Back to Journals » Journal of Pain Research » Volume 16
T Lymphocyte Subsets Profile and Toll-Like Receptors Responses in Patients with Herpes Zoster
Authors Chen W , Zhu L , Shen LL , Si SY, Liu JL
Received 17 January 2023
Accepted for publication 5 May 2023
Published 17 May 2023 Volume 2023:16 Pages 1581—1594
DOI https://doi.org/10.2147/JPR.S405157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Wei Chen,1,* Lu Zhu,1,* Li-Ling Shen,1 Shao-Yan Si,2 Jun-Lian Liu3
1Dermatology, Zhejiang Provincial Dermatology Hospital, Huzhou, Zhejiang, People’s Republic of China; 2Department of Comprehensive Basic Experiment, Strategic Support Force Medical Center, Bejing, People’s Republic of China; 3Dermatology, Chui Yang Liu Hospital Affiliated Tsinghua University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun-Lian Liu, Chui Yang Liu Hospital affiliated Tsinghua University, 2 Chuiyangliu South Street, Chaoyang District, Beijing, 100022, People’s Republic of China, Tel +86-13801214526, Email [email protected]
Purpose: Herpes zoster (HZ) is caused by the varicella-zoster virus (VZV), and 20% of healthy humans and 50% of people with immune dysfunction have a high probability of suffering from HZ. This study aimed to screen dynamic immune signatures and explore the potential mechanism during HZ progression.
Patients and Methods: Peripheral blood samples from 31 HZ patients and 32 age-sex-matched healthy controls were collected and analyzed. The protein levels and gene levels of toll-like receptors (TLRs) were detected in peripheral blood mononuclear cells (PBMCs) by flow cytometry and quantitative real-time PCR. Further, the characteristics of T cell subsets and cytokines were detected via a cytometric bead array.
Results: Compared to healthy controls, the mRNA levels of TLR2, TLR4, TLR7, and TLR9 mRNA in PBMCs were significantly increased in HZ patients. The protein level of TLR4 and TLR7 was significantly increased in HZ patients, but the levels of TLR2 and TLR9 were dramatically decreased. The CD3+ T cells were constant in HZ and healthy controls. CD4+ T cells were decreased in HZ patients, while CD8+ T cells were increased, resulting in an improved CD4+/CD8+ T cells ratio. Further, it was found that Th2 and Th17 were not changed, but the decreased Th1 and upregulated Treg cells were found in HZ. The Th1/Th2 and Th17/Treg ratios were significantly decreased. Last, the levels of IL-6, IL-10, and IFN-γ were significantly increased, but IL-2, IL-4, and IL-17A had no significant changes.
Conclusion: The dysfunction of host’s lymphocytes and activation of TLRs in PBMCs were the important mechanism in varicella-zoster virus induced herpes zoster. TLRs might be the core targets for the therapy drug development in treating HZ.
Keywords: herpes zoster, peripheral blood mononuclear cells, T lymphocyte, toll-like receptors
Introduction
Herpes zoster (HZ) is an infectious skin disease caused by the reactivation of varicella-zoster virus (VZV) which has been hidden in the dorsal root ganglion of the spinal cord. It is mainly characterized by erythema, clustered vesicles, and neuralgia distributed in unilateral cutaneous ganglia.1 The incidence rate of herpes zoster infection is 2.67 per 100 person-years.2 In the United States, nearly 1 in 3 people suffer HZ in their lifetime.3 In China, its incidence rate in individuals aged ≥50 years is 6.64 per 1000 person-years.4 It is noted that about 20% of the HZ patients will suffer from postherpetic neuralgia (PHN)-a common complication.5 Meanwhile, the risk of HZ increases with age, leading to long-term sequelae such as blindness, the increased risk of stroke, and heart attack.6 At present, the treatment of HZ is still limited to symptom control, such as using acyclovir, valacyclovir, or famciclovir, ideally within 72 hours of the development of the rash.5 Thus, clearly and actually revealing the pathogenesis of HZ is of great importance to develop methods to prevent and treat HZ.
Generally, the virus infection disease is always related to the host’s immunity, such as influenza,7 COVID-19,8 etc. As the first line of defense against pathogens, the innate immune system recognizes and combines pathogen-related molecular patterns through pattern recognition receptors (PRRs).9 Then, it activates downstream signal transduction pathways and mediates the activation of immune responses, inducing innate immune cells to secrete proinflammatory cytokines and inflammatory factors.10 Toll-like receptors (TLRs) were the most deeply studied PRRs, playing an important role in the pathogenesis of infectious diseases, such as human papilloma virus (HPV), herpes simplex virus (HSV), human immunodeficiency virus (HIV).11 TLRs could induce macrophages to release inflammatory cytokines to mediate the host’s immune defense response, including tumor necrosis factor α (TNF-α), interferon-γ (IFN-γ), etc. In the meantime, T lymphocytes were activated and released a large number of cytokines to mediate the host’s antiviral response.12
Till now, TLRs have 13 family members in mammals, including TLR1~ TLR13. According to their cellular localization, TLRs were classified into two major subfamilies: the cell surface subfamily (TLRs 1, 2, 4, 5, 6, and 10) and the endolysosomal compartment subfamily (TLRs 3, 7, 8, and 9).13 TLRs could induce the activation of the signal pathways, leading to the expression of inflammatory cytokines and chemokines, which determined the differentiation trend of T cells. For example, TLRs activated nuclear factor kappa B signal (NF-κB) through the myeloid differentiation factor 88 (MyD88) transduction pathway.14 Subsequently, various cytokines were produced, including IFN-α/β, TNF-α, interleukin-2 (IL-2), IL-4, IL-6, IL-10 and IL-12.15 These cytokines were the key factors in regulating the T cell differentiation to Th1, Th2, Th17, and Treg cells.16 TLRs were also expressed on immune cells in the skin, mucosa, and blood system.17 Recently, it was found that activation of TLR2, TLR7, and TLR9 proteins could induce VZV antigen peptide-specific immune response.5,6 The mRNA levels of TLR2, TLR4, and TLR7 were upregulated in peripheral blood mononuclear cells (PBMC) of HZ patients.18,19 Further, Trudler et al found that most satellite glial cells around VZV-infected neurons highly expressed TLR9, suggesting that TLR9 might participate in VZV recognition and the host’s antiviral immunity.20,21
The pathogenesis of HZ is more closely related to cellular immunity.22 In vitro experiments indicated that VZV could depress the expression of major histocompatibility complex I (MHC-I), CD80, CD86, and other immune molecules on the cell surface. VZV could inhibit the expression of signal pathways related to T cell activation, restricting T lymphocyte activation and proliferation. This will make VZV-infected cells escape T-cell immune recognition, and reactivate the virus.23 Different lymphocyte subsets were important to the host’s immune balance. From VZV reactivation to clinical symptoms, this pathogenesis could result in disturbed T lymphocyte subsets and an imbalanced immune response.
Despite the importance of TLRs, the comprehensive characterization of TLRs in mRNA and protein levels in HZ was not reported, limiting the elucidation of the pathogenesis of HZ. Moreover, the role and mechanism of T lymphocytes and their subsets in the pathogenesis of HZ were still unclear. In this study, flow cytometry and RT-PCR were used to profile T lymphocytes and their subsets (Th1, Th2, Th17, and Treg cells) in peripheral blood, TLRs expression in BPMCs, and cytokines in the serum of HZ patients.
Materials and Methods
Patient and Samples
In this work, 31 patients (aged 18 to 63 years old) at different time points (day 1 to day 10) after the onset of the rash were classified into HZ groups, including 16 males and 15 females. At the same time, 32 age-sex-matched healthy donors (aged 19 to 70 years old) were treated as normal controls (NC), including 14 males and 18 females. Patients with HZ were diagnosed clinically by a typical vesicular rash in dermatome distribution, and medications prescribed were in standard criterion.24 Exclusion criteria included minors, known as serious immunity disorders or cardiocerebrovascular, diabetes, breastfeeding or pregnancy, and accompanying fungal, bacterial, or viral infection in one month. Clinical pain-related scores, including Numbering Rating Score (NRS), Touch induced NRS, Numbness degree, DN4, ID-pain, GAD7, PHQ9, the impact of pain on mood, and the impact of pain on daily life were documented in each patient. The clinical characteristics of HZ patients and healthy controls were summarized in Table 1. Ethical Review Methods for Biomedical Research involving Humans was approved by the ethics committee at Zhejiang Provincial Dermatology Hospital (NO.LL2021-1), and the written informed consent was signed by all participants before study entry. Meanwhile, this study had been conducted in compliance with the protocol, the current version of the Declaration of Helsinki and the national legal and regulatory requirements of China.
 |
Table 1 The Comparison of Clinical Data Between Herpes Zoster Patients (HZ) and Control Group (NC). Data Were Presented as mean±SD and the Significance Was Analyzed by Chi-Square Test |
Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
The blood samples from HZ patients and healthy control were collected and put into the 10 mL EDTA-coated vacutainer tubes (BD Biosciences). 4 mL blood and 4 mL physiological saline solution were mixed in a tube, and subsequently, 6 mL human lymphocyte separation medium (No.LTS1077, TBD Science, China) was added. Then, it was centrifuged at 2000 rpm for 20 min. The supernatant was removed and the residues were reconstituted by 5-times physiological saline solution. Further, it was centrifuged at 2000 rpm for 20min. The supernatant was removed and the residues were PBMCs.
Real-Time PCR
Based on the cDNA sequences of human β-actin, TLR2, TLR4, TLR7, and TLR9 in GenBank, their primer sequences were developed using Primers 5 software (Table 2) and further produced by Sangon Biotech.LTD (Shanghai, China). Total RNA in the peripheral blood mononuclear cells was obtained with TRIzol reagent (Invitrogen, USA), and cDNA was synthesized from RNA using a reverse transcription kit (Takara, RR047A). Real-time PCR was performed using fluorescent quantitative PCR kits (Promega Corporation) with specific primers based on the manufacturer’s instructions. β-actin was used as an endogenous reference gene in each reaction.
 |
Table 2 Primers Sequences and the Size of Amplified Fragments for Toll-Like Receptors |
Flow Cytometry
The analysis of toll-like receptors: The PBMCs in HZ and control group were surface-stained with Percp Cyanine5.5-conjugated CD14 (No.45–0149-42, eBioscience, USA) for 30 min at 4 °C. The cells were then either intracellularly stained with anti-human TLR2-FITC (No.11–9922-42, eBioscience, USA), anti-human TLR4-PE (No.12–9917-42, eBioscience, USA), anti-human TLR9-PE (No.12–9099-82, eBioscience, USA), or anti-human TLR7-FITC antibody (No.NBP2-25274F, Novus Biologicals, USA) by an intracellular fixation and permeabilization kit (No.88–8824-00, eBioscience, USA) according to the manufacturer’s instructions. All plots shown on populations were gated on lymphocytes by forward and side scatter. Compensation settings were adjusted using isotype controls. Flow cytometry analysis was performed on a BD FACS Calibur (BD Biosciences, USA). Data analysis was performed by using FlowJo software (Ashland, OR, USA).
The analysis of T lymphocytes: The 50 μL blood was incubated with 10 μL CD3+/CD4+/CD8+/CD45+(340,490, BD bioscience, USA) or CD3+/CD16+CD56+/CD45/CD19 antibody (340,499, BD bioscience, USA) for 20 min under room temperature. Then, 1mL hemolysin was added, mixed, and incubated for 10 min (room temperature and in a dark place). After centrifugation at 1200 rpm for 5 min, the supernatant was removed and the residue was reconstituted by 2 mL sheath fluid. Then, they were centrifugated at 1200 rpm for 5 min, the supernatant was removed and the residue was reconstituted by 20 Ml sheath fluid. Then, they analyzed a BD FACS Calibur (BD Biosciences, USA).
The analysis of T lymphocytes subsets: The PBMCs in HZ and control group were surface-stained with APC-conjugated CD4 (No.561841, BD bioscience, USA), APC-conjugated CD25 (No.560987, BD bioscience, USA) or FITC-conjugated CD4 (No.561842, BD bioscience, USA) for 30 min at 4 °C. The cells were then either intracellularly stained with PE-conjugated IL-17A (No.560436, BD bioscience, USA) by an intracellular fixation and permeabilization kit (No.88–8824-00, eBioscience, USA) or intranuclearly stained with PE-conjugated Foxp3 by the Foxp3/Transcription Factor Staining Buffer kit (No.00–5521-00, eBioscience, USA) according to the manufacturer’s instructions. All plots shown on populations were gated on lymphocytes by forward and side scatter. Compensation settings were adjusted using isotype controls. Flow cytometry analysis was performed on a BD FACS Calibur (BD Biosciences, USA). Data analysis was performed by using FlowJo software (Ashland, OR, USA).
The analysis of cytokines: Serum levels of IL-2, IL-4, IL-6, IL-10, and IFN-γ were established by flow cytometry utilizing a cytometric bead array (CBA) (BD Biosciences, CA, USA). The results were obtained by BD CBA Analysis Software (BD Biosciences).
Statistical Analysis
In this work, data were processed with GraphPad Prism 9 and were presented as the means ± SD. Unpaired Student’s t-test was applied to analyze the difference between two groups, nsP>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Results
As shown in Table 1, a total of 63 subjects were used to sample collection and data analysis, including 31 herpes zoster patients and 32 health control. For gender (P=0.53) and age (P=0.94), there was no significance between these two groups, which was analyzed by the chi-square test, indicating that their blood samples could be effectively used to further analysis.
The Disordered Lymphocytes Were Found in the Blood of Herpes Zoster Patients (HZ)
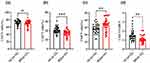
Lymphocytes are the important cellular components for the host’s immune response, and they are regarded as the front-line “soldier” to fight against external infection and monitor the host’s cell variation. Herpes zoster is caused by the varicella-zoster virus, and the lymphocytes’ response may be disturbed in HZ patients. As shown in Figure 1, the lymphocytes were analyzed by flow cytometry, including CD3+, CD4+, and CD8+ T lymphocytes. For CD3+ T cells, it was found that there was no significant difference between HZ patients and control groups (P>0.05, Figure 1a). It was noted that, as compared to control groups, the CD4+ T cells were dramatically decreased in HZ patients (P<0.001), while the CD8+ T cells were significantly improved (P<0.01, Figure 1b and c). Indeed, it could be found that the CD4/CD8+ T cell ratio was significantly decreased in HZ patients (P<0.01, Figure 1d).
Further, the subsets of lymphocytes were analyzed, including Th1, Th2, Th17, and Treg cells. As shown in Figure 2a, significantly decreased Th1 cells were found in HZ patients, while Treg cells were significantly increased. For the Th2 and Th17 cells, there was no significance between HZ patients and control groups. Further, the Th1/Th2 ratio and Th17/Treg ratio were significantly decreased in HZ patients (Figure 2b).
The Disordered Cytokines Were Found in the Serum of Herpes Zoster Patients (HZ)
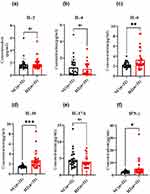
The disordered lymphocytes always resulted in imbalanced cytokines. Then, the cytokines were detected in the serum of HZ patients and the control group, including IL-2, IL-4, IL-6, IL-10, IL-17A, and IFN-γ (Figure 3). For the IL-2, IL-4, and IL-17A, there was no significance between HZ patients and the control group (P<0.05, Figure 3a, b and e). Notably, it was found that increased IL-6, IL-10, and IFN-γ were found in HZ patients (Figure 3c, d and f).
The Activation of Toll-Like Receptors Were Found in Blood Mononuclear Cells of Herpes Zoster Patients (HZ)
Toll-like receptors (TLRs) were responsible for inflammation cytokines’ s production. The mRNA and protein expression of TLRs in peripheral blood mononuclear cells of HZ patients and health hosts were detected by RT-PCR and flow cytometry, respectively. In Figure 4a, as compared to the control groups, the mRNA expression of TLR2, TLR4, TLR7, and TLR9 (Figure 4a–d) was dramatically up-regulated in HZ patients. Further, their protein expression was also investigated. As shown in Figure 5a, the blood mononuclear cells (R1) were selected, and CD14-positive mononuclear cells (R2) were subjected to further analysis. In line with the mRNA results, TLR4 and TLR7 were significantly increased (Figure 5b). It was noted that the mRNA of TLR2 and TLR9 was increased, while their protein levels were decreased.
Discussion
The Host’s Immune Response Was Involved in HZ Pathogenesis
VZV is a human varicella virus α herpes virus responsible for herpes zoster (HZ). It is mainly transmitted by aerosol and direct contact with vesicular secretions.25 During the initial infection stage of VZV, the virus was inoculated on the upper respiratory tract mucosa and replicated in epithelial cells. Subsequently, the virus infected mononuclear cells located in local lymph nodes, causing viremia. Further, VZV is transferred to peripheral immune organs, such as the liver and spleen. After 10–14 days, the virus transferred to the skin to form the second viremia.26 During the primary infection of VZV, the neurons in the ganglion are invaded by the virus mainly in two ways. One was the retrograde axonal transport of the skin replication site, and the other is the transmission of VZV from the infected T cells to the ganglion through the cell-related viremia. As reported in the previous work, it was found that after VZV infection, the TLRs-mediated immune regulation mechanism could depress the expression of MHC-I, MHC-II, and costimulatory molecules on the surface of T cells. Subsequently, VZV-infected cells could escape the immune recognition by CD4+ T lymphocytes and CD8+ T lymphocytes, and the virus was reactivated.23 The reactivated VZV spreads from the sensory ganglia to the skin and infects keratinocytes and fibroblasts, resulting in a strong inflammatory reaction and pain feeling of the skin.27
T lymphocytes and their subset are the main effector cells that mainly mediated cellular immune response. Generally, T cells are divided into CD4+ T cells and CD8+ T cells according to the different molecules on the cell membrane surface. Given the functions, T cells are divided into Th cells, CTL cells, and regulatory T cells (Treg). CD4 Th cells can differentiate into Th1, Th2, and Th17 cells, which are regulated by different cytokines. For example, IL-2 and IFN- γ could induce Th0 to differentiate into Th1; IL-4 induced Th0 to differentiate into Th2; TGF- β and IL-6 induced Th0 to differentiate into Th17; and TGF- β and IL-2 induce Th0 to differentiate into Treg cells. Th1 and Th2 cells play an important role in regulating cellular and humoral immune responses, respectively. Th17 cells participate in innate immunity and some infectious diseases by secreting IL-17. Treg cells inhibit target cell activation and secret TGF-β, IL-10, and other cytokines to inhibit immune response. When VZV infection occurred, the host’s immune response started to exert protective effects.
T Lymphocytes and Their Cytokines’ Production Were Important for the Host’s Immune Response in HZ
The above information indicated that T lymphocytes, TLRs, and cytokines were the three core indexes for VZV-caused diseases, such as HZ. The effective monitor of host’s immune modulation of HZ patients in the early stage could help the treatment of HZ. When subjects were exposed to children experiencing varicella (chicken pox), antigen-specific CD4+ T cells in peripheral blood were increased.28 Vukmanovic-Stejic et al found that the numbers of circulating IFN-γ-secreting VZV-specific CD4+ T cells were significantly decreased in old subjects.29 The animal model of rhesus monkeys infected with simmian varicella virus (SVV) was used to simulate human infection with VZV, and it was found that all T cell subsets (CD4+ T cells) were decreased during immunosuppression, except for CD8 effectors.30 Zhang et al profiled the peripheral blood T cell subsets of HZ patients (n=73) and healthy people (n=80), and they found that the CD4+ T cells in HZ patients (infection time was 17±4 day) were significantly reduced, which was earlier than the changes of CD8+ T cells and CD4+T/CD8+T.31 In our work, the CD3+ T lymphocytes were not changed in HZ patients, while decreased CD4+ T cells and upregulated CD8+ T cells were found (Figure 1). This was in line with work of Vossen et al,28 Stejic et al29, James et al30 and Zhang et al.31 However, in other work, significantly increased CD4+ and CD8+ T cells were found in PBMC samples of HZ patients (2 weeks infection), while decreased CD4+ and CD8+ T cells were found in patients after 3, or 4 weeks infection.32 It was noted that the decreased CD4+T and CD8+ T cells were found in the patients with 3, 7 days’ infection.32 In our work, HZ patients’ infection time ranged from 1 to 10 days, which was less than two weeks (14 days). Our data could reflect the early stage of HZ with decreased CD4+ T cells, which was in line with data of Peng et al.32 As compared to Peng et al,32 the opposite CD8+ T cells’ expression was found in our work (n=31), and smaller sample size (n=6) in their work might be the reason.
The immune response mediated by memory CD8+ T cells also played an important role in immune defense in HZ patients.33 After VZV activation, the host’s immune response was mainly mediated by cytotoxic CD8+ T cells. Steain et al found that a large number of CD8+ T cells infiltrated around dorsal root ganglion neurons in HZ patients, indicating that CD8+ T cells participated in the HZ cellular immune response.34 The increased CD8+ T cells in the early infection time found in our work (Figure 1c) were in line with the reported data.30,32 In addition, a decreased tendency of CD4+/CD8+ T cell ratio was observed in our work (Figure 1d), indicating a faster proliferation velocity of CD8+ T cells.32 It has been found that the ratio of CD4+T and CD8+T cells decreased significantly after VZV activation and before the appearance of the typical rash. When rash began to appear, the ratio of CD4+T/CD8+T began to increase gradually until the skin lesions subsided and the ratio returned to the normal range.35
After VZV infection, initial CD4+ T cells could differentiate into Th1, Th2, Th17, and Treg cell subsets to achieve different functions. Th1 cells mainly secreted IL-2, IFN-γ, and TNF-α, participating in the host’s antiviral immune response and enhancing the cytotoxicity of cytotoxic T lymphocytes and natural killer cells. Th2 mainly secreted cytokines, such as IL-4 and IL-10, inhibiting the differentiation of Th1 cells and their cytokines’ production.36 Th17 was a unique CD4+ T cell subset different from Th1 and Th2 cells. At the early stage of infection, with the help of IL-6 and TGF-β, the retinoic acid related nuclear lonely receptor γt (Retinoid related orphan receptor γt, RORγt) in the initial CD4+T cells was induced and activated, promoting CD4+T cells to differentiate into Th17 and produce IL-17, IL-21, IL-23, etc.37 Regulatory T cell (Treg) was a subset of CD4+T cells independent of Th1 and Th2 cells. Together with Th17, they regulated and inhibited each other in viral infectious diseases, and jointly maintain the host’s immune balance.38
In our work, Treg cells in HZ patients were significantly increased, while the Th2 and Th17 cells were constant. The Th1 cells were decreased (Figure 2). IFN-γ secreted by Th1 cells could inhibit the transcription of viral DNA and controls viral replication by forming an obstacle between VZV early protein IE62 and the transcriptional mediator complex.39 It was found that IFN-γ, TNF-α, and IL-6 levels in the skin lesions of HZ patients were significantly increased.40 The levels of Th1 cytokines (IL-2 and TNF-α) in the blister fluid from HZ patients’ skin lesions were significantly lower than in healthy control, whereas the levels of Th2 cytokines (IL-10 and IL-4) were significantly decreased.41 Elevated IL-1β, IL-6, TNF-α, IL-8, and IL-10 were also found in the serum of HZ and PHN patients.42 Along with constant IL-2, IL-4, and IL-17A, the elevated IL-6, IL-10, and IFN-γ were also found in the serum of HZ patients (Figure 3). As a sensitive indicator of early tissue damage, IL-6 exerted an important role in the early inflammatory response of HZ. The highly expressed IL-6 in HZ patients could inhibit the activation of VZV and reduce the damage to skin and neurons.43 Agata et al found that the levels of Th17 cytokines (IL-17, IL-23, IL-21) and Th2 cytokines (IL-4 and IL-12) in peripheral blood serum of HZ patients were higher than those of healthy control groups.44 Cytokines such as IL-4, IL-10, and IL-13 secreted by Th2 cells in turn inhibit the differentiation and development of Th17 cells, resulting in a decrease in the expression of Th17 type cytokines IL-17, IL-23, and IL-21.45 According to the above results, Th2 cells, which were dominant in the HZ eruption stage, inhibit the proliferation and differentiation of Th17, showing high expression of Th2 type cytokines.
Treg cells could indirectly participate in the occurrence and development of HZ by regulating CD4+T cells and Th1 cells. Decreased CD4+T cells and significantly increased CD4+CD25+Foxp3+ T cells were found in the peripheral blood of HZ patients, and CD4+T cells were negatively correlated with Treg cells.46 It indicated that Treg cells were activated in HZ patients, and the activated Treg cells could inhibit the proliferation and activation of CD4+ T cells. This was also found in our work. Moreover, the increased IL-10 could effectively inhibit Th1 cell-mediated antiviral response. Treg cells and Th17 cells maintain the host’s immune balance by interacting with each other. When their ratio was too high, the host could induce the differentiation of Th17 cells and inhibit the growth of Treg cells under the cooperation of IL-6.41 In our work, the ratio of Th17/Treg cells in the HZ group shifted and the proportion of Treg cells increased, suggesting that the balance of Th17/Treg cells was destroyed. The proportion of Th17 remained unchanged in the pathogenesis of HZ, which was not only related to the dominant Th2, but also might be the increased inhibition of Treg cells on Th17 cells.
Toll-Like Receptors Were the Core Signal Targets for the Host’s Immune Response in HZ
After VZV infection, TLRs could induce the activation of the signal pathways, leading to the expression of inflammatory factors and chemokines, determining the differentiation trend of T cells. In our work, the mRNA and protein levels of TLR2, TLR4, TLR7, and TLR9 were characterized. Among them, TLR2 and TLR4 could be expressed on the cells’ surface. Wang et al found that after VZV infection with, TLR2 expression was up-regulated in PBMCs, along with the production of IL-6. Suppressing TLR2 mRNA could reduce IL-6 response to VZV in human monocyte-derived macrophages.47 In our work, the TLR2 mRNA and IL-6 levels in HZ patients were significantly higher than that in healthy controls, indicating that TLR2 participated in the pathogenesis of HZ. We speculated that after VZV activation, TLR2 was highly expressed in PBMC of HZ patients, triggering a strong immune inflammatory reaction, leading to pathological damage to skin and nerve tissue, blisters, and neuralgia. In this study, the low level of TLR2 protein expression might be related to the loss of TLR2 post transcriptional regulatory genes, which needed further study. In the previous work, it found that TLR4 could reduce the viral load by mediating antiviral immune response after recognizing viral protein.48 The mRNA levels of TLR2 and TLR4 in peripheral blood mononuclear cells of HZ showed an upward trend, indicating that TLR2 and TLR4 were involved in VZV recognition and antiviral immune response.19 Our result was consistent with them, including overexpression of TLR4 mRNA and its protein. This indicated that TLR4 could specifically recognize VZV, promote the release of cytokines, and induce an antiviral immune response.
TLR7 and TLR9 were classified into the endolysosomal compartment subfamily of TLRs. TLR7 mainly recognized single stranded RNA, and generates type I interferon and proinflammatory cytokines by recognizing viral nucleic acid to trigger an immune response. TLR7 agonist could activate Langerhans cells in the skin and promote the polarization of Th1 cells, defensing on viral infection.29 Till now, the role of TLR7 in the pathogenesis of HZ had not been reported. In this work, we found that the mRNA level of TLR7 and its protein in PBMCs of HZ patients were significantly higher than that of healthy people, indicating that activated TLR7 was involved in the pathogenesis of HZ. A preliminary research basis for the new target of clinical drug therapy for HZ was also provided.
TLR9, as a signal transduction “sensor” for recognizing VZV, activates NF-κB and IRF signal transduction pathways by recognizing VZV DNA CpG sequence, mediating the production of inflammatory cytokines. Most satellite glial cells around VZV-infected neurons expressed TLR9, and a large amount of IFN-α was released, indicating that TLR9 could induce host’s antiviral immune response by recognizing VZV.20,21 After blocking STAT-3 signal pathway in peripheral blood leukocytes, macrophages and NK cells stimulated by TLR9 agonist CpG released a large amount of IFN-α and TNF-α, suggesting that TLR9 could rapidly activate macrophages and NK cells to release cytokines involved in the innate immune response.49 In our work, the TLR9 mRNA of PBMCs in HZ patients was significantly higher than that in healthy controls, indicating that TLR9 was involved in the pathogenesis of HZ, and the expression level of antiviral inflammatory cytokines was regulated by TLR9. The upregulation of TLR9 enhanced MyD88 dependent NF-κB and IRF signal transduction pathway, inhibited STAT3 signal transduction pathway, and induced IFN-α to exert anti-virus function. IFN-α and IFN-β activated JAK-STAT pathway via positive feedback to induce a proinflammatory reaction and produce cytokine TNFα, IL-6, and IL-1β. These cytokines and chemokines could induce lymphocyte infiltration, magnifying the inflammatory reaction. This was the role of TLR 9 in the pathogenesis of HZ.
Conclusion
Herpes zoster (HZ) was caused by the varicella-zoster virus (VZV), affecting 20% of healthy humans and 50% of people with immune dysfunction in the world. The host’s immune system exerts an important role in the defense of HZ, but the dynamic immune profiles and molecular mechanisms remain unclear. The dynamic immune signatures and the potential mechanism during HZ progression were profiled. It was found that decreased protein expression of TLR2 and TLR9 and increased TLR4 and TLR7 were found in PBMCs of HZ patients. Meanwhile, the T lymphocytes in the PBMCs of HZ were also characterized, including decreased CD4+ T, Th1 cells Th1/Th2, Th17/Treg ratios, and increased CD8+ T, CD4+/CD8+ T cells ratio. Last, the levels of IL-6, IL-10, and IFN-γ were significantly increased, but IL-2, IL-4, and IL-17A had no significant changes (Figure 6). It was the first time to present the TLR7 expression in PBMCs of HZ patients, including gene and protein levels. Moreover, the protein level of TLR2, 4, and 9 were also characterized in this work for the first time. This work provided the immune information and potential targets for the therapy drug development in treating HZ. However, in our work, the deep pathological mechanism of HZ was not provided, and the mice model and cell model should be used to validate the linkage among T lymphocytes, TLRs, and HZ in the further work.
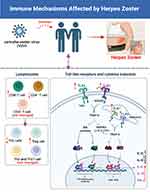 |
Figure 6 Summarized immune mechanisms affected by herpes zoster (HZ). Created with BioRender.com. |
Acknowledgments
This research was funded by National Natural Science Foundation of China, grant number 81071402.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, et al. Clinical characteristics and outcomes in a population with disseminated herpes zoster: a retrospective cohort study. [Caracteristicas clinicas y evolutivas de una poblacion con herpes zoster diseminado: un estudio de cohorte retrospectiva]. Actas Dermosifiliogr. 2017;108(2):145–152. doi:10.1016/j.ad.2016.10.009
2. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–1573 e12. doi:10.1053/j.gastro.2020.01.001
3. McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125–e134. doi:10.1093/cid/ciz1090
4. Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: data from an integrated health care network. J Infect. 2021;82(2):253–260. doi:10.1016/j.jinf.2020.12.013
5. Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96(10):656–663.
6. Sun Y, Kim E, Kong CL, Arnold BF, Porco TC, Acharya NR. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. Clin Infect Dis. 2021;73(6):949–956. doi:10.1093/cid/ciab121
7. Gu Y, Zuo X, Zhang S, et al. The mechanism behind influenza virus cytokine storm. Viruses. 2021;13(7):Jul. doi:10.3390/v13071362
8. El Karoui K, De Vriese AS. COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment. Kidney Int. 2022;101(5):883–894. doi:10.1016/j.kint.2022.01.022
9. Gao LA, Wilkinson ME, Strecker J, et al. Prokaryotic innate immunity through pattern recognition of conserved viral proteins. Science. 2022;377(6607):eabm4096. doi:10.1126/science.abm4096
10. Snoeck S, Abramson BW, Garcia AGK, Egan AN, Michael TP, Steinbrenner AD. Evolutionary gain and loss of a plant pattern-recognition receptor for HAMP recognition. Elife. 2022;11. doi:10.7554/eLife.81050
11. Sun L, Liu W, Zhang LJ. The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J Immunol Res. 2019;2019:1824624. doi:10.1155/2019/1824624
12. Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29(1):15–26. doi:10.1007/s00281-007-0061-8
13. Zhu KC, Wu M, Zhang DC, et al. Toll-like receptor 5 of golden pompano Trachinotus ovatus (Linnaeus 1758): characterization, promoter activity and functional analysis. Int J Mol Sci. 2020;21(16). doi:10.3390/ijms21165916
14. Hu YH, Wang Y, Wang F, et al. SPOP negatively regulates Toll-like receptor-induced inflammation by disrupting MyD88 self-association. Cell Mol Immunol. 2021;18(7):1708–1717. doi:10.1038/s41423-020-0411-1
15. Manik M, Singh RK. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J Med Virol. 2022;94(3):869–877. doi:10.1002/jmv.27405
16. Ruterbusch M, Pruner KB, Shehata L, Pepper M. In Vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 Paradigm. Annu Rev Immunol. 2020;38:705–725. doi:10.1146/annurev-immunol-103019-085803
17. Huang X, Yang Y. Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses. Expert Opin Ther Targets. 2010;14(8):787–796. doi:10.1517/14728222.2010.501333
18. Wang LF. Expression and significance of toll-like receptor 4 and 9 mRNA in peripheral blood mononuclear cells of children with herpes zoster. National Medl Front Chin. 2012;7(10):1–2.
19. Wu JL, Liu Y, Gong SZ, Zhao M, Zhang XJ. Expression of TLR2, TLR4 mRNA in peripheral blood mononuclear cells of patients with herpes zoster. Chin Med Abstrac. 2010;27(05):273–274.
20. Trudler D, Farfara D, Frenkel D. Toll-like receptors expression and signaling in glia cells in neuro-amyloidogenic diseases: towards future therapeutic application. Mediators Inflamm. 2010;2010:1–12. doi:10.1155/2010/497987
21. Yu HR, Huang HC, Kuo HC, et al. IFN-alpha production by human mononuclear cells infected with varicella-zoster virus through TLR9-dependent and -independent pathways. Cell Mol Immunol. 2011;8(2):181–188. doi:10.1038/cmi.2010.84
22. Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. doi:10.3390/v14020192
23. van Besouw NM, Verjans GM, Zuijderwijk JM, Litjens NH, Osterhaus AD, Weimar W. Systemic varicella zoster virus reactive effector memory T-cells impaired in the elderly and in kidney transplant recipients. J Med Virol. 2012;84(12):2018–2025. doi:10.1002/jmv.23427
24. Dayan RR, Peleg R. Herpes zoster - typical and atypical presentations. Postgrad Med. 2017;129(6):567–571. doi:10.1080/00325481.2017.1335574
25. Laing KJ, Ouwendijk WJD, Koelle DM, Verjans G. Immunobiology of Varicella-Zoster Virus Infection. J Infect Dis. 2018;218(suppl_2):S68–S74. doi:10.1093/infdis/jiy403
26. Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200(7):917–925. doi:10.1084/jem.20040634
27. Arvin AM, Moffat JF, Sommer M, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol. 2010;342:189–209. doi:10.1007/82_2010_29
28. Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. Development of virus-specific CD4+ T cells on reexposure to Varicella-Zoster virus. J Infect Dis. 2004;190(1):72–82. doi:10.1086/421277
29. Vukmanovic-Stejic M, Sandhu D, Seidel JA, et al. The characterization of varicella zoster virus-specific T cells in skin and blood during aging. J Invest Dermatol. 2015;135(7):1752–1762. doi:10.1038/jid.2015.63
30. James SF, Traina-Dorge V, Deharo E, et al. T cells increase before zoster and PD-1 expression increases at the time of zoster in immunosuppressed nonhuman primates latently infected with simian varicella virus. J Neurovirol. 2014;20(3):309–313. doi:10.1007/s13365-014-0237-7
31. Zhang MF, Ma J, Yang L, Zhang J, Zhang XY. Detection of Serum CD4+ T cell and T-lymphocyte subsets in patients of herpes zoster. Chin J Integr Med. 2009;23(04):205–206+235.
32. Peng Q, Guo X, Luo Y, et al. Dynamic Immune Landscape and VZV-Specific T cell responses in patients with herpes zoster and postherpetic neuralgia. Front Immunol. 2022;13:887892. doi:10.3389/fimmu.2022.887892
33. Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–357. doi:10.1007/82_2010_31
34. Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88(5):2704–2716. doi:10.1128/JVI.03445-13
35. Harrer A, Wipfler P, Pilz G, et al. Adaptive immune responses in a multiple sclerosis patient with acute varicella-zoster virus reactivation during treatment with fingolimod. Int J Mol Sci. 2015;16(9):21832–21845. doi:10.3390/ijms160921832
36. Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28(4):163–171. doi:10.1093/intimm/dxw006
37. Ciofani M, Madar A, Galan C, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi:10.1016/j.cell.2012.09.016
38. Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol. 2011;187(9):4440–4450. doi:10.4049/jimmunol.1002860
39. Ku CC, Chang YH, Chien Y, Lee TL. Type I interferon inhibits varicella-zoster virus replication by interfering with the dynamic interaction between mediator and IE62 within replication compartments. Cell Biosci. 2016;6:21. doi:10.1186/s13578-016-0086-6
40. Nikkels AF, Sadzot-Delvaux C, Pierard GE. Absence of intercellular adhesion molecule 1 expression in varicella zoster virus-infected keratinocytes during herpes zoster: another immune evasion strategy? Am J Dermatopathol. 2004;26(1):27–32. doi:10.1097/00000372-200402000-00005
41. Zhang M, Wu N, Yang L, et al. Study on the T-helper cell 1/2 cytokine profile in blister fluid of patients with herpes zoster and its clinical significance. J Dermatol. 2011;38(12):1158–1162. doi:10.1111/j.1346-8138.2011.01289.x
42. Zhu SM, Liu YM, An ED, Chen QL. Influence of systemic immune and cytokine responses during the acute phase of zoster on the development of postherpetic neuralgia. J Zhejiang Univ Sci B. 2009;10(8):625–630. doi:10.1631/jzus.B0920049
43. Bayat A, Burbelo PD, Browne SK, et al. Anti-cytokine autoantibodies in postherpetic neuralgia. J Transl Med. 2015;13:333. doi:10.1186/s12967-015-0695-6
44. Zajkowska A, Garkowski A, Swierzbinska R, et al. Evaluation of chosen cytokine levels among patients with herpes zoster as ability to provide immune response. PLoS One. 2016;11(3):e0150301. doi:10.1371/journal.pone.0150301
45. Kumagai J, Hirahara K, Nakayama T. [Pathogenic Th cell subsets in chronic inflammatory diseases]. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39(2):114–123. Japanese. doi:10.2177/jsci.39.114
46. Xing Q, Hu D, Shi F, Chen F. Role of regulatory T cells in patients with acute herpes zoster and relationship to postherpetic neuralgia. Arch Dermatol Res. 2013;305(8):715–722. doi:10.1007/s00403-013-1367-0
47. Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79(20):12658–12666. doi:10.1128/JVI.79.20.12658-12666.2005
48. Lagos D, Vart RJ, Gratrix F, et al. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe. 2008;4(5):470–483. doi:10.1016/j.chom.2008.09.012
49. Kortylewski M, Kujawski M, Herrmann A, et al. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res. 2009;69(6):2497–2505. doi:10.1158/0008-5472.CAN-08-3031
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.