||
Ni YH, Huo LJ, Li TT. Antioxidant axis Nrf2-keap1-ARE in inhibition of alcoholic liver fibrosis by IL-22. World J Gastroenterol. 2017;23(11):2002–2011. | ||
Pellicoro A, Iredale JP. Reply to Elastin-based molecular MRI of liver fibrosis. Hepatology. 2013;58(4):1518–1519. | ||
Atta HM. Reversibility and heritability of liver fibrosis: implications for research and therapy. World J Gastroenterol. 2015;21(17):5138–5148. | ||
Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015;30(5):580–589. | ||
Zou L, Chen S, Li L, Wu T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp Toxicol Pathol. 2017;69(7):451–460. | ||
Tang N, Zhang Y, Liu Z, Fu T, Liang Q, Ai X. Correlation analysis between four serum biomarkers of liver fibrosis and liver function in infants with cholestasis. Biomed Rep. 2016;5(1):107–112. | ||
Bansal R, Nagórniewicz B, Prakash J. Clinical advancements in the targeted therapies against liver fibrosis. Mediators Inflamm. 2016;2016(3):1–16. | ||
Yang N, Shi JJ, Wu FP, et al. Caffeic acid phenethyl ester up-regulates antioxidant levels in hepatic stellate cell line T6 via an Nrf2-mediated mitogen activated protein kinases pathway. World J Gastroenterol. 2017;23(7):1203–1214. | ||
Reza HM, Tabassum N, Sagor MA, et al. Angiotensin-converting enzyme inhibitor prevents oxidative stress, inflammation, and fibrosis in carbon tetrachloride-treated rat liver. Toxicol Mech Methods. 2016;26(1):46–53. | ||
Ilavenil S, Kaleeswaran B, Ravikumar S. Protective effects of lycorine against carbon tetrachloride induced hepatotoxicity in Swiss albino mice. FundamClinPharmacol. 2012;26(3):393–401. | ||
Edeas M, Attaf D, Mailfert AS, Nasu M, Joubet R. Maillard reaction, mitochondria and oxidative stress: potential role of antioxidants. PatholBiol (Paris). 2010;58(3):220–225. | ||
Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690(1–2):12–23. | ||
Shin SM, Yang JH, SH Ki. Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev. 2013;2013(6):763257. | ||
Shu M, Huang DD, Hung ZA, Hu XR, Zhang S. Inhibition of MAPK and NF-κB signaling pathways alleviate carbon tetrachloride (CCl4)-induced liver fibrosis in Toll-like receptor 5 (TLR5) deficiency mice. Biochem Biophys Res Commun. 2016;471(1):233–239. | ||
Wang R, Yu XY, Guo ZY, Wang YJ, Wu Y, Yuan YF. Inhibitory effects of salvianolic acid B on CCl 4-induced hepatic fibrosis through regulating NF-κB/IκBα signaling. J Ethnopharmacol. 2012;144(3):592–598. | ||
Sasaki Y, Iwai K. Roles of the NF-κB pathway in B-lymphocyte biology. Curr Top Microbiol Immunol. 2015;393:177–209. | ||
Herpers B, Wink S, Fredriksson L, et al. Activation of the Nrf2 response by intrinsic hepatotoxic drugs correlates with suppression of NF-κB activation and sensitizes toward TNFα-induced cytotoxicity. Arch Toxicol. 2016;90(5):1163–1179. | ||
Köhler UA, Böhm F, Rolfs F, et al. NF-κB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma. J Hepatol. 2016;64(1):94–102. |
Supplementary material
  | Table S1 Primer sequences for real-time polymerase chain reaction assay |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.