Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Tailored botulinum toxin type A injections in aesthetic medicine: consensus panel recommendations for treating the forehead based on individual facial anatomy and muscle tone
Authors Anido J, Arenas D, Arruabarrena C, Dominguez-Gil A, Fajardo C, Mira M, Murillo J, Ribé N, Rivera H , Ruiz del Cueto S, Silvestre H, Tirado M
Received 29 March 2017
Accepted for publication 20 July 2017
Published 19 October 2017 Volume 2017:10 Pages 413—421
DOI https://doi.org/10.2147/CCID.S138274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Javier Anido,1 Daniel Arenas,2 Cristina Arruabarrena,3 Alfonso Domínguez-Gil,4 Carlos Fajardo,5 Mar Mira,6 Javier Murillo,7 Natalia Ribé,8 Helga Rivera,9 Sofia Ruiz del Cueto,6 Helder Silvestre,10 Marisa Tirado11
1A-Clinic, Madrid, 2Hospital Cruz Roja, Madrid, 3Clinic Cristina Arruabarrena, San Sebastiá, 4Salamanca University, Salamanca, 5Clinic Fajardo, Malaga, 6Clinic Mira+Cueto, Madrid, 7Clinic CIR, Seville, 8Institute Natalia Ribé, Barcelona, 9Clinic Helga Rivera, Vigo, Spain; 10Clinic Europa, Lisbon, Portugal; 11Clinic Derma Alemar, Castellón, Spain
Background: Facial lines and wrinkles are strongly influenced by individual differences in anatomy and muscle activity and no single injection protocol will suit all patients. However, there is only limited information in the published literature on how to develop a tailored approach to botulinum toxin treatment.
Methods: An expert panel of physicians was convened to establish a consensus on developing an individualized approach to treatment of the forehead with incobotulinumtoxinA. Separate treatment protocols were developed for men and women and subdivided by background level of muscle activity: kinetic, hyperkinetic, and hypertonic. Each muscle tone category was then further subdivided to take account of individual characteristics that can influence treatment.
Results: Consensus members describe how to perform a dynamic assessment to optimize the dose and injection technique for each patient. A tailored treatment protocol is described for men and women with a wide range of forehead presentations. For each presentation, units of toxin as well as the precise location of injection points were defined by creating a 12-zone map of the forehead.
Conclusion: These recommendations depart from traditional consensus documents by providing detailed incobotulinumtoxinA injection protocols for the forehead based on the major parameters that differ between patients, including muscular anatomy, size, and tone. It is expected that the use of this document will lead to more satisfactory, natural, and individualized aesthetic outcomes for patients.
Keywords: incobotulinumtoxinA, Xeomin, Bocouture, forehead lines, frontalis
Introduction
Three botulinum toxin type A products are currently approved for aesthetic use in Western markets: onabotulinumtoxinA (ONA; Botox®/Vistabel®, Allergan Inc., Irvine, CA, USA), abobotulinumtoxinA (ABO; Dysport®/Azzalure®, Ipsen, Paris, France), and incobotulinumtoxinA (INCO; Xeomin®/Bocouture®, Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany). All share the same mode of action, with differences between products occurring because of proprietary manufacturing processes, purification methods, and inactive ingredients in the formulation. INCO is the only product free from complexing proteins or neurotoxin-associated proteins. These play no role in the neuronal mode of action of the toxin complex and have no effect on product diffusion.1 Stability is unaffected by their absence, with INCO having a shelf-life of 3–4 years at room temperature, compared with 2–3 years for ONA and ABO and a requirement for refrigeration. A lack of complexing proteins also reduces the potential antigenicity of a product and thus the risk of developing secondary neutralizing antibodies.2
All three toxins are US Food and Drug Administration (FDA)-approved for the treatment of moderate-to-severe glabellar frown lines. ONA and INCO are also approved for the treatment of lateral periorbital lines (crow’s feet) in the EU. For INCO only, European regulatory authorities reached a consensus in 2016 on approvability for the combined treatment of upper facial lines (glabellar frown lines, lateral periorbital lines, and horizontal forehead lines). Results from head-to-head clinical trials have demonstrated that INCO and ONA have similar efficacy and tolerability when used in a 1:1 dose conversion ratio for the treatment of glabellar frown lines3,4 and lateral periorbital lines.5,6 A recent consensus review suggests that a conversion ratio of 1:2.5 (INCO/ONA:ABO) may be assumed in aesthetic indications.7 In addition to the above-mentioned approved indications, all the products are also widely used off-label for a number of other aesthetic indications, including lifting and reshaping the eyebrows, softening perioral lines, treating platysmal bands, and correcting facial asymmetry.
The most important goal of botulinum toxin treatment in aesthetic medicine is to achieve a balance between dynamic wrinkles caused by hyperactive muscles while maintaining natural facial animation. This is influenced by a number of factors including individual anatomy, structure, action and mass of the muscles, and personal aesthetic preferences. Tailored treatment taking into account all these factors is required for optimal results and consequently patient satisfaction with treatment and their physician.
Why is this expert consensus required in aesthetics?
Clinical data and consensus articles provide helpful guidance on aesthetic indications, but their consideration of individual patient differences is more limited.7–16 Patients differ enormously in their facial anatomy both within and between genders, and treating all patients at the same injection points and with the same doses will leave many with less than optimal results. The current document was put together to help physicians develop an individualized approach to botulinum toxin treatment of the forehead.
Consensus methodology
Twelve experts in the fields of aesthetic medicine, dermatology, and plastic surgery convened four times between July 2014 and February 2015 in Madrid, Spain, to develop independent, consensus-based recommendations for the use of INCO for aesthetic indications in patients with varying degrees of muscular activity and wrinkle severity. During these meetings, the group developed a series of recommendations covering the clinical history and physical evaluation of the patient as well as a muscular map of each treatment area illustrating the points of injection. The following text summarizes the recommendations for the treatment of the forehead. The content reflects the opinions of the authors only. The recommendations of the group for the treatment of other facial muscles will be published in a separate paper.
Consensus recommendations
Patient evaluation and classification
A number of patient characteristics and anatomical features help define their suitability for botulinum toxin injection. The positions, strength, and insertion points of the facial muscles can be determined by inspecting them at rest, by observing their movements while the patient makes varying facial expressions, and by palpating them. Signs for areas of stronger contraction include greater dynamic movement, deeper lines, and larger apparent mass during use.
Consensus members classified patients into three groups based on their facial muscle contractions and line severity prior to treatment: kinetic, hyperkinetic, and hypertonic.17 Kinetic patients are those with regular muscle contraction and wrinkles during active expression but not at rest. Hyperkinetic patients have more excessive muscle contraction and may require more frequent treatment and higher doses to achieve the desired effect. Finally, hypertonic patients are those with an inability to relax specific muscles and with visible wrinkles at rest. They may still be candidates for treatment, but should be advised that while botulinum toxin treatment may result in some improvements, the wrinkles will not completely disappear and additional use of an injectable dermal filler may be necessary. Deep static lines due to loss of skin elasticity are not suitable for botulinum toxin injection.
The aim of treatment is to eliminate lines when the patient is at rest, but to leave the ability for some movement and minimal wrinkling when the patient is animated or actively expressing emotion.
Treatments
The dosing units described in this document are applicable to INCO and ONA and use standard reconstitution volumes. The units of ABO are different, but a conversion ratio of INCO/ONA:ABO of 1:2.5 or 1:3 is generally assumed in aesthetic indications.7
Forehead
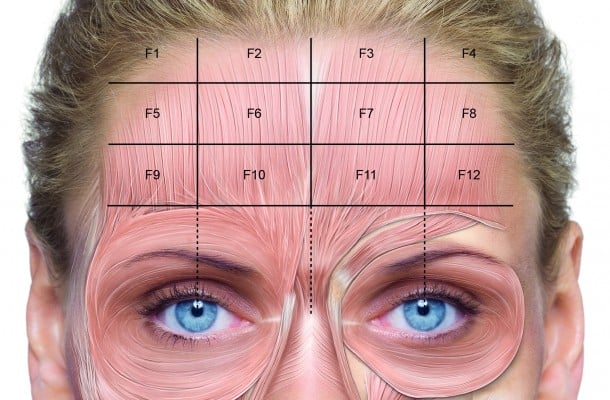
Horizontal forehead lines are caused by the contraction of the frontalis, a large pair of muscles whose fibers are oriented vertically and whose medial fibers are joined in the glabellar region, where they intersect with the procerus.18 The central and lateral fibers merge with the corrugator supercilii and the inner part of the orbicularis oculi (Figure 1). Contraction of the frontalis raises the eyebrows and the upper eyelid, wrinkling the forehead in the process.8 The corrugator supercilii work antagonistically as depressors of the brow, and the forehead should not undergo treatment without treatment of the corrugator. The simultaneous treatment of all upper facial lines is an approved indication for INCO and is effective and commonly performed in aesthetic practice.19
  | Figure 1 Injection points for the treatment of horizontal forehead lines. |
Before beginning the treatment, the physician should evaluate the patient for expressivity, muscle mass, symmetry, lateral versus medial movement, compensation for brow ptosis, and brow width and height. The intensity of contractions along the height of the frontalis can differ substantially from individual to individual, and variations in muscle function should be taken into account when deciding on the dose of botulium toxin and where the injections will be placed. This can be detected by light palpation over the area while the patient actively raises and lowers the eyebrows.
Treatment of the frontalis muscles can not only reduce horizontal forehead lines but can also affect eyebrow shape and height. Brow shape is influenced by the complex interplay between the frontalis and the lateral (lateral orbicularis oculi) and medial (procerus, corrugator supercilii, medial orbicularis oculi) brow depressors, and the use of botulinum toxin treatments to shape the brow is not considered in this document. To precisely define the location of injection points for individual patients, the consensus group divided the forehead into 12 zones positioned 1.5−2.0 cm above the eyebrow to avoid any risk of brow ptosis (Figure 1).
Injection points and units vary between men and women due to differences in anatomy and patient preferences, and they were considered separately when designing a treatment protocol for the forehead.20,21 The point of divergence of the two frontalis muscles is generally lower in men than women, which has implications for injection sites.22 Men typically also have greater muscle mass and a larger forehead surface area than women and require higher doses. Eyebrows are naturally positioned lower in men, and excessive relaxation of the lower frontalis can result in brow ptosis. Men and women were then subdivided by muscle tone prior to treatment (kinetic, hyperkinetic, and hypertonic). Each muscle tone category was further subdivided to take account of individual characteristics that can influence treatment. In this manner, a tailored treatment protocol was developed for women (Table 1) and men (Table 2) with a wide range of forehead presentations.
  | Table 1 Individualized treatment protocol for the forehead in women Note: *Injection between both points (F/F). |
  | Table 2 Individualized treatment protocol for the forehead in men Note: *Injection between both points (F/F). |
Women with kinetic frontalis
Average-size forehead
In women with an average size forehead and dynamic forehead lines, the group recommends intramuscular injection at four injection points across the midline of the forehead (F5 to F8) with 1−2 U of botulinum toxin per point depending on the strength of the frontalis. The injection points should be ~1.5−2.0 cm apart and placed on alternating sides of the targeted horizontal lines.
High forehead
Women with a high forehead and dynamic wrinkles are treated as mentioned previously with 1−2 U at each of injection points F5 to F8. If required, a second line of injections can be placed above the first with the addition of two injection points at F2 and F3, and subcutaneous injection of 1 U of botulinum toxin at each point.
Tendency to develop Mephisto sign
The so-called Mephisto sign occurs in some patients when lateral movement of the frontalis remains after treatment and produces visible wrinkles. It is more common when treatment of the forehead is restricted to the area between the midpupillary lines. Women with strong lateral frontalis fibers should receive 1 U intramuscularly at F6 and F7 and 1 U subcutaneously at points F9 and F12.
Palpebral weakness
Upper eyelid ptosis may occur when treatment of the frontalis muscle unmasks subtle pre-existing weakness of the levator palpebrae superioris muscle. Botulinum toxin product labels recommend evaluation of the upper eyelid, especially in patients with a history of glabellar trauma or surgery, for the presence of levator palpebra muscle separation or weakness.9 These patients are treated by intramuscular injection of 1−2 U at points F5 to F8, but with the addition of 1 U of botulinum toxin subcutaneously at points F6 and F7.
V-shaped frontalis
The frontalis may form either a uniform band across the forehead or be V-shaped with a relative absence of fibers medially. Women with the latter presentation are treated by subcutaneous injection of 1 U of botulinum toxin on the midline between F6 and F7 in addition to intramuscular injection of 1−2 U at points F5 and F8.
Women with hyperkinetic frontalis
Average-size forehead
Treatment is with the same pattern of intramuscular injection as for women with a kinetic frontalis (F5 to F8), but using the higher end of the dose range (2 U) per injection point.
High forehead
In the first row of injections, treatment is with 2 U intramuscularly at each of points F5 to F8. Women with a high forehead can receive a second line of injections placed above the first. However, in those with a hyperkinetic frontalis, the two additional injection points at F2 and F3 are injected intramuscularly rather than subcutaneously with 1 U of botulinum toxin at each point.
Tendency to develop Mephisto sign
Treatment is with 2 U of toxin injected intramuscularly at injection sites F6 and F7. Two lateral injections are also placed on each side of the forehead by injecting 1 U of toxin subcutaneously at the lateral limit of zones F1/F5 and F5/F9, and on the other side of the forehead 1 U at the lateral limit of zones F4/F8 and F8/F12.
Palpebral weakness
These women should receive 1 U of botulinum toxin subcutaneously at each of two sites defined as the midline of F5/F6 and F7/F8 in addition to the standard F5–F8 injection points.
V-shaped frontalis
Treatment is with intramuscular injection of 2 U of botulinum toxin at each of points F5 to F8 and with 1 U subcutaneously on the midline of F6/F7.
Women with hypertonic frontalis
Average-size forehead
Hypertonic patients are difficult to treat, and the limitations of botulinum toxin treatment should be explained beforehand. The recommended treatment protocol is 1 U of botulinum toxin subcutaneously at sites F5 and F8, and 1 U intramuscularly at sites F6 and F7. Hypertonic patients are particularly susceptible to brow ptosis, and injection in the lateral limit of the frontalis should be avoided.
High forehead
Treatment is as for women with an average-size forehead with the recommendation that patients are evaluated after several days at the follow-up appointment to determine if further treatment is necessary.
Other forehead presentations
These are uncommon in women with a hypertonic frontalis, and the details of their treatment are presented in Table 1.
Men with kinetic frontalis
Average-size forehead
For men with regular frontalis contractions, dynamic forehead wrinkles, and an average size forehead, the group recommends intramuscular injection at four injection points across the midline of the forehead (F5 to F8) with 2 U of botulinum toxin per point. As in women, the injection points should be ~1.5−2.0 cm apart and placed on alternating sides of the targeted horizontal lines.
Prominent forehead
It is important not to accentuate a prominent forehead due to a receding hairline or shaved head. Smoothing out the entire forehead or leaving muscle activity above the level of the normal hairline would draw attention to the upper third of the face. In addition to intramuscular injection of 2 U at points F5 to F8, these men can also be treated with additional intramuscular injection of 1 U at points F1 and F4 (1 cm below the muscle attachment).
Tendency to develop Mephisto sign
In addition to intramuscular injection of 2 U at points F5 to F8, 1 U of toxin can be injected intramuscularly at each of injection sites F9 and F12 coinciding with the point of maximum contraction.
Palpebral weakness
Men with palpebral weakness should receive intramuscular injection of 2 U at points F5 and F8 plus 3 U at points F6 and F7.
V-shaped frontalis
Standard treatment is with intramuscular injection of 2 U at points F5 to F8 plus subcutaneous injection of 1 U of botulinum toxin on the midline of F6/F7.
Short forehead
Treatment should be restricted to 1 U intramuscularly at points F6 and F7 and 1 U subcutaneously at points F9 and F12.
Men with hyperkinetic frontalis
Average-size forehead
The group recommends the same pattern of intramuscular injection as for men with a kinetic frontalis (F5 to F8) with the addition of two further intramuscular injections of 1U each on the midline of points F1/F2 and F3/F4.
Prominent forehead
A second line of injections can be placed above the first as in men with a kinetic frontalis, but a higher dose may be used. In addition to 2 U intramuscularly in each of points F5 to F8, they are treated with 2 U intramuscularly in the midline of points F1/F2 and 2 U intramuscularly in the midline of points F3/F4.
Tendency to develop Mephisto sign
In addition to intramuscular injection of 2 U at points F5 to F8, 1 U of toxin can be injected intramuscularly at each of injection sites F9 and F12 (coinciding with the point of maximum contraction) plus 1 U intramuscularly on the midline of points F1/F2 and points F3/F4.
Palpebral weakness
The group recommends intramuscular injection of 1 U at points F5 to F8 plus 2 U of botulinum toxin intramuscularly on the midline of points F1/F2 and points F3/F4.
V-shaped frontalis
Standard treatment is with intramuscular injection of 2 U at points F5 to F8. In addition, these patients can receive intramuscular injection of 1 U of botulinum toxin on the midline between points F1/F2 and F3/F4 as well as 1 U subcutaneously at each of points F6 and F7.
Short forehead
Men with a short forehead and hyperkinetic frontalis receive the standard treatment of 2 U intramuscularly at each of points F5 to F8.
Lateral superciliary wrinkles
Prominent lateral superciliary wrinkles will require additional injections in the lateral frontalis. In addition to intramuscular injection of 2 U at points F5 to F8, 1 U can be injected intramuscularly on the midline of points F1/F2 and F3/F4, and 1 U subcutaneously in the lateral limit of points F9 and F12.
Men with hypertonic frontalis
Average-size forehead
The limitations of botulinum toxin treatment in these patients should be explained before starting the treatment to avoid disappointment. The recommended treatment protocol is 1 U of botulinum toxin intramuscularly at sites F5 to F8.
Prominent forehead
Two rows of injection are used. In the first row, toxin is administered intramuscularly as 1 U at each of points F5 to F8. In the second row, 1 U is administered intramuscularly at the midline of points F1/F2 and F3/F4.
Palpebral weakness
One unit of toxin may be injected intramuscularly at the midline of points F1/F5, F2/F6, F3/F7, and F4/F8. When performing this treatment, the brow depressor muscles (corrugator supercilii, procerus, depressor supercilii, and superolateral portion of the orbicularis oculi) should be treated at the same time.
Short forehead
Men with a short forehead and hypertonic frontalis should be treated with intramuscular injection of 1 U at each of points F5 to F8.
Other forehead presentations
The Mephisto sign and V-shaped frontalis are rare in men with a hypertonic frontalis, and the details of their treatment are presented in Table 2.
Discussion
Patient satisfaction with botulinum toxin treatment depends on the physician’s ability to visualize and recommend a treatment plan that meets the patient’s goals and needs. A number of consensus documents have been published to assist physicians on the use of botulinum toxin in aesthetic medicine.7–16 However, to the authors’ knowledge, this is the first document to provide an individualized approach to treatment with detailed injection protocols for the forehead based on the major parameters that differ between patients, including muscular anatomy, size, and tone.
The recommendations were developed on the premise that no single injection protocol can suit all patients. However, few studies in the literature account for differences in facial anatomy and muscle tone when evaluating treatment with botulinum toxin.23–26 For example, a review on the use of botulinum toxin in men found only two studies that accounted for gender in either the study design or subgroup analysis and only one dose-ranging study.21 As the number of male patients seeking treatment has been increasing, physicians need to account for gender when evaluating and treating their cosmetic patients. Sexual dimorphism in facial anatomy and cutaneous physiology is well documented, yet these differences are rarely accounted for in clinical practice.27 Men and women also differ in a number of other facial features including the size of the forehead, the position and shape of the eyebrows, and the shape of the jaw. Such anatomical variations between genders result in differences in aging and consequently in how individuals should be treated.
For an optimal aesthetic outcome, each patient should undergo a static and dynamic assessment of muscle position, mass, and functional status prior to treatment. In the current consensus, muscle tone is divided into kinetic, hyperkinetic, and hypertonic, and each must be treated differently for optimal results. Careful observation of the extent of dynamic movement of the skin will identify areas of stronger or weaker muscle contraction. In this way, the physician can determine why certain wrinkles are formed and which muscles are creating them. This information is needed to balance the effects of opposing muscles and minimize the risk of unwanted outcomes. The static and dynamic evaluation may also identify other more subtle variations in facial musculature that should be considered during the planning of an effective botulinum toxin treatment regimen, such as palpebral weakness, compensatory muscle use, and facial asymmetry. The dynamic assessment is therefore essential to optimize the dose and injection technique for each patient.
It is hoped that the current consensus document will be of use to a wide range of aesthetic physicians from beginners to experts. It departs from the single template of dosing and injection points described in most consensus guidelines by tailoring treatment protocols to individual patients, which will lead to more satisfactory, natural, and individualized aesthetic outcomes.
Acknowledgments
The consensus meetings were funded by Merz Pharma España S.L., Madrid, Spain. Editorial support for manuscript development was provided by Jenny Grice and funded by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
Disclosure
The authors report no conflicts of interest in this work.
References
Kerscher M, Roll S, Becker A, et al. Comparison of the spread of three botulinum toxin type A preparations. Arch Dermatol Res. 2012;304:155–161. | ||
Torres S, Hamilton M, Sanches E, Starovatova P, Gubanova E, Reshetnikova T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2013;7:11–17. | ||
Kane MA, Gold MH, Coleman WP 3rd, et al. A randomized, double-blind trial to investigate the equivalence of incobotulinumtoxinA and onabotulinumtoxinA for glabellar frown lines. Dermatol Surg. 2015;41:1310–1319. | ||
Sattler G, Callander M, Grablowitz D, et al. Non-inferiority of NT201, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg, 2010;36(Suppl 4):2146–2154. | ||
Prager W, Wissmüller E, Kollhorst B, et al. Comparison of two botulinum toxin type A preparations for treating crow’s feet: a split-face, double-blind, proof-of-concept study. Dermatol Surg. 2010;36:2155–2160. | ||
Muti G, Harrington L. A prospective rater- and subject-blinded study comparing the efficacy of incobotulinumtoxinA and onabotulinumtoxinA to treat crow’s feet: a clinical crossover evaluation. Dermatol Surg. 2015;41(Suppl 1):S39–S46. | ||
Yutskovskaya Y, Gubanova E, Khrustaleva I, et al. IncobotulinumtoxinA in aesthetics: Russian multidisciplinary expert consensus recommendations. Clin Cosmet Investig Dermatol. 2015;8:297–306. | ||
Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit) – Part I: upper facial wrinkles. J Eur Acad Dermatol Venereol. 2010;24:1278–1284. | ||
Kane M, Donofrio L, Ascher B, et al. Expanding the use of neurotoxins in facial aesthetics: a consensus panel’s assessment and recommendations. J Drugs Dermatol. 2010;9:s7–s22. | ||
Raspaldo H, Baspeyras M, Bellity P, et al. Consensus Group. Upper- and mid-face anti-aging treatment and prevention using onabotulinumtoxin A: the 2010 multidisciplinary French consensus – part 1. J Cosmet Dermatol. 2011;10:36–50. | ||
Raspaldo H, Niforos FR, Gassia V, et al; Consensus Group. Lower-face and neck antiaging treatment and prevention using onabotulinumtoxin A: the 2010 multidisciplinary French consensus – part 2. J Cosmet Dermatol. 2011;10:131–149. | ||
Ahn BK, Kim YS, Kim HJ, Rho NK, Kim HS. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg. 2013;39:1843–1860. | ||
Carruthers A, Kane MA, Flynn TC, et al. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine – a global, evidence-based botulinum toxin consensus education initiative: part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg. 2013;39:493–509. | ||
Carruthers J, Fournier N, Kerscher M, Ruiz-Avila J, Trindade de Almeida AR, Kaeuper G. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine – a global, evidence-based botulinum toxin consensus education initiative: part II: incorporating botulinum toxin into aesthetic clinical practice. Dermatol Surg. 2013;39:510–525. | ||
Lorenc ZP, Kenkel JM, Fagien S, et al. Consensus panel’s assessment and recommendations on the use of 3 botulinum toxin type A products in facial aesthetics. Aesthet Surg J. 2013;33(Suppl 1):35S–40S. | ||
Sundaram H, Signorini M, Liew S, et al; Global Aesthetics Consensus Group. Global Aesthetics Consensus: botulinum toxin type A – evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast Reconstr Surg. 2016;137:518e–529e. | ||
De Maio M, Rzany B. Patient selection. In: De Maio M, Rzany B, editors. Botulinum Toxin in Aesthetic Medicine. Berlin: Springer; 2007:11–19. | ||
Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal aesthetic botulinum toxin type A therapy. Aesthetic Plast Surg. 2013;37:975–983. | ||
Kerscher M, Rzany B, Prager W, Turnbull C, Trevidic P, Inglefield C. Efficacy and safety of incobotulinumtoxinA in the treatment of upper facial lines: results from a randomized, double-blind, placebo-controlled, phase III study. Dermatol Surg. 2015;41:1149–1157. | ||
Flynn TC. Botox in men. Dermatol Ther. 2007;20:407–413. | ||
Keaney TC, Alster TS. Botulinum toxin in men: review of relevant anatomy and clinical trial data. Dermatol Surg. 2013;39:1434–1443. | ||
Spiegel JH, Goerig RC, Lufler RS, Hoagland TM. Frontalis midline dehiscence: an anatomical study and discussion of clinical relevance. J Plast Reconstr Aesthet Surg. 2009;62:950–954. | ||
Kane MA, Brandt F, Rohrich RJ, et al. Evaluation of variable-dose treatment with a new US botulinum toxin type a (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124:1619–1629. | ||
De Almeida AR, da Costa Marques ER, Banegas R, Kadunc BV. Glabellar contraction patterns: a tool to optimize botulinum toxin treatment. Dermatol Surg. 2012;38:1506–1515. | ||
Monheit G, Lin X, Nelson D, Kane M. Consideration of muscle mass in glabellar line treatment with botulinum toxin type A. J Drugs Dermatol. 2012;11:1041–1045. | ||
Xie Y, Zhou J, Li H, Cheng C, Herrler T, Li Q. Classification of masseter hypertrophy for tailored botulinum toxin type A treatment. Plast Reconstr Surg. 2014;134:209e–218e. | ||
Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55:144–149. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
