Back to Journals » Clinical Ophthalmology » Volume 16
Tailoring Screening Guidelines for Retinopathy of Prematurity in Egypt: An Exploratory Multicentric Study
Authors Abdel Aziz I, Alsoda MF, Elmenofy TM, Sakhsoukh MM, Abd el Azim NM, Ahmed AM , Abd El-Halim SA, Baris SSH, Fouad YA , Elghonemy AM , Metwally H, El Gendy WM , Ali R, Basha YM, Mohamed EAE, Amin WM, Naguib MA, Elnashar HA
Received 24 August 2022
Accepted for publication 20 October 2022
Published 2 November 2022 Volume 2022:16 Pages 3625—3630
DOI https://doi.org/10.2147/OPTH.S383497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Ihab Abdel Aziz,1 Mohamed Fawzy Alsoda,2 Tarek Mohamed Elmenofy,1 Mohamed Medhat Sakhsoukh,1 Noha Mohamed Abd el Azim,1 Amr Mahmoud Ahmed,1 Sohaila Ali Abd El-Halim,3 Sherine Salaheldin Hassan Baris,3 Yousef Ahmed Fouad,4 Ayman Mohyieldin Elghonemy,1 Heba Metwally,1 Wael Mohamed El Gendy,1 Raghdaa Ali,2 Yehia Mahmoud Basha,5 Eman Abo ElMaaty Mohamed,6 Wafaa Mohamed Amin,6 Maged Adly Naguib,1 Hazem Abdallah Elnashar1
1Department of Ophthalmology, The Memorial Institute for Ophthalmic Research, Giza, Egypt; 2Department of Pediatrics and Neonatology, Ahmed Maher Teaching Hospital, Cairo, Egypt; 3Department of Pediatrics and Neonatology, Mataria Teaching Hospital, Cairo, Egypt; 4Department of Ophthalmology, Ain Shams University Hospitals, Cairo, Egypt; 5Department of Pediatrics and Neonatology, Damanhour Teaching Hospital, Beheira, Egypt; 6Department of Pediatrics and Neonatology, El-Galaa Teaching Hospital, Cairo, Egypt
Correspondence: Hazem Abdallah Elnashar, Department of Ophthalmology, The Memorial Institute for Ophthalmic Research, Giza, 23314, Egypt, Tel +201227011043, Email [email protected]
Background: Retinopathy of prematurity (ROP) is increasing in incidence in developing nations, including Egypt. Secondary prevention requires timely detection through the development of regional screening guidelines, which should be preceded by large-scale studies to characterize the population at risk.
Methods: A prospective, multicentric exploratory study that included five large tertiary institutions in an urban Egyptian setting. All infants born with gestational age (GA) < 37 weeks and/or birth weight (BW) ≤ 2000 grams were screened. More mature and heavier infants with unstable clinical course were also included. The primary outcome measure was the rate of ROP and high-risk disease occurrence in relation to underlying risk factors.
Results: Of the 768 eyes (384 screened infants), 347 eyes (45.2%) had stage 1 or higher disease, and 43 eyes (5.6%) had high-risk disease. Eyes with stage 1 or higher ROP and treatment-requiring ROP had a mean (± SD) GA of 33.4 (± 2.6) weeks and 32.8 (± 3.2) weeks, and BW of 1842.3 (± 570.1) grams and 1747.6 ± (676.2) grams, respectively. Treatment-requiring eyes belonged to infants that had significantly lower GA and significantly higher prevalence of co-morbidities than non-treatment-requiring eyes.
Conclusion: The incidence of ROP and high-risk disease in an urban Egyptian setting are similar to those in comparable settings elsewhere and locally. This exploratory study supports tailoring local screening criteria for ROP, and may aid the future development of national guidelines.
Keywords: retinopathy of prematurity, ROP, screening, plus disease, preterm
Introduction
Retinopathy of prematurity (ROP) is a vision-threatening condition that remains a leading cause of childhood blindness. It occurs due to arrested development of neurovascular retinal components that stimulate aberrant vascular proliferation.1 The incidence of the disease varies across different regions, reflecting the state of neonatal care. As the quality of the care initially improves with subsequent reduction in infant mortality, the rate of ROP may paradoxically increase; this probably reflects the early suboptimal care and inadequate number of ophthalmologists trained on ROP screening.2 Thus, middle-income countries with developing health-care systems have been registering the highest rates of ROP in the recent years.3,4
Although low gestational age (GA) and birth weight (BW) are classically considered the major risk factors for ROP development, heavier and more mature infants with co-morbidities or those receiving suboptimal care can also develop severe forms of the disease.2,5 Consequently, unified international screening criteria do not exist; regional guidelines are developed in accordance to local neonatal care standards and available screening data.6,7 This necessitates organized screening efforts to establish the burden of the condition and its regional risk factors, followed by planned prevention measures. Prevention is either primary with better access to safe and standardized prenatal care, secondary with tailored and enforced screening guidelines for early detection and intervention, or tertiary with management of complications and visual rehabilitation.8,9
In Egypt, few reports10–12 suggest a high burden of ROP over the past decade, reflecting the increase in neonatal survival rates. However, no established screening guidelines exist to date. Further, rural regions within the country are likely to be inflicted by more severe forms of the disease due to lower standard of care.13 The discrepancy in the criteria used for screening and in regional conditions of neonatal care, together with the small sample sizes, result in wide variability in reporting and indefinite conclusions.
In this prospective, multicentric screening effort, we utilized expanded ROP screening criteria to establish the incidence rate and risk factors for ROP development in an urban setting within greater Cairo, Egypt. The aim was to provide reference data as a preliminary step for establishing national tailored guidelines for ROP screening.
Methods
This is a preliminary analysis of prospectively acquired screening data started by the Giza Memorial Institute for Ophthalmic Research (MIOR), one of the oldest and largest dedicated academic eye institutes in Egypt. The screening began in December 2020 and is ongoing as of the time of writing. The final date included in this analysis was April 2022. Four large tertiary health-care institutes within the same region with maternal and neonatal services were also selected for participation and informed of the study’s inclusion criteria. The study adhered to the tenants of the Helsinki Declaration, and ethical approval from the General Organization for Teaching Hospitals and Institutes was obtained (approval ID: IOP00074). All parents were adequately informed about the screening procedure and value and provided written informed consent prior to the screening procedure. All data were de-identified before analysis to ensure anonymity of study participants.
As this was an exploratory study to establish adequately inclusive criteria for screening, we set out to adopt to lowest possible threshold for screening (Table 1). Infants born with GA < 37 weeks (wk) and/or BW ≤ 2000 grams (g) were included in our screening. More mature and heavier infants were also included in the screening if they had an unstable clinical course or known associated risk factors (eg, poor weight gain, neonatal respiratory distress syndrome [RDS], prolonged oxygen requirement, neonatal sepsis, or blood transfusion).
 |
Table 1 Screening Criteria for ROP in Comparison to Published Criteria and Guidelines |
All screening was performed by adequately trained ophthalmologists from a single institution (MIOR). All infants underwent screening at the age of 4 weeks or at 31 weeks of postmenstrual age, even if the infant was in the neonatal intensive care unit (NICU). Charts of infants were reviewed for GA, BW, duration of NICU admission, and systemic risk factors. Dilated fundus examination was conducted in accordance to the American Academy of Pediatrics (AAP)14 and the Royal College of Pediatrics and Child Health (RCPCH) guidelines.15 We used readily-available combination eye drops for pupillary dilation (phenylephrine 2.5% and cyclopentolate 1%). A maximum of three drops were instilled in the eye at 15-minute intervals to avoid systemic toxicity. We then employed topical anesthesia (Benoxinate Hydrochloride 0.4%) during examination of infants that were placed in a swaddling position, with the eyes kept open by an appropriately sized speculum. Binocular indirect ophthalmoscopy utilizing a 28-diopter handheld lens was used to examine the fundus, with rotation of the eye aided by an indentor.
Classification and staging of the disease was based on the second edition of the international classification of ROP;18 the third edition19 was made available in the middle of the study and was utilized in later screening episodes. In case of positive or equivocal findings, colored fundus photographs were obtained using a contact fundus camera (3nethra neo, Forus Health, India). The same camera was used for subjects from all centers and the image analysis was made by two experienced retina specialists (more senior staff members) with open adjudication in case of disagreement. Treatment decision was based on the early treatment of ROP trial.20 Type I (high risk) disease was considered in zone I when any stage ROP was associated with plus disease, or stage 3 ROP without plus disease, and in zone II when stage 2 or 3 ROP were associated with plus disease.
Data were collected and tabulated using Excel software. The Statistical Package for Social Science (IBM SPSS Version 25) was used for analysis. Normality and hetereoskedasticity of continuous data were assessed with Shapiro–Wilk and Levene’s test, respectively. Data were described in terms of mean (± SD) in case of normal distribution, and median with interquartile range (IQR) in case of non-normally distributed. Grouping in terms of treatment requirement allowed comparing GA, BW, and comorbidities between groups. Continuous outcomes were compared with unpaired Student’s t-test or Mann–Whitney U-test according to data distribution. Discrete outcomes were compared with chi-squared or Fisher’s exact test accordingly. The alpha risk was set to 5%.
Results
The final analysis included 768 eyes of 384 screened infants. The percentage of infants admitted to the NICU that fulfilled the screening criteria in the four auxiliary tertiary centers during the study interval were 46%, 39.5%, 31%, and 22%. Table 2 demonstrates the demographic and co-morbidity distribution of the examined infants. The median (IQR) GA of the examined infants was 34 (32–36) wk, and the median (IQR) BW was 1875 (1555–2410) g. Infants were admitted to the NICU for a median (IQR) of 24 (15–33) days. Over half of the infants (206, 53.6%) did not develop an associated co-morbidity. In those with co-morbidities (178, 46.4%), the most common were RDS (132, 34.4%) and neonatal sepsis (77, 20.1%).
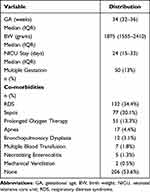 |
Table 2 Distribution of Demographic and Co-Morbidity Variables in the Studied Sample (n = 384) |
The results of ROP screening are given in Table 3. The majority of the eyes (421, 54.8%) had stage 0 disease, and only a small proportion had pre-plus (62, 8.1%) or plus (31, 4%) disease. The eyes that had stage 1 or higher disease (347, 45.2%) belonged to infants with a mean (± SD) GA and BW of 33.4 (± 2.6) wk and 1842.3 (± 570.1) g.
 |
Table 3 Screening Results in the Studied Eyes (n = 768) |
Forty-three eyes (5.6%) had type I disease and were candidates for intervention. When analyzing risk factors in infants with treatment-requiring disease, their mean (± SD) GA and BW were found to be 32.8 (± 3.2) wk and 1747.6 (± 676.2) g, respectively, which were lower than those of non-treatment-requiring infants (34.2 ± 2.8 wk [p = 0.0245], and 1998.6 ± 627.8 g [p = 0.0706], respectively). A significantly higher proportion of infants that required treatment (63.6%) had developed co-morbidities compared to those that did not require treatment (45.3%, p = 0.0257). All infants with treatment-requiring disease that fell outside the AAP screening criteria for GA and BW (11, 2.9%) had one or more co-morbidities and an unstable clinical course.
Discussion
Secondary prevention of ROP entails timely detection and treatment of cases. This requires the development of regional screening guidelines, which is an evolving process with continuous updates from large-scaled studies.7,21,22 Although the disease is emerging in African nations, no formal screening guidelines exist in most of the continent’s countries, including Egypt.23 In this exploratory, relatively-large prospective study of ROP in an urban Egyptian setting, we demonstrate that the incidence of high-risk disease (type I ROP, 5.6%) was within reported ranges, but with a distribution of risk factors that is more consistent with developing nations like India,17 rather than developed ones like the United States14 or the United Kingdom.15
The optimum ROP screening guidelines are ones that have the highest sensitivity for detecting high-risk disease.2,24 This stimulated our adoption of the most encompassing screening criteria in Egypt to date in order to better characterize our studied population. Although direct comparison to other cohorts is not possible due to differences in cutoff screening criteria of GA and BW, the incidence of ROP (excluding stage 0, 45.2%) and of high-risk disease (5.6%) in our sample were relatively comparable to previous reports. In a study from Turkey that included all preterm infants from 49 NICUs, the overall incidence of ROP was 30% and of severe ROP was 5%.25 In a prospective, multicentric study in China that screened infants with GA ≤ 34 wk and/or BW ≤ 2000 g, the incidence of ROP and type 1 ROP were 17.8% and 6.8%, respectively.26 A study from Alexandria, Egypt that screened infants with BW < 1250 g and maternal postmenstrual age <32 wk found the incidence of ROP to be 34.4% and of type 1 ROP to be 9.8%.11 The largest retrospective report of screened Egyptian infants to date included 2318 preterm infants, without specifying the utilized screening criteria, and found that the incidence of type 1 disease was 5.7%.27
National screening protocols should initially adopt more inclusive criteria to accommodate for regional inconsistence in neonatal care standards and ROP prevalence.24,28 With gradual improvement in neonatal care over the years, a shift in occurrence of severe disease towards smaller and less mature infants is often seen, allowing for later adjustments to screening guidelines and reducing their financial burden.22,29,30 Regional discrepancy is also noted across different settings within the same nation and could necessitate tailored screening; for instance, a higher prevalence of severe ROP among heavier and more mature infants was found in rural compared to urban settings in India.31,32 This may also be the case in Egypt. A recent study by Tawfik et al13 that screened infants in 32 NICUs in rural Egypt with cutoffs GA ≤ 34 wk and/or BW ≤ 2000 g found the incidence of treatment-requiring ROP to be 47.4%, much higher than the rate of high-risk disease in our study (5.6%).
We found that the main risk factors for developing treatment-requiring disease were a low GA (mean; 32.8) and BW (mean; 1747.6 g), and the presence of co-morbidities (prevalence; 63.6%). This is in line with published findings. Low GA and BW, and the use of supplemental oxygen are considered the major risk factors linked to ROP development within the literature.2 Other associated risk factors include RDS, sepsis, intraventricular hemorrhage, apnea, continuous positive pressure ventilation, and frequent blood transfusion.33
A strength of this study is including one of the largest screening samples for ROP to date in Egypt. Further, the prospective nature, the inclusion of multiple urban centers, and the wide-range screening criteria contribute to the strength of study design and limit missing of any heavier and more mature infants that may develop the disease. A limitation of the work is that the results cannot be generalizable to a nation-wide level due to heterogeneity in neonatal healthcare provision. Another limitation is that the number of treatment-requiring infants was insufficient to allow for multivariate regression analysis of risk factors.
In conclusion, we provide a large analysis of incidence and risk factors of ROP in an urban Egypt setting. The incidence rates of ROP and of high-risk disease were comparable to national and international rates, although occurrence of treatment-requiring disease was significantly less than in a large recent study from a rural setting.13 This exploratory study can serve in tailoring local criteria for ROP screening, and in the future development of national guidelines. With national plans for digitalization of the healthcare system, easier documentation and referral of infants that require screening could be expected, and a greater contribution of telemedicine could limit the proportion of missed screening encounters.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi:10.1016/S0140-6736(13)60178-6
2. Kim SJ, Port AD, Swan R, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63:618–637. doi:10.1016/j.survophthal.2018.04.002
3. Darlow BA, Gilbert C. Retinopathy of prematurity - a world update. Semin Perinatol. 2019;43:315–316. doi:10.1053/j.semperi.2019.05.001
4. Azad R, Gilbert C, Gangwe AB, et al. Retinopathy of prematurity: how to prevent the third epidemics in developing countries. Asia Pacific J Ophthalmol. 2020;9:440–448. doi:10.1097/APO.0000000000000313
5. Yu CW, Popovic MM, Dhoot AS, et al. Demographic risk factors of retinopathy of prematurity: a systematic review of population-based studies. Neonatology. 2022;119:151–163. doi:10.1159/000519635
6. Bowe T, Nyamai L, Ademola-Popoola D, et al. The current state of retinopathy of prematurity in India, Kenya, Mexico, Nigeria, Philippines, Romania, Thailand, and Venezuela. Digit J Ophthalmol. 2019;25:49–58. doi:10.5693/djo.01.2019.08.002
7. Sen P, Wu W-C, Chandra P, et al. Retinopathy of prematurity treatment: Asian perspectives. Eye. 2020;34(4):632–642. doi:10.1038/s41433-019-0643-4
8. Sai Kiranmayee P, Kalluri V. India to gear up to the challenge of ‘third epidemic’ of retinopathy of prematurity in the world. Indian J Ophthalmol. 2019;67:726–731. doi:10.4103/ijo.IJO_700_18
9. Campbell JP, Singh P, Redd TK, et al. Applications of artificial intelligence for retinopathy of prematurity screening. Pediatrics. 2021;147:e2020016618. doi:10.1542/peds.2020-016618
10. Nassar MM. Screening for retinopathy of prematurity: a report from upper Egypt. Int J Ophthalmol. 2016;9:262–265. doi:10.18240/ijo.2016.02.15
11. Hadi AMA, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol. 2013;7:831–837. doi:10.2147/OPTH.S40136
12. Hakeem AHAA, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19:289–294. doi:10.4103/0974-9233.97927
13. Tawfik S, Mansour A, Selim NL, et al. Analysis of a two-year independent screening effort for retinopathy of prematurity in rural Egypt. BMC Ophthalmol. 2021;21:1–6. doi:10.1186/s12886-021-02193-x
14. Fierson WM, Chiang MF, Good W. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142(6):e20183061. doi:10.1542/peds.2018-3061
15. Royal College of Pediatrics and Child Health. UK screening of retinopathy of prematurity guideline; 2022. Available from: https://www.rcpch.ac.uk/sites/default/files/2022-06/RetinopathyGuidelinesJune.pdf.
16. Bas AY, Demirel N, Koc E, et al. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol. 2018;102:1711–1716. doi:10.1136/bjophthalmol-2017-311789
17. Shukla R, Murthy GVS, Gilbert C, et al. Operational guidelines for ROP in India: a summary. Indian J Ophthalmol. 2020;68:S108–S114. doi:10.4103/ijo.IJO_1827_19
18. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–999. doi:10.1001/archopht.123.7.991
19. Chiang MF, Quinn GE, Fielder AR, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;128:e51–e68. doi:10.1016/j.ophtha.2021.05.031
20. Good WV; Group ET for R of PC. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233.
21. Jalali S, Matalia J, Hussain A, et al. Modification of screening criteria for retinopathy of prematurity in India and other middle-income countries. Am J Ophthalmol. 2006;141(5):966–968. doi:10.1016/j.ajo.2005.12.016
22. Larsen PP, Müller A, Lagrèze WA, et al. Incidence of retinopathy of prematurity in Germany: evaluation of current screening criteria. Arch Dis Childhood Fetal Neonatal Ed. 2021;106(2):189–193. doi:10.1136/archdischild-2020-319767
23. Wang D, Duke R, Chan RP, et al. Retinopathy of prematurity in Africa: a systematic review. Ophthalmic Epidemiol. 2019;26(4):223–230. doi:10.1080/09286586.2019.1585885
24. Alizadeh Y, Behboudi H, Dourandeesh M, et al. Retinopathy of prematurity: applicability of international and national screening guidelines in the north of Iran. Turk J Pediatr. 2022;64(2):221. doi:10.24953/turkjped.2021.1943
25. Bas AY, Koc E, Dilmen U. Incidence and severity of retinopathy of prematurity in Turkey. Br J Ophthalmol. 2015;99:1311–1314. doi:10.1136/bjophthalmol-2014-306286
26. Xu Y, Zhou X, Zhang Q, et al. Screening for retinopathy of prematurity in China: a neonatal units–based prospective study. Invest Opthalmol Vis Sci. 2013;54:8229. doi:10.1167/iovs.13-12297
27. Bassiouny RM, Gaafar WM, El Nokrashy A, et al. Clinical outcome following reinjection of Ranibizumab for reactivation of retinopathy of prematurity. Eye. 2021;36(11):2137–2143. doi:10.1038/s41433-021-01814-5
28. Jalali S, Anand R, Kumar H, et al. Programme planning and screening strategy in retinopathy of prematurity. Indian J Ophthalmol. 2003;51:89–99.
29. Trzcionkowska K, Schalij‐Delfos NE, van den Akker‐van Marle EME. Cost reduction in screening for retinopathy of prematurity in the Netherlands by comparing different screening strategies. Acta Ophthalmol. 2022. doi:10.1111/aos.15205
30. Barjol A, Lux AL, Dureau P, et al. Evaluation and modification of French screening guidelines for retinopathy of prematurity. Acta Ophthalmol. 2022;100:e1451–e1454. doi:10.1111/aos.15091
31. Hungi B, Vinekar A, Datti N, et al. Retinopathy of prematurity in a rural neonatal intensive care unit in South India—a prospective study. Indian J Pediatr. 2012;79:911–915. doi:10.1007/s12098-012-0707-y
32. Sanghi G, Sawhney JS, Kaur S, et al. Evaluation of clinical profile and screening guidelines of retinopathy of prematurity in an urban level III neonatal intensive care unit. Indian J Ophthalmol. 2022;70(7):2476–2479. doi:10.4103/ijo.IJO_1925_21
33. Azami M, Jaafari Z, Rahmati S, et al. Prevalence and risk factors of retinopathy of prematurity in Iran: a systematic review and meta-analysis. BMC Ophthalmol. 2018;18:83. doi:10.1186/s12886-018-0732-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
