Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Systemic Therapy for Hepatocellular Carcinoma: Current Updates and Outlook
Received 12 January 2022
Accepted for publication 15 March 2022
Published 30 March 2022 Volume 2022:9 Pages 233—263
DOI https://doi.org/10.2147/JHC.S358082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmed Kaseb
Yinjie Fan,1,2 Hang Xue,2 Huachuan Zheng2
1College of Integrated Chinese and Western Medicine, Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning, 110847, People’s Republic of China; 2Department of Oncology and Experimental Center, the Affiliated Hospital of Chengde Medical University, Chengde, Hebei, 067000, People’s Republic of China
Correspondence: Huachuan Zheng, Department of Oncology and Experimental Center, the Affiliated Hospital of Chengde Medical University, Chengde, Hebei, 067000, People’s Republic of China, Tel +86-0314-2279458, Fax +86-0314-2279458, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) has emerged the culprit of cancer-related mortality worldwide with its dismal prognosis climbing. In recent years, ground-breaking progress has been made in systemic therapy for HCC. Targeted therapy based on specific signaling molecules, including sorafenib, lenvatinib, regorafenib, cabozantinib, and ramucirumab, has been widely used for advanced HCC (aHCC). Immunotherapies such as pembrolizumab and nivolumab greatly improve the survival of aHCC patients. More recently, synergistic combination therapy has boosted first-line (atezolizumab in combination with bevacizumab) and second-line (ipilimumab in combination with nivolumab) therapeutic modalities for aHCC. This review aims to summarize recent updates of systemic therapy relying on the biological mechanisms of HCC, particularly highlighting the approved agents for aHCC. Adjuvant and neoadjuvant therapy, as well as a combination with locoregional therapies (LRTs), are also discussed. Additionally, we describe the promising effect of traditional Chinese medicine (TCM) as systemic therapy on HCC. In this setting, the challenges and future directions of systemic therapy for HCC are also explored.
Keywords: hepatocellular carcinoma, targeted therapy, immunotherapy, traditional Chinese medicine, locoregional therapies, adjuvant therapy, neoadjuvant therapy
Introduction
Hepatocellular carcinoma (HCC), as the most common type of liver cancer, remains a major public-health challenge worldwide.1,2 Because of risk factors for HCC, such as hepatitis B virus (HBV) and hepatitis C virus (HCV), excessive alcohol consumption, cigarette smoking, diabetes, obesity, and dietary habits that are modifiable, its incidence is growing annually.1,3 And it is predicted that by 2025, the number of newly diagnosed HCC cases will be over 1 million annually.4
The management of HCC is mainly based on a staging system for liver cancer with regard to tumor size and number, tumor location, liver function reserves (Child–Pugh Classifications), and performance status (PS).5,6 Recently, the Barcelona Clinic Liver Cancer (BCLC) system has been commonly adopted in clinical studies and practice, which provides the most effective means for assessing patient prognosis and guiding treatment.7,8 For patients with early- or intermediate-stage HCC, hepatectomy, liver transplantation (LT), locoregional therapies (LRTs) such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and radiation therapy are curative treatments that improve survival.9,10 However, up to 70% of HCC cases who received curative surgery or ablation therapy experienced recurrence within 5 years.11 In comparison, systemic therapies are the only treatments available to improve survival for patients with advanced HCC (aHCC).12 Nevertheless, the development and efficacy of systemic therapy has been challenging.13 Coupled with the dismal fact that more than half of HCC cases are diagnosed at an advanced or incurable stage, this disease has been the second most common cause of cancer mortality, with a 5-year survival rate of only 3%.1,3
Gratifyingly, substantial progress has been made in understanding the mechanisms underlying hepatocarcinogenesis and progression, as well as in the development of novel drugs to regulate specific steps of these mechanisms, over several decades.14 Since first-line sorafenib, an oral multi-tyrosine kinase inhibitor (TKI), was first demonstrated to prolong the survival of patients with aHCC in 2007, molecular-targeted agents have made tremendous strides in the enrichment of systemic therapy.15 Tyrosine kinase inhibitors (lenvatinib, regorafenib and cabozantinib) and a vascular endothelial growth factor (VEGF) receptor inhibitor (ramucirumab) have been established as first- or second-line therapies for patients with aHCC.16–19 Recently, immune checkpoint inhibition, which mainly modulates programmed cell death-1 (PD-1), cytotoxic T lymphocyte- associated protein 4 (CTLA-4), and their ligands (programmed cell death ligand 1/2 [PD-L1/2] and B7-1/2, respectively) has developed into a potent anti-cancer strategy.20 Anti–PD-1 antibodies (nivolumab and pembrolizumab), as well as the combination of immune checkpoint inhibitors (ICIs) and VEGF inhibitors (atezolizumab and bevacizumab, nivolumab and ipilimumab), have been approved for the treatment of aHCC.21–23
Importantly, a growing body of preclinical studies and clinical trials based on systemic therapies are ongoing globally. Despite recent advancements, there remain some critical questions and challenges regarding the management of HCC. In this article, we summarized the systemic therapy relying on mechanisms of hepatocarcinogenesis and progression, and then elaborate the practice treatment protocols for HCC applied to clinical practice. In light of this, we finally discuss the challenges and directions in the development of systemic therapy for HCC.
Treatment Advances in Systemic Therapy
Molecular Targeted Therapy
Targeting Molecular Pathways in HCC
The pathophysiology of HCC is known to be a complex multistep process associated with aberrant molecular events and diverse signaling pathways.2 Currently, the most concerning molecular signaling pathway is tyrosine kinase–related signaling mainly related to cell survival, proliferation, differentiation, migration, and angiogenesis. Depending on where they function, tyrosine kinases can be divided into receptor tyrosine kinases (RTKs) and non-RTKs.24 Receptor tyrosine kinases transduce extracellular signals into cells, while non-RTKs regulate intracellular signal transduction and molecular communication.24,25 Furthermore, a large number of receptors exist in the receptor tyrosine kinase group, such as VEGF receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor, hepatocyte growth factor receptor (HGFR), TIE2, FLT3, and RET.26,27 Once the above-mentioned receptor binds its cognate ligand, the latter is phosphorylated and subsequently recruits intracellular signaling molecules.24 These recruited molecules subsequently activate multiple downstream signaling pathways, such as the Ras/Raf/MEK/ERK and phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), inducing carcinogenesis, invasion, and metastasis in an oncogenic setting.25 Therefore, besides extracellular growth or angiogenic factors and their receptors, intracellular signaling molecules represent potential molecular targets.
First-Line Therapy
Sorafenib
Sorafenib is an oral multi-TKI, and holds dual anti-proliferative and anti-angiogenic effects by blocking Raf/MEK/ERK and JAK/STAT, and inhibiting VEGFRs, PDGFR-β, c-Kit, FLT3, and RET.28 The sorafenib HCC Assessment Randomized Protocol (SHARP) trial revealed the benefit of sorafenib for patients with aHCC in 2007 and made it as a first-line treatment of aHCC firstly approved by the Food and Drug Administration (FDA).15 In the SHARP trial, 602 aHCC patients without previous systemic treatments were randomly allocated to receive sorafenib or placebo, presenting a significant improvement in overall survival (OS) (10.7 vs 7.9 months; hazards ratio [HR] = 0.69; P < 0.001) and time to radiologic progression (5.5 vs 2.8 months; HR = 0.58; P < 0.001). Similar findings were also found in an Asia-Pacific (AP) trial with 226 patients, showing an improved OS (6.5 vs 4.2 months; HR = 0.68; P = 0.014) and time-to-progression (TTP; 2.8 vs 1.4 months; HR = 0.57; P = 0.0005).29
However, some patients underwent dose reductions or even treatment interruptions due to sorafenib-related adverse events (AEs) during the trial.15 The most frequent AEs were hand-foot skin reaction (HFSR), desquamation, rash, fatigue, weight loss, hypertension as well as gastrointestinal symptoms such as anorexia, nausea, abdominal pain, and diarrhea.15,29 Therefore, coping with AEs is pivotal to improve the efficacy of sorafenib. A randomized controlled trial conducted by Ren and colleagues showed that urea-based creams improved HFSR-associated quality of life and medication compliance during sorafenib treatment.30 Paradoxically, there exists a significant correlation between AEs and the survival of patients with aHCC treated with sorafenib, and skin-related AEs such as HFSR may act as potential biomarkers of sorafenib efficacy.31,32 Thus, caution should be exercised while managing AEs.
Interestingly, a greater OS was apparent in the SHARP trial compared with the AP trial (10.7 vs 6.5 months). Based upon varied etiologies of HCC, the different subgroups of patients in both trials were further studied. We found that the percentages of patients with HBV in the SHARP and AP trial were 19% and 71%, respectively; while the percentages of patients with HCV were 29% and 11%, respectively. Subgroup analyses showed a better survival benefit from sorafenib in patients with HCV compared to those with HBV.33,34 A consistent result was also reported that the TTP of patients with HCV was significantly longer than that of patients with HBV (6.5 vs 4.0 months, respectively, P = 0.05).35 Together with this result, a meta-analysis of Phase III trial results confirmed that there was an improved OS for patients with HCV rather than HCV and the hepatitis status may be a dependent risk factor for the effect of sorafenib.36 Furthermore, improved OS from sorafenib treatment was achieved mainly by inhibiting the rate of tumor growth and deterioration of liver function among patients with HCV.37 Mechanistically, sorafenib suppresses the high activity of Raf-1 sustained by HCV-1 core protein, preventing the mitosis and oncogenesis of HCV-infected liver cells.38 Besides HCV infection, a low neutrophil-to-lymphocyte ratio (NLR), and the liver-confined disease without extrahepatic spread are predictive factors of a superior sorafenib response.39 Recently, the eligibility criteria of patients for treatment with sorafenib require that patients must have a good liver function reserve, commonly Child–Pugh A. The study revealed that patients with Child–Pugh B had a shorter OS and a higher incidence of AEs than those with Child–Pugh A.40 Although no clear limitation exists regarding sorafenib for patients with Child–Pugh B, special attention should be paid to AEs when treating individuals with poor liver functional reserve.41,42
Lenvatinib
Lenvatinib is also an oral multi-TKI that exerts an anti-tumor effect by targeting VEGFR1-3, FGFR1-4, PDGFR-α, RET and c-Kit, and has been approved as first-line treatment by the REFLECT trial in 2018.43 In the REFLECT trial, 954 eligible patients, mainly from Asia, were randomly assigned to lenvatinib (n=478) or sorafenib (n=476) treatment groups.44 The lenvatinib treatment group was found to show no noticeable improvement compared with the sorafenib group regarding the primary endpoint (OS, 13.6 vs 12.3 months). However, lenvatinib treatment caused a higher objective response rate (ORR) by modified Response Evaluation Criteria in Solid Tumors (24.1% vs 9.2%), and a longer progression-free survival (PFS) (7.4 vs 3.7 months) and TTP (8.9 vs 3.7 months). Further, the tumor shrinkage and necrotizing effect in lenvatinib treatment group was noted.16 Thus, the higher ORR of lenvatinib may increase the tolerability and adherence to therapy of aHCC patients. On the other hand, lenvatinib resulted in HCC downstaging to achieve surgical resection. A post hoc analysis of data from the REFLECT trial indicated a greater efficacy of lenvatinib than sorafenib as assessed by OS, ORR and PFS regardless of liver function, in line with the above-mentioned results.45 In terms of AEs, patients who received lenvatinib experienced more serious AEs than those who received sorafenib, such as proteinuria, anemia, dyspnoea, hypertension, thrombocytopenia and hypothyroidism.16 Therefore, lenvatinib is also more prone to treatment discontinuation (40% vs 32%) due to AEs. This might partially account for the shorter treatment duration (5.7 months) than TTP in lenvatinib treatment group.16
The inclusion criteria of the REFLECT trial required a Child-Pugh A hepatic function, and patients with ≥50% liver tumor occupation as well as obvious invasion of the bile duct and/or the main portal vein were excluded.16 Vogel et al argued that the baseline liver function was a significant prognostic factor, and a better liver function may be predictive of a higher efficacy of lenvatinib or sorafenib.45 Further subgroup analyses showed that patients with HBV infection and a high alpha-fetoprotein (AFP) level in serum (>200 ng/mL) may benefit more from lenvatinib, which was quite different from sorafenib treatment.16,43,44 In addition, the evaluation of health-related quality of life (HRQOL) during treatment demonstrated that to some extent, lenvatinib delayed functional deterioration, such as fatigue, pain and diarrhea, supporting the use of lenvatinib in clinical domains.46,47 With respect to the cost-effectiveness of treatment, lenvatinib may also achieve a better efficacy at a lower cost than sorafenib.48
Second-Line Therapy
Regorafenib
In 2017, regorafenib was approved by the FDA as second-line therapy for patients with HCC progression during sorafenib treatment according to the RESORCE trial.17 Hence, regorafenib, as a fluorinated analog of sorafenib, shares a broader spectrum of molecular targets (such as VEGFR 1–3, PDGFR, FGFR, TIE2, c-Kit, RET, Raf-1, and BRAF) and performs more robust anti-proliferative and anti-angiogenic effects than sorafenib.49,50 The double-blind RESORCE phase III trial randomized 573 patients with sorafenib-refractory aHCC to regorafenib (n=379) and placebo (n=194) groups.17 Regorafenib significantly prolonged OS compared with the placebo (10.6 vs 7.8 months; HR = 0.63; P < 0.0001). Moreover, PFS (3.1 vs 1.5 months; HR = 0.46; P < 0.001) and TTP (3.2 vs 1.5 months; HR = 0.44; P < 0.001) were also significantly extended in regorafenib treatment, compared with placebo. In addition, this trial further demonstrated that regorafenib showed a better ORR (10.6% vs 4.1%, P = 0.005) as well as disease control rate (DCR) (65.2% vs 36.1%, P = 0.001) than placebo.49 Subsequently, the efficacy of regorafenib also arose in all relevant subgroups such as region, portal vein thrombosis, and serum AFP levels. Also, an exploratory analysis derived from the RESORCE trial described that regorafenib clinically benefited aHCC patients, independent of their last sorafenib dose and the stage of their disease progression with prior sorafenib treatment.51 However, the inclusion criteria of the RESORCE trial were highly strict, and only patients who not only had documented radiological progression of HCC after tolerating at least 400 mg of sorafenib per day for 20 of the last 28 days, but also preserved good liver function (Child–Pugh A) were enrolled.17 This determines regorafenib is not suitable for all post-sorafenib patients with aHCC in clinical practice. Kim et al also concluded that patients with Child–Pugh B had a poorer clinical prognosis and higher incidences of severe AEs during regorafenib treatment compared to those with Child-Pugh A.52 Hence, cautious patient selection is crucial, especially with respect to liver function.
There were AEs similar to sorafenib during regorafenib treatment, including HFSR, fatigue, hypertension, diarrhoea as well as increased serum aspartate aminotransferase (AST) level and bilirubin. Moreover, the rates of AEs were largely similar regardless of the last sorafenib dose (800 mg/day).51 Nevertheless, some serious AEs, such as HFSR and increased AST level, may result in treatment discontinuation in regorafenib and placebo groups (10% vs 4%). Interestingly, HFSR related to regorafenib treatment correlated with an improved OS, as previously shown for sorafenib.53 Remarkably, a further analysis pointed out that the sorafenib–regorafenib sequential therapy significantly outperformed the sorafenib–placebo therapy to improve OS (26.0 vs 19.2 months).51 Additionally, Bruix et al found that the patients with an AFP response could experience an improved OS from regorafenib than those without an AFP response.54 Thereby, patient selection based on single or multiple good biomarkers may maximize the efficacy of regorafenib. Another exploratory study identified that plasma proteins (angiopoietin 1, cystatin B, the latency-associated peptide of transforming growth factor-β [TGF-β] 1, oxidized low-density lipoprotein receptor 1, and C-C motif chemokine ligand 3) were negatively correlated with increased OS after regorafenib treatment, while the converse was true for plasma miRNAs (miR-30A, −122, −125B, −200A, −374B, −15B, −107, −320, and −645).55 These results may guide the individualized prescription of regorafenib for aHCC in the clinic, although the mechanisms involved have yet to be established.
Cabozantinib
Cabozantinib is another oral multi-TKI targeting VEGFR 1–3, MET, RET, KIT, TIE2, FLT3, c-MET, and AXL,56 due to which cabozantinib has also been shown to abrogate sorafenib-resistance by suppressing tumor cell proliferation and angiogenesis.57,58 Based on the CELESTIAL trial, cabozantinib was recommended as second-line treatment for aHCC patients in January 2019.18 Patients with aHCC who suffered from disease progression when receiving at least one systemic regimen including sorafenib were randomly assigned into cabozantinib and placebo treatment group. The trial showed a significantly longer OS (10.2 vs 8.0 months; HR = 0.76, P < 0.001) and PFS (5.2 vs 1.9 months; HR = 0.44; P < 0.001) in cabozantinib than placebo groups. Moreover, cabozantinib resulted in a significant improvement in ORR (4% vs 0.4%, P = 0.009) and DCR (64% vs 48%, P < 0.001). These clinical outcomes were evaluated based on albumin–bilirubin (ALBI) in the CELESTIAL trial. The patients in the cabozantinib cohort had longer OS as well as PFS than those in placebo cohort, regardless of ALBI grades. However, subgroup analysis of cabozantinib cohort revealed that outcomes were significantly better in the subgroup of ALBI grade 1 than grade 2. In addition, subgroup analysis indicated that cabozantinib was more favorable for the patients with extrahepatic spread, a high serum concentration of AFP (>400 ng/mL), or good PS (0–1).59 In terms of etiologic factors, cabozantinib tended to achieve better therapeutic effects in cases with HBV than those with HCV, which was consistent with lenvatinib rather than sorafenib.34,43,59 Hypertension, palmar–plantar erythrodysesthesia (PPE), HFSR, diarrhea, fatigue, and elevated AST were most frequently observed during cabozantinib treatment. Similar to sorafenib and regorafenib, a negative relationship existed between AEs and survival outcomes. Patients with severe hypertension or PPE from cabozantinib therapy had better OS and PFS than those without these two AEs.60 Despite a relatively high incidence of serious AEs (68%), the discontinuation of treatment due to AEs occurred in only 16% of cases treated with cabozantinib.18 As previously described, cabozantinib has been shown to play a major part in addressing the c-MET-induced sorafenib resistance.58,61 Together, these results suggest that cabozantinib may be a well-tolerated oral drug for the aHCC patients with intolerance to sorafenib or other therapies.
Of patients enrolled in the CELESTIAL trial, 72% who had previously received only sorafenib and 28% received two systemic therapies, suggesting that cabozantinib may act as second- or third-line treatment. For patients with prior sorafenib, cabozantinib still prolonged OS from 7.2 to 11.3 months, irrespective of the duration of previous sorafenib treatment.18,60 More interestingly, sorafenib–cabozantinib sequential therapy appeared to improve PFS more than sorafenib–regorafenib sequential therapy, albeit of a similar OS between both sequential therapies.62 Nevertheless, cost-effectiveness analysis showed that cabozantinib was a poorly cost-effective drug as a second-line treatment for aHCC.63 Thus, modestly controlling costs seems to favor the clinical use of cabozantinib. Additionally, the CELESTIAL trial revealed that low levels of MET, GAS6, HGF, ANG2, VEGF-A, as well as Interleukin (IL)-8, and high levels of insulin-like growth factor 1 in serum were associated with a good prognosis of aHCC patients treated with cabozantinib.64 However, the translation of these serum biomarkers into the clinic has yet to be achieved.
Ramucirumab
Unlike TKI, ramucirumab is an intravenous recombinant monoclonal antibody against VEGFR-2 that blocks the ligand–receptor interaction and its downstream signaling to exert an antitumor effect.65 Ramucirumab did not achieve a significant improvement of OS for the patients with prior sorafenib regimen compared to placebo in the Ramucirumab After Sorafenib in Patients With Advanced Hepatocellular Carcinoma (REACH) trial (9.2 vs 7.6 months, HR = 0.87, P = 0.14).66 However, a subgroup analysis substantiated that the patients with an elevated AFP (≥400 ng/mL) obtained a better survival benefit from ramucirumab treatment than those with AFP<400 ng/mL (OS, 7.8 vs 4.2 months; HR = 0.67; P = 0.006).67,68 The REACH-2 trial was subsequently conducted in aHCC patients with AFP ≥400 ng/mL, and showed that ramucirumab produced a longer OS (8.5 vs 7.3 months; HR = 0.71; P = 0.0199) and PFS (2.8 vs 1.6 months; HR = 0.45; P < 0.0001) than placebo with no difference in ORR (5% vs 1%, P = 0.1697). Furthermore, ramucirumab also potently improved DCR (59.9% vs 38.9%; P = 0.0006) compared to placebo.19 On the basis of REACH and REACH-2 trials, a pooled efficacy analysis also favored the above results that aHCC patients with AFP ≥400 ng/mL enjoyed a better outcome than those with placebo (OS 8.1 vs 5.0 months; HR = 0.69; P = 0.0002).69
Ramucirumab was shown to be well tolerated and have a manageable safety profile in aHCC patients with AFP ≥ 400 ng/mL as well as a prior sorafenib treatment.69 The grade 3 or worse AEs related to ramucirumab, including hypertension, hyponatremia and increased AST, were not common. In addition, treatment discontinuation from emergent AEs was observed in only 9.5% of patients receiving ramucirumab.19 It is well known that the Child–Pugh classification plays a pivotal role in evaluating severity of liver disease and prognosis of a patient with liver disease.70 Thus, several subgroup analyses based on REACH and REACH-2 trials were carried out to assess the relationship between several variables of the Child–Pugh classification (ALBI grade and ascites) and outcomes of aHCC patients during ramucirumab treatment. Patients with ABLI grade 1 or better had a longer OS than those with grade 2 in ramucirumab arm,71 while a great survival benefit from ramucirumab was obtained in all of patients irrespective of ascites.72,73 More importantly, ramucirumab is the first FDA approved drug for aHCC patient depending on the biomarker-selected group (AFP≥400ng/mL).19,74 However, the molecular mechanism underlying the ramucirumab selective trend is still unknown.
Other Targeted Agents
As is well known, hepatocarcinogenesis and its subsequent progression is a complex multistep process, accompanied with the sophisticated crosstalk of numerous signaling pathways. And a large number of agents targeting signaling molecules have been emerging over recent decades. In light of this, these targeted agents, apart from drugs approved by FDA, will be briefly elucidated based on where they function directly.
Agents Targeting the Extracellular Space or Membrane
As already mentioned above, RTKs transduce extracellular signals into the cell and subsequently regulate a variety of pathophysiological process, particularly in hepatocarcinogenesis. These RTKs include VEGF, FGFR, PDGFR, EGFR, HGFR, TIE2, FLT3, and RET, among others. To date, targeting these receptors has been shown to be a powerful treatment modality. In a randomized multiCenter Phase II–III trial clinical trial, donafenib, as a deuterated sorafenib derivative, brought a significant therapeutic advantage over sorafenib (OS, 12.1 vs 10.3 months; HR = 0.831; P = 0.0245) with a lower incidence of serious AEs (grade ≥3) than sorafenib (38% vs 50%; P = 0.0018) in aHCC patients from China.75,76 This exciting result thereby renders it as the first monotherapy agent due to OS superior to sorafenib for aHCC, with a favorable safety and tolerability. Yet, it remains to be buttressed by an international multi-center clinical trial. Similarly, another oral multi-TKI, nintedanib (BIBF 1120), was documented to have a similar OS to sorafenib in aHCC cases (10.2 vs 10.7 months; HR = 0.94), while it may lead to different but generally manageable AEs.77 Unfortunately, the development of some novel targeted agents have been halted before entering into clinical application. The clinical trial might not be further conducted due to the low efficacy of several agents, including dovitinib,78 brivanib,79 vandetanib,80 and erlotinib.81 In addition, the terrible safety frequently erodes the feasibility of some agents in clinical practice, such as linifanib (ABT-869)82 and sunitinib.83 Nevertheless, the combination of these agents significantly improved the outcomes of aHCC patients. The combination of sunitinib and TACE prolonged OS of aHCC patients in comparison to TACE plus placebo (20.5 vs 25 months).84
In addition to RTKs, a variety of non-RTKs also exist in cellular membrane, like the receptors binding to TGF-β which are serine/threomine kinases.85 TGF-β signaling pathway was documented to promote cancer progression in the late phase.86 Thus, targeting TGF-β pathway is still a potential strategy for the management of HCC. Galunisertib (LY2157299), a small molecule inhibitor of TGF-β1 receptor I, was identified to provide a longer OS for aHCC patients with AFP or TGF-β responders.87 Moreover, several glycoproteins that bind to the cellular membrane may be promising targets in treatment regimens for HCC. For instance, endoglin (CD105), as a co-receptor for TGF-β, is strongly associated with angiogenesis, inflammation, and fibrogenesis in hepatocarcinogenesis.88 Studies revealed that TRC105 alone, as an anti-CD105 monoclonal antibody, inhibited tumor angiogenesis and exhibited a significant effect on ORR (25%) of HCC combining with sorafenib.89 Recently, a multi-center Phase II study is undergoing to evaluate this effect.
Agents Targeting the Intracellular Space
Several important downstream signaling pathways located inside of cell play crucial roles in tumor development. The PI3K/AKT/mTOR pathway may be involved in vascular invasion, intrahepatic metastasis, and sorafenib resistance.90,91 SF1126 is a novel dual target inhibitor against PI3K/BRD4 and Ras/Raf/MAPK pathways,92 and had a significant antitumor effect as either a single agent or in combination with sorafenib.93 In terms of mTOR inhibitors, everolimus alone or combined with sorafenib rarely showed better survival benefits, despite the strongly reversing effects of everolimus on sorafenib resistance in aHCC.91,94,95 In addition, aberrant expression of canonical Ras/Raf/MAPK signaling molecules was frequently observed in HCC, and associated with a poor prognosis.96,97 MEK inhibitors (selumetinib and refametinib) and sorafenib appear to have a synergistic effect on aHCC.98,99 Likewise, sorafenib also exerted a synergistic effect with several small molecule inhibitors targeting nuclear signaling molecules, including resminostat (a histone deacetylases inhibitor),100 palbociclib (PD-0332991),101 and ribociclib (cyclin-dependent kinase 4/6 inhibitors).102 Nevertheless, OPB-111077 (STAT3 inhibitor)103 and CT-707 (YAP signaling inhibitor)104 demonstrated limited efficacy only in vivo or in vitro HCC models. Further clinical trials remain to be required to validate the efficacy of the agents.
Cytotoxic Chemotherapy
Cytotoxic chemotherapy for aHCC has been challenging since several decades. Few clinical trials demonstrate that cytotoxic agents provide significant survival benefits for aHCC patients, particularly in those with cirrhosis and poor hepatic function that may increase the toxicity of cytotoxic drugs and the difficulty of delivery to HCC lesions.105,106 Moreover, HCC cells frequently express intrinsic drug resistance via drug-resistant genes such as MDR1 gene, resulting in a RR below 25% of cytotoxic agents in aHCC patients.107,108 Considering the cost and availability of treatment regimens for aHCC, cytotoxic chemotherapy is still an alternative in less-developed countries and regions.109 More recently, emerging novel chemotherapeutic agents and combination regimens were shown to greatly improve the survival of aHCC patients.
Single-Agent Chemotherapy
Doxorubicin rarely increases survival benefits in aHCC patients with a low ORR (< 20%), although numerous studies and clinical trials have been conducted to assess its efficacy.110,111 A randomized controlled trial demonstrated that aHCC patients did obtain a longer OS after doxorubicin treatment compared to those without (10.6 vs 7.5 weeks; P = 0.036). However, doxorubicin led to serious AEs, such as neutropenia and cardiotoxicity. And the mortality related to AEs was up to 25%.112 Capecitabine, as a prodrug of 5-fluorouracil (5-FU), has been shown to be a standard therapy for HCC with its good antitumor activities and low toxicity.113 For aHCC patients with who discontinued treatment owing to disease progression or AEs, metronomic capecitabine may be a potent second-line systemic therapy, extending OS to 9.5 months from 5.0 months of the control.114,115 Remarkably, capecitabine also reduced the risk of HCC recurrence and even improved the survival of such patients after surgery as one of several adjuvant therapies.116,117 Nonetheless, other single agents, such as gemcitabine,118 oxaliplatin,119 and irinotecan,120 were only used in aHCC patents with a good PS and liver function, for which these agents may exist intolerant toxicity or their antitumor effect were just dependent on a small sample study.
Combination Chemotherapy
Currently, combination chemotherapy has been well recognized to improve the survival of aHCC patients in comparison to single-agent treatment. A multicenter, open-label, randomized, phase III study (the EACH study) from China demonstrated that aHCC patients with the FOLFOX4 regimen (fluorouracil, leucovorin, and oxaliplatin) showed a better PFS and ORR than those with doxorubicin alone, whereas the median OS of this study was not significantly changed (6.4 vs 4.97 months; HR = 0.80; P = 0.07).121 However, OS in FOLFOX4 arm tended to increase and presented a significant advantage over that in the single-agent treatment arm as the follow-up continued (5.9 vs 4.3 months; HR = 0.74; P = 0.03).122 The FOLFOX4 regimen had also a relatively well tolerance in treatment of Chinese aHCC patients. Although a higher occurrence of hematological toxicity (neutropenia, leukopenia, and thrombocytopenia) was observed in the FOLFOX4 treatment group, the difference was not significant.123 Therefore, the FOLFOX4 regimen was approved as an alternative strategy for aHCC in China. As aforementioned, sorafenib was not recommended for aHCC patients with Child–Pugh B or more severe, particularly in cirrhosis patients. However, oxaliplatin-based chemotherapy appears to exert strong anti-tumor role for these patients. An exploratory study observed that aHCC patients with Child–Pugh B cirrhosis did not show a compromising OS (5.5 vs 4.0 months; P = 0.19) and PFS (3.0 vs 2.4 months; P = 0.42) compared to those with Child–Pugh A in the gemcitabine plus oxaliplatin (GEMOX) regimen, without more AEs like thrombocytopenia and peripheral neuropathy.123 Thus, GEMOX was feasible and tolerated for aHCC patients who were ineligible for sorafenib due to Child–Pugh B cirrhosis. More than that, GEMOX regimen was also confirmed to act as a second-line therapy in aHCC patients after failure of sorafenib, with 8.3 months of OS and 3.1 months of PFS, despite the lack of control group.124 A prospective study remains to be required to validate this conclusion. Of note, GEMOX regimen was detected to induce tumor sharp shrinking and downsizing, providing a secondary local therapy for 8.5% of the patients without local therapy option initially in the multicenter AGEO study.125
Several other platinum-based regimens, such as XELOX (oxaliplatin plus capecitabine),126 PIAF (cisplatin, interferon, doxorubicin, and 5-FU),127 GP (gemcitabine plus cisplatin),128 and cisplatin plus capecitabine129 were mostly explored in phase II trials for aHCC treatments. It is noteworthy that oxaliplatin-based regimens seem to show a superiority over cisplatin-based regimens with a less toxicity profile (toxicity-related mortality rate: less than 3% vs 9%). In light of this, a systematic review and pooled analysis indicated that GEMOX combination may exert better antitumor activities than other oxaliplatin-based regimens, with FOLFOX4 regimen not included.130 Given that all of these results are based on small scale or single arm studies, such chemotherapy regimens are just alternative options in aHCC cases with good PS as well as the infeasibility of sorafenib owing to liver function or economic status.
Immunotherapy
HCC is confirmed to be a strongly immunogenic tumor showing potential anticancer immunity.131 Disappointingly, the impaired antigen recognition and presentation process, as well as the immunosuppressive tumor microenvironment (TME) orchestrated by tumor and stromal cells, assist HCC cells to escape from this anticancer immunity.132 Tumor-associated antigens (TAAs) are recognized and then eliminated by cytotoxic T-lymphocytes (CTLs) only after they are processed and presented by major histocompatibility complex class 1 (MHC-1).133 Clinical findings revealed that cytokines (IL-1, −4, and −5) were upregulated in aHCC, and closely associated with vascular invasion and distant metastasis.134 Alterations in these cytokines may lead to a higher ratio of CD4+ to CD8+ T cells and a lower expression of MHC-1, preventing TAAs from being recognized by CTLs and inducing immune escape.135 Moreover, myeloid-derived suppressor cells (MDSCs) incur immune tolerance by impeding T-cell activation and upregulate regulatory T cells (Tregs) via the release of immunosuppressive factors (IL-10 and TGF-β).136 In turn, Tregs can also prevent immune surveillance against HCC with negative regulatory immune activity.137 In addition, as immune suppressor cells, M2-polarized tumor-associated macrophages facilitate an immunosuppressive TME.138 The upregulation of co-inhibitory lymphocyte signals also acts as a crucial role in the immunosuppressive TME. And these signals include immune checkpoint ligands, such as PD-1, CTLA-4, lymphocyte-activation gene 3 (LAG3), T-cell immunoglobulin, mucin domain containing-3 (TIM-3), and their receptors.139 Furthermore, other machinery of immune evasion also exists, including up-regulated levels of tolerogenic enzymes, the presence of a metabolically unfriendly milieu for immune cells, and the attenuated Ig-mediated opsonization.139 Any attempt to address these barriers in order to eliminate tumor cells through the host immune system conceives a promising immunotherapy.
Second-Line Therapy
Nivolumab
Nivolumab is the first fully-humanized Ig G4 monoclonal antibody against PD-1, restoring host immune activity against tumor cells by the competitive blockade of PD-1 immune checkpoint signaling.140 In the phase I/II Checkmate 040 trial, nivolumab was demonstrated to provide a substantial ORR of 15–20% and DCR of 58–64% for aHCC patients regardless of prior therapies like sorafenib, with a manageable safety profile. Importantly, theaHCC patients with prior sorafenib treatment still have an encouraging ORR (19%) and OS (13.2 months) in nivolumab treatment.140 Based on these results, nivolumab was approved by the FDA as a second-line drug for aHCC patients. Thereafter, a randomized multicenter phase III study (Checkmate 459) was conducted to compare nivolumab with sorafenib as first-line therapy in aHCC patients without systemic therapy.141 Nivolumab was not inferior to sorafenib regarding mean OS (16.4 vs 14.7 months; HR = 0.85; P = 0.0752), although OS did not reach the predefined standard of statistical significance (HR = 0.84, P = 0.0419). Furthermore, there was a better ORR in nivolumab group than sorafenib group (15% vs 7%). Additionally, nivolumab also showed a less toxic profile than sorafenib, with a lower incidence of grade 3/4 treatment-related AEs (22% vs 49%) such as rash, diarrhea, pruritus, and other immune-related diseases.141,142 More recently, some studies also compared the efficacy and safety of nivolumab with regorafenib in aHCC patients after sorafenib failure and demonstrated a better ORR and lower treatment-associated AEs in nivolumab group than those in regorafenib group.143,144 Therefore, nivolumab may serve as a first-line or second-line drug for such patients. Further analysis of CheckMate 040 Cohort 5 suggested that nivolumab might be suitable for aHCC patients with Child-Pugh B.145 However, such patients showed poor clinical survival compared to those with Child–Pugh A, with a short OS and low ORR.146,147 Surprisingly, the sub-analyses of CheckMate-040 revealed that aHCC patients treated with nivolumab underwent the disease progression, but had a nonconventional benefit.148 It indicates that the reliable response biomarkers for nivolumab is of importance for evaluating clinical outcome. A handful of biomarkers are currently identified to be associated with an improved survival and response in nivolumab treatment, such as PD-1 and PD-L1 expression,141,149 a good AFP response,150 inflammatory cytokines (CD3 and CD8)149 and peripheral blood mononuclear cells (effector T cells and nonclassical monocytes).151 This algorithm remains yet to be confirmed in some large prospective studies.
Pembrolizumab
Similarly, another monoclonal PD-1 antibody (pembrolizumab) was approved for second-line treatment of aHCC on the basis of the efficacy and safety profile in the single-arm Keynote-224 trial.152 In the global, randomized, double-blind, phase III trial Keynote-240, however, pembrolizumab did not satisfy the preplanned statistical threshold (P=0.0174), although it achieved a statistically improved OS (13.9 vs 10.6 months, P=0.0238) and PFS (3.0 vs 2.8 months, P=0.0022) in aHCC patients after sorafenib failure compared to placebo.153 Gratifyingly, the latest clinical data from the phase III Keynote-394 study conducted in Asia demonstrated that the significantly improved OS (13.6 vs 13.0 months, P = 0.0180) and PFS (2.6 vs 2.3 months, P = 0.0032) for patients with previously treated aHCC compared with placebo, which met its prespecified statistical criteria of 0.019307 and 0.013447, respectively. The incidence of treatment-related AEs was relatively higher in pembrolizumab arm than that in placebo arm (66.9% vs 49.7%), which was consistent with that in the trial Keynote-224 and Keynote-240.154 In addition, the post hoc analysis of Keynote-240 revealed that pembrolizumab did not significantly affect liver function in comparison to placebo in aHCC, and exhibited an improved survival regardless of ALBI grade.155 The HRQOL assessment documented that pembrolizumab could provide a good HRQOL to aHCC patients.156 Together with these results they potently establish the second-line treatment of pembrolizumab for aHCC patients receiving sorafenib previously. However, pembrolizumab was shown not to be a cost-effective therapy for HCC.157 Moreover, the clinical study (NCT04442581) comparing cabozantinib with pembrolizumab in the first-line setting is ongoing.
Other Immunotherapies
With the encouraging clinical survival benefit of aHCC patients from nivolumab and pembrolizumab, interest in other immunotherapies, such as other ICIs (PD-1/PD-L1 and CTLA-4 inhibitors), cytokines, adoptive T-cell transfer therapy, and HCC vaccines, has dramatically increased.139,158
In addition to nivolumab and pembrolizumab, several other anti–PD-1 antibodies were also shown to exhibit a certain antitumor effect in aHCC. An example is camrelizumab, which yielded an OS at 6 months of 74.4% and OR of 14.7% for pretreated Chinese patients with aHCC, with a manageable toxicity like increased AST and decreased neutrophils.159 Based on a Phase I trial of tislelizumab showing an antitumor activity for HCC, a phase II trial (NCT03419897) assessing its efficacy and safety profile in pretreated aHCC patients and a global randomized phase III RATIONALE-301 trial (NCT03412773) comparing tislelizumab to sorafenib as a first-line agent in aHCC patients are currently in progress.160 The positive results of such studies deserve attention as tislelizumab can attenuate a potentially negative impact on other immune cells like macrophages with its high binding affinity and specificity for PD-1, which may address the anti-PD-1 therapy resistance.160 In addition, durvalumab, a fully-humanized IgG1 against PD-L1, was evaluated in aHCC patients with prior sorafenib treatment in a phase I/II clinical trial. Interestingly, the patients with HCV appear to enjoy a better survival than the overall cases (OS 19.3 vs 13.2 months; ORR 25% vs 10.3%) in durvalumab treatment.161 The underlying mechanisms of its etiological trend remain to be identified.
CTLA-4 is an immune system checkpoint molecule that maintains immune response in check by limiting the over-activation of effector T cells and mediating the impact of Tregs on immune response, rendering it a promising target for immunotherapy.162 An anti–CTLA-4 antibody, tremelimumab, was observed to have a partial response rate of 17.6% and TTP of 6.48 months with a manageable toxicity like rash in a phase II trial.163 Thereafter, the development of tremelimumab focuses on the combination with locoregional therapy such as RFA or TACE.164 Another anti–CTLA-4 antibody, ipilimumab, exhibited a great antitumor activity as combination therapy rather than single-agent regimens.165 To date, interferon-α (IFN-α), IL-12, and other cytokines appear not to provide a clear survival advantage for HCC.166 Apart from anti–PD-L1/PD-1 and anti–CTLA-4 antibodies, nevertheless, other novel targets, such as LAG3, TIM-3, and immune-receptor tyrosine-based inhibitory motif domain, and immune checkpoint bi-specific antibodies against CTLA-4 and PD-1, like MEDI5752, may be promising immunotherapies for aHCC patients.133,167
Adoptive Cell Transfer
In contrast to restore or augment the preexisting immune responses with checkpoint inhibition therapy, adoptive cell transfer (ACT) therapy induces novel or different immune responses by re-transferring autologous or allogeneic immune cells back to patients following their expansion and modification in vitro.168 This also determines the highly specificity and individualization of ACT. Of ACT therapies, chimeric antigen receptor T cells (CAR-T cells), T cell receptor (TCR) engineered T cells, cytokine-induced killer cells (CIKs), and tumor- infiltrating lymphocytes (TILs) currently show promising antitumor activities for HCC.168,169
ACT therapy largely depends on the modification of various and specific TAAs in vitro. The identification of TAAs that trigger an efficient immune response to eliminate tumor cells appears particularly important.170 Modified TCR-engineered T cells present the ability of recognizing and binding to the MHC of antigen-presenting cells and TAAs of tumor cells. Unlike conventional T cells, antigens recognized by these T cells are not confined to membrane-bound antigens.171,172 TCR engineered T cells specific to HBV antigens were successfully established and revealed to provide antitumor activities in recurrent HBV-related HCC after LT with a good safety profile.173,174 In addition, TCR-engineered T cells targeting AFP or glypican-3 (GPC-3) were shown to inhibit the progression of HCC in vitro and in vivo.175,176 There are currently several ongoing trials involving in TCR-engineered cells recognizing various TAAs in HCC, such as AFP (NCT03971747, 04368182, and 03132792), HBV antigen (NCT03899415), and melanoma antigen gene protein (NCT03441100). CAR-T cells recognize and kill hepatoma cells expressing specific TAAs without MHC restriction, which may prevent the tumor immune escape induced by MHC down-regulation.177 In prospective phase I clinical studies, autologous GPC-3–CAR-T cell therapy showed favorable survival (OS rates at 3 years, 1 years, and 6 months: 10.5%, 42.0% and 50.1%, respectively) in patients with GPC-3 positive aHCC. Its concomitant toxic effects were tolerable and reversible, while one patient suffered from grade 5 cytokine release syndrome.178 Over half of patients with CD133-positive unresectable HCC obtained a median OS of 12 months and overall PFS of 6.8 months after CD133-CAR-T cells reinfusion.179 Besides CAR-T cells and TCR engineered T cell therapies, CIKs, and TILs are also undergoing clinical trials with excellent Experimental results.171,180 ACT therapy will provide a potential alternative for the treatment of HCC.
Vaccines
Therapeutic vaccines mainly involve dendritic cell (DC), peptides, and oncolytic viruses. DC vaccines, as a cellular vaccine, can incur strong antitumor immune response via the recruitment of effector T cells and subsequent release of TAAs derived from tumor lysis.181 Phase I and II clinical trials confirmed that DCs pulsed with tumor lysis exhibited antitumor efficacy and a safety profile in aHCC patients, with a mean survival of 5.5 months.182,183 Moreover, the feasibility and tolerability of peptide vaccines, such as AFP,184 GPC-3,185 and multidrug resistance-associated protein 3,186 were proved in clinical studies. Nevertheless, these clinical studies on DC and peptide vaccines revealed marginal antitumor activity probably owing to a lack of large-scale studies and a control group. In contrast, oncolytic virus therapy is a promising option in the development of tumor vaccines. Oncolytic viruses are gene-modified viruses that replicate and lyse specific tumor cells with its specific cellular tropism obtained, releasing TAAs for the activation of antitumor immune response.187,188 The modified poxvirus, JX-594, inserted into the human granulocyte-macrophage colony stimulating factor gene was demonstrated to confer aHCC patients with a longer OS in high than low doses (OS,14.1 vs 6.7 months) in a randomized phase II trial (TRAVERSE).189 However, the subsequent phase III trial (PHOCUS) comparing JX-594 plus sorafenib with sorafenib alone indicated that JX-594 did not provide improved antitumor efficacy.190 The result may be associated with the immunosuppressive TME of HCC. Therefore, the clinical trials comparing JX-594 with nivolumab (NCT03071094) and other ICIs in aHCC are ongoing.191
Combination Therapy
As abovementioned, the development and progression of HCC is a sophisticated process mediated by multiple pathways. Additionally, single-agent therapies frequently result in dose- or time-dependent severe AEs, thereby leading to treatment interruption due to intolerance. Thus, the efficacy of single-agent, such as tyrosine kinase inhibitors (TKIs), ICIs or cytotoxic chemotherapy, may have reached a plateau at an OS of 14–16 months. Various combination regimes of ICIs and anti-VEGF monoclonal antibodies have altered this picture and greatly improved survival of aHCC patients. Since various combination regimes of ICIs and anti-VEGF monoclonal antibodies emerged in 2020, however, the therapeutic efficacies in aHCC have achieved an unprecedented improvement.22,23
ICI–Anti-Angiogenic Therapy Combination
Antiangiogenic therapy enhances the functions of effector T-cells and immune cells infiltration into TME by normalizing aberrant vasculature, and blunts the functions of suppressive immune cells (Treg cells and MDSCs), finally to augment tumor responsiveness to immunotherapy.192
First-Line Therapy
Atezolizumab Plus Bevacizumab
The combination of atezolizumab (anti-PD-L1 antibody) and bevacizumab (anti-VEGF antibody) was firstly proved to greatly improve OS (19.2 vs 13.4 months; HR = 0.66; P = 0.0009), ORR (29.8% vs 11.3% per Response Evaluation Criteria in Solid Tumors version 1.1) and complete response rate (CRR) (7.7% vs 0.6%) of aHCC patients over sorafenib in the IMbrave150 trial.22,193 Importantly, the HRQOL in atezolizumab plus bevacizumab group was not inferior to the sorafenib group, with a longer median time to the deterioration of quality of life (11.2 vs 3.6 months). Moreover, there was not significantly different in the risk of grade 3 or 4 AEs between both groups, such as proteinuria, diarrhea and autoimmune events.22 Notably, bevacizumab may induce bleeding, and the assessment of varices via upper-gastrointestinal endoscopies and treatment when necessary was required at least 6 months before enrollment. In addition, this improved survival benefit was also confirmed in aHCC patients in China.194 To date, the combination regime of atezolizumab and bevacizumab has been approved as first-line therapy for aHCC by American Society of Clinical Oncology, European Society for Medical Oncology, Chinese Society of Clinical Oncology (CSCO), and National Comprehensive Cancer Network guidelines.195–197 In a single-center study, aHCC patients with a low NLR had a prolonged PFS in comparison to those with a high NLR (cumulative PFS at 150 days: 64% vs 20%), favoring the pretreatment NLR value as a potent predictor of the response to atezolizumab-bevacizumab therapy for aHCC.198 Furthermore, atezolizumab-bevacizumab therapy was demonstrated to enabled aHCC patients to receive a long-term disease-free status (19 months) after hepatectomy.199 As later-line therapy, the doublet did not provide great clinical efficacy for the aHCC patients with prior TKIs treatment, which may be associated with resistance to anti-VEGF TKIs.200 Certainly, further clinical studies are still needed. Regarding of cost-effectiveness in clinical practice, atezolizumab-bevacizumab treatment was no cost-effective alternative compared to sorafenib for aHCC.201 Therefore, the economic status of HCC patients has to be considered for a better clinical benefit from this regimen.
Other ICI–Antiangiogenic Therapies
The great success of the combination of atezolizumab and bevacizumab has changed the therapeutic regimens for HCC. The phase II/III ORIENT-32 trial conferred the combination of sintilimab (anti-PD-L1) and bevacizumab as the first-line treatment for unresectable HCC because this combination exhibited a better survival benefit than sorafenib.23 In addition to the synergistic antitumor effect of anti-angiogenic therapy and immunotherapy, adding anti-PD-1 antibody to lenvatinib treatment enhanced the antitumor efficacy by increasing the proportion of CD8+ T cells in a HCC model.202 This was supported by the phase Ib study where lenvatinib plus pembrolizumab had improved ORR (46%), PFS (9.3 months), and a prolonged median OS of 22 months in patients with unresectable HCC, with manageable toxicities.203 On the basis of promising results from this study, the double-blind randomized controlled phase III LEAP-002 trial comparing this combination with lenvatinib plus placebo is currently in progress. And the promising result of this phase III trial might establish a new therapeutic benchmark in the treatment of aHCC in the near future.204 Another combination of lenvatinib plus nivolumab also achieved similar antitumor activities in aHCC patients in a preliminary study.205 In addition, the VEGF Liver 100 trial evaluating the efficacy and safety of avelumab (anti-PD-L1 antibody) plus axitinib (TKI) as a first-line regimen for aHCC patients indicated that such patients without prior treatment in this combination received a favorable ORR (31.8%) and PFS (5.5 months) with a manageable safety profile, while OS data were unavailable at cut-off date.206 As for HBV-positive aHCC patients, a multicenter, open-label phase II trial was carried out to assess the efficacy and safety of the anti-PD-1 antibody, camrelizumab (SHR-1210), plus apatinib (anti-VEGFR2) as first- or second-line therapy foraHCC patients in China on the basis of their previous study.207,208 In this phase II trial, the combination of camrelizumab and apatinib showed an exciting survival benefit in either the first- or second-line setting in aHCC patients. The ORR, 12-month survival rate, and PFS were 34.3% vs 22.5%, 74.7% vs 68.2% and 5.7 vs 5.5 months, in first- vs second-line therapy, respectively.208 Along with this, the ongoing randomized, open-label, multicenter, phase III trial (NCT03764293) comparing this combination with sorafenib in aHCC is key. Interestingly, synergic effects may exist in the combination of the immune checkpoint bispecific antibodies AK104 (anti-PD-1/CTLA-4 bispecific antibody) and lenvatinib owing to its promising antitumor effects and an acceptable safety in aHCC.209
Currently, various combinations of ICIs and anti-angiogenic drugs dramatically improve clinical survival for aHCC patients. The triplet combination of TKI agents and two ICIs, for example, may achieve a surprising OS of over 25 months. However, the concomitant toxicity should be taken into considerations. Therefore, a number of trials weighing the efficacy and safety of combinations of ICIs and anti-angiogenic drugs or TKIs are under way (Table 1).
 |
Table 1 Selected Ongoing Trials of Combination Therapies in aHCCa |
ICI–ICI Combinations
Non-redundant and complementary checkpoint signals may provide evidence for the combination of two ICIs.210 Furthermore, blocking the B7-CTLA-4 pathway mediated by anti-CTLA-4 antibody exerts antitumor effects by increasing the activated CD8+ T cell level in lymph nodes and subsequently increasing activated CD8+ T cells infiltrating into tumor tissues. In turn, only when the required CD8+ T cells exist in tumor tissues, the inhibition of the PD-1/PD-L1 pathway activates tumor immunity. Moreover, anti-CTLA-4 antibody may potently attenuate Treg cells in the immunosuppressive TME (Figure 1).211
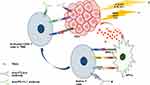 |
Figure 1 Brief mechanisms of action mediating synergistic effects of combined immunotherapies. (a) Blocking the PD-1/L1 pathway alone does not induce an antitumor immune response, but inhibition of the CTLA-4 pathway via anti-CTLA-4 antibody promotes activated CD8+ T cells accumulating in lymph nodes and then infiltrating into TME, enhancing the antitumor effects of anti-PD1/L1 antibody.211 (b and c) LRTs or metronomic CTs trigger the release or exposure of immunostimulatory molecules like TAAs by damaging cancer cells, followed by the blockade of the PD-1/L1 and CTLA-4 pathway by anti-PD1/L1 and anti-CTLA-4 antibody, resulting in robust antitumor immune response229,234,246. Abbreviations: HCC, hepatocellular carcinoma; LRTs, locoregional therapies; TACE, transarterial chemoembolization; TA, thermal ablation; CTs, chemotherapies; TAAs, tumor-associated antigens; APCs, antigen-presenting cells; MHC-1, major histocompatibility complex class 1; TCR, T cell receptor; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; TME, tumor microenvironment. |
Second-Line Therapy
Nivolumab Plus Ipilimumab
An important exploration involving the combination therapy of two ICIs comes from the CheckMate 040 study evaluating the efficacy and safety of nivolumab plus ipilimumab (anti-CTLA-4 antibody) in aHCC patients previously receiving sorafenib.212 In this phase I/II study, the best survival benefits, with the OS of 22.8 months and ORR of 32%, presented at the highest ipilimumab dose arm (ipilimumab 3 mg/kg plus nivolumab 1 mg/kg, 4 doses administered every 3 weeks, and then nivolumab 240 mg every 2 weeks). And the duration of response (DOR) even reached 4 years in some patients.213 Of the three different doses of ipilimumab arms, a higher dose of ipilimumab combined with nivolumab appeared to exhibit a better survival outcome. However, a higher ipilimumab dose conceived the more immune-mediated toxicities, rendering almost everyone in the highest ipilimumab dose arm experience AEs (94%).212 Fortunately, these immune-related AEs were manageable by systemically administering corticoid. In light of the efficiency and safety profile of the combination regimen, nivolumab in combination with ipilimumab was approved by the FDA as second-line treatment in aHCC.214 A further meta-analysis validated that the combination of nivolumab (1 mg/kg) plus ipilimumab (3 mg/kg) seemed to provide a superior OS and PFS over regorafenib (160 mg), nivolumab (3 mg/kg), and cabozantinib (60 mg) monotherapy for aHCC patients as second-line therapy.215 Of note, this combination regimen may act as a rescue strategy for the aHCC patients with anti-PD-1/L1 therapy failure, achieving encouraging survival benefits (mean OS = 10.9 months, ORR = 16%) with tolerable toxicity.216 As aforementioned, the synergistic effect between nivolumab and ipilimumab may explain this situation. To explore clinical efficacy with this combination, a phase III trial CheckMate 9DW (NCT04039607) comparing nivolumab plus ipilimumab with sorafenib or lenvatinib monotherapy in the first-line setting for aHCC is under way.
Other ICI–ICI Combination Therapies
The efficacy of anti-CTLA4 dose dependence also appeared in another combination of tremelimumab plus durvalumab for aHCC. A phase I/II trial testing the efficacy and safety of the combination showed that the higher tremelimumab dose group (tremelimumab 300 mg+durvalumab 1500 mg followed by durvalumab every 4 weeks) obtained a better OS (18.7 months) and ORR (24%) with positive tolerability than other arms as a second-line treatment.217,218 In a subsequent clinical study, this novel treatment regimen also displayed the better clinical benefit-risk profile than the lower tremelimumab dose regiment, single agent tremelimumab or durvalumab, suggesting that a higher dose of tremelimumab may trigger a stronger immune response and thus strengthen the anti-tumor activity of durvalumab.219 The latest findings of the multicenter, phase III HIMALAYA study based on this revealed the superiority of the higher tremelimumab dose regimen over sorafenib on survival benefits (OS, 16.4 vs 13.8 months, P = 0.0035), thus strongly supporting the use of the Single Tremelimumab Regular Interval Durvalumab regimen (tremelimumab 300 mg+durvalumab 1500 mg followed by durvalumab every 4 weeks) as first-line therapy for aHCC.220 Moreover, numerous ICI combination regimes (Table 1), like IBI310 (anti-CTLA-4 antibody) plus sintilimab, are undergoing to explore a highly efficacious systemic therapy with a favorable safety profile.
Chemotherapy-Based Combinations
We have previously described the superiority of oxaliplatin-based chemotherapies over other combination chemotherapies in terms of antitumor effects and toxicity profiles. Accordingly, the combinations with oxaliplatin-based regimens are of considerable interest as promising alternatives for aHCC patients, particularly in less developed regions. In a single-arm phase II study, the combination of sorafenib and modified FOLFOX achieved encouraging clinical efficacy with an ORR of 18% and TTP of 7.7 months in aHCC patients without prior systemic therapies.221 Considering its concomitant AEs, especially in hepatotoxicity, this regimen may be limited to those patients with a good liver function reserve.221 Furthermore, the regimens of sorafenib plus XELOX (SECOX) or GEMOX were observed to have a relatively satisfying TTP of 5.92 months and 6.2 months, respectively.222,223 Both regimes showed a tolerable safety profile. As a consequence of these results, the combinations of oxaliplatin-based regimens plus sorafenib have become the front-line therapy for aHCC patients with good PS and liver function in China.197 Nevertheless, subsequent studies argued that adding GEMOX or XELOX to sorafenib did not seem to improve survival benefits, resulting in an inferior OS than sorafenib alone (13.5 vs 14.8 months and 7.1 vs 12.5 months, respectively).224,225 Although there seems to be a better clinical outcome for aHCC regarding TTP (6.4 vs 2.8 months) and OS (13.7 vs 6.5 months) in the combination of sorafenib and doxorubicin than doxorubicin plus placebo in a phase II clinical trial, this combination failed to significantly improve survival for aHCC patients in comparison to sorafenib alone (OS, 9.3 vs 9.4 months) in a phase III CALGB 80802 clinical trial.226,227 Taken together, the combinations of chemotherapy with TKIs are not recommended as a standard treatment for aHCC, in the setting of the availability of first-line (lenvatinib) or second-line (cabozantinib and ramucirumab).
Similar to the synergistic effects of anti-CTLA-4 antibody when combined with anti-PD-1/L1 antibody, selected immunogenic chemotherapeutics sensitized tumors to host antitumor T cell immunity, instigating CD8+ T cells to infiltrate into tumor to facilitate ICIs against various cancers.228 Furthermore, metronomic chemotherapy also triggers the secretion or exposure of immunostimulatory molecules, like TAAs, by damaging cancer cells to elevate responsiveness to ICIs (Figure 1).229 Based on this rationale, a single-arm phase II study was conducted to evaluate the efficacy and safety of camrelizumab plus FOLFOX4 or GEMOX chemotherapy as first-line therapy for aHCC. An encouraging ORR of 26.5% and PFS of 5.5 months were obtained and severe immune-related AEs were found in only 5.9% of aHCC patients.230 Currently, a multicentered randomized Phase III trial (NCT03605706) is underway to compare camrelizumab combined with FOLFOX4 regimen to placebo combined with FOLFOX4 as first-line therapy in aHCC patients. Further investigations are warranted to identify the administration strategy of chemotherapy that facilitates rather than attenuates the immune system in these combination treatments.231
Combination of Systemic and LRTs
LRTs (thermal ablation [TA], stereotactic body radiotherapy [SBRT], and TACE) are already widely used for HCC since they directly impair tumors and reduce the tumor burden. Yet, quite a few HCC patients suffered from disease progression following LRTs, largely TACE. Accordingly, whether the addition of systemic therapies to LRTs could address this issue is to be explored.
Clinical trials of the combination of sorafenib to TACE showed mixed results. The phase II TACTICS trial showed a significantly improved PFS in sorafenib plus TACE group than TACE alone group (25.2 vs 13.5 months; P = 0.006), with a better OS at 2 years (77.2% vs 64.6%).232 Another observational study demonstrated that the combination of TACE plus sorafenib significantly improved OS compared to sorafenib or TACE alone arm regardless of HCC patients with BCLC stage B or C.233 Sorafenib seems to synergize TACE to improve survival benefit in HCC. Theoretically, VEGF and other angiogenic pathways, which are activated by the hypoxic environment created by TACE, may promote angiogenesis and revascularization to induce the residual viable tumor to grow. It is the anti-angiogenic agent, sorafenib, that disables angiogenic pathways to achieve a synergistic effect.234 In the phase III STAH and TACE 2 trials, sorafenib combined with TACE failed to improve OS (12.8 vs 10.8 months, P = 0.29) and PFS (238 vs 235 days, P = 0.94).235,236 In addition, meta-analyses concluded that sorafenib combined with TACE did not improve clinically relevant outcomes for HCC patients,237,238 with more AEs237 and less cost-effectiveness.239 Thus, this regimen is not recommended for HCC patients to date. Combinations of sorafenib and hepatic arterial infusion chemotherapy (HAIC) appear to exhibit diametrically opposed clinical outcomes depending on the chemotherapeutic drugs infused. HAIC of FOLFOX added to sorafenib was observed to improve survival benefit240 and HAIC of cisplatin with fluorouracil or not was not.241,242 Moreover, sorafenib in combination with radiotherapy, SBRT, and Yttrium-90 radioembolization showed an improved survival compared to sorafenib alone to some extent.243–245 These results should be confirmed by more ongoing studies to identify the duration, sequencing, dose, and timing of administration. A slew of clinical trials, such as the efficacy of lenvatinib plus TACE (NCT03838796), are ongoing (Table 1).
There also exists a synergistic effect in the combination of immunotherapy and LRTs. LRTs like TACE and TA, not only release plenty of TAAs triggering potent antitumor immune responses, but also produce some danger signals that awake host innate immune system and develop effector T cell immunity.234,246 In this setting, LRTs enable itself to couple with immunotherapy. Duffy et al combined LRTs (TACE or ablation) with tremelimumab to assess their safety and efficacy in HCC.164 There were a remarkably increase of CD8+ T cells and immune cells infiltrating into tumor after ablation (Figure 1). The combination of ablation and tremelimumab resulted in a partial response of 26.3%, as well as an OS of 12.3 months and median time to progression of 7.4 months.164 Clinical trials comparing this regimen with ablation or tremelimumab alone are warranted. More recently, the phase III EMERALD-1 clinical trial to evaluate TACE coupled with durvalumab and bevacizumab is underway. Similarly, numerous clinical trials are ongoing to explore the efficacy and safety of the combination of ICIs and LRTs (Table 1).
Adjuvant and Neoadjuvant Therapy
Although surgical tumor removal, including resection and LT, is a potentially curative therapy for HCC, recurrence after surgery is extremely common, presenting in over 70% of HCC cases within 5 years.5,247 By and large, the recurrence within 2 years may arise from the remnants and aggressiveness of the primary tumor, while the recurrence beyond 2 years largely results from de novo hepatocarcinogenesis related to underlying liver diseases like hepatitis or liver cirrhosis.248 Furthermore, the frequent diagnosis at advanced stages and a terrible liver function reserve always deprive patients of surgical resection, with only 20% of HCC patients eligible for curative resection.249 Therefore, in the setting of recent inspiring achievements of systemic therapies, preoperative and postoperative strategies based on the aforementioned risk factors deserve exploration to improve surgical resectability and decrease postoperative recurrence with the ultimate aim of improving clinical outcomes for HCC patients.
Adjuvant Therapy
As noted above, adjuvant therapy reduces the early recurrence of HCC through eradicating remnant tumor cells or inhibiting hepatocarcinogenesis.250 Some relevant studies indeed improve recurrence-free survival (RFS) and OS of HCC patients after surgery despite the lack of guidelines recommending adjuvant treatment.11,251,252 Immunotherapy was shown to effectively prevent metastases or de novo tumorigenesis via booting or modifying the host immune function.253 Several randomized controlled trials demonstrated that the adjuvant immunotherapy with CIKs might improve clinical benefits to HCC patients in terms of RFS and OS after curative resection. The phase III multi-center study explored the adjuvant effects of activated CIKs on HCC patients after curative resection with a follow-up of up to 68.5 months. The mean RFS was significantly higher in the immunotherapy group than in no adjuvant therapy group (44 vs 30 months, P = 0.01). It was the same for RFS rate at 5 years (44.8% vs 33.1%).254,255 Nonetheless, another similar trial suggested that CIK therapy failed to extend the DFS and OS of HCC patients although its primary outcome, time to recurrence, was prolonged to 13.6 months from 7.8 months of no CIK treatment group.256 Meta-analysis of the above studies concluded that adoptive immunotherapies like CIKs could eradicate the small intrahepatic metastases from the primary tumor but not multicentric relapse, resulting in the improvement of the early (less than 3 years) rather than late recurrence.257 Additionally, the GPC3-derived peptide vaccine,185 autologous DCs,258 and DC vaccine plus activated T-cell transfer259 were revealed to reduce HCC recurrence and improve clinical outcomes for HCC patients after curative operation. Clinical trials assessing the efficacy and safety of ICIs as adjuvant therapy for HCC have been lacking until now. However, the results of multiple ongoing trials, such as nivolumab (NCT03383458) and pembrolizumab (NCT03867084), are worthy of awaiting (Table 2). Of these, the phase III CheckMate 9DX trial evaluating nivolumab as an adjuvant therapy for HCC is extremely exciting, since one half of the sales of nivolumab is forecasted to be attributed to its sales in the adjuvant setting in 2027.260
 |
Table 2 Selected Ongoing Trials of Adjuvant and Neoadjuvant Therapies in HCC |
Molecular targeted drugs as an adjuvant therapy look dismal owing to the failure of the phase III STORM trial. This trial indicated that sorafenib failed to significantly prolong RFS for HCC following resection or ablation in comparison to the placebo arm, with a more frequent incidence of AEs (28% vs less than 1%).261 Yet, the pity of the STORM trial does not seem equivalent to the ineffectiveness of molecular targeted agents as an adjuvant therapy for HCC. And the development of molecular targeted agents in postoperative adjuvant therapy needs further investigations. An example is sorafenib treatment following curative hepatectomy that improved RFS and OS in HCC patients with microvascular invasion.262 Two phase III trials, IMbrave 050 and EMERALD-2, exploring the molecular targeted agents plus ICIs as adjuvant therapy are underway. The feasibility of chemotherapy in the adjuvant treatment for HCC has been assessed in several studies. The HCC patients receiving capecitabine after curative resection had a lower risk of tumor recurrence, but no extended OS.116 However, chemotherapy is not supported in the adjuvant treatment for HCC because of its toxicity and uncertain efficacy based on meta-analyses and clinical trials to date.263 Notably, traditional Chinese medicine (TCM) has achieved outstanding breakthroughs in reducing HCC recurrence and metastasis currently. Erzhu Qinggan Jiedu Recipe,264 Huaier granule265 and traditional herbal medicine266 were confirmed to significantly improve RFS and OS in the adjuvant setting for HCC.
Neoadjuvant Therapy
Neoadjuvant therapy facilitates the tumor downstage to increase surgical resectability as a bridging treatment, and decreases the recurrence following operation. Like adjuvant therapy for HCC, neoadjuvant therapy for HCC is not also endorsed in current clinical guidelines given the potentially invasive and metastatic properties and thereby lack of adequate clinical studies. Despite several clinical trials, including BIOSHARE trial proved that preoperative sorafenib yielded significantly downstage for patients with resectable HCC with a good safety, more studies substantiating this result remain to be warranted for the limited scale and patient population of these trials.267,268 The outstanding overall response achieved by immunotherapy in aHCC has renewed the interest in neoadjuvant treatment of resectable HCC. The interim report from a small size, randomized phase II trial validated that perioperative immunotherapy (nivolumab with or without ipilimumab) produced a notable pathological CRR (29%), without delay of surgical resection.269 And the final result may improve the dismal situation of neoadjuvant therapy for HCC. Furthermore, the combination of molecular targeted agents and ICIs also showed surprising clinical benefits in the neoadjuvant setting for the patients with resectable HCC.270,271 Yarchoan et al indicated that cabozantinib combined with nivolumab induced a marked pathologic response and conferred surgical resection to patients initially beyond traditional resection criteria.270 Meanwhile, other combination therapies (NCT03222076 and NCT04425226) as tumor downstage treatments are being evaluated to provide more radical surgical opportunities to HCC patients (Table 2).
TCM
TCM is based on thousands of years of clinical experience against HCC with the unique philosophic regimen of theories, diagnosis and treatments. In theoretical systems of TCM, “a concept of holism” and “the treatment according to syndrome differentiation” are highly valued. The former supports the human systems as a holism to maintain a dynamic equilibrium of external environments and internal organism system. And the loss of relative equilibrium will incur the occurrence of diseases. It is via the latter that TCM restores this equilibrium to cure diseases. In the setting of TCM theory and accumulating clinical experience, HCC fits into the category of “Zheng Jia” and “Ji Ju”, with basic pathogenic mechanisms including spleen deficiency, qi stagnation, blood stasis, excessive heat, yin deficiency, and dampness.272,273 In view of this, there are numerous traditional Chinese medicines (TCMs) usually subdivided into formulas and single herbs fighting against HCC through clearing heat, detoxifying the body, eliminating dampness, calming the liver wind, or strengthening qi. The antitumor effects against HCC of TCMs, particularly formulas, possess multi-component, multi-target, and various biological pathways features on the basis of the integrity of organism, which coincides with the complex mechanisms of hepatocarcinogenesis and progression.274
TCMs were shown to potently alleviate AEs from conventional treatments, such as chemotherapy and molecular targeted therapy, but also enhance the therapeutic effect of other treatments for HCC.275 YIV-906 (PHY906), derived from a Chinese herbal formula named Huang-Qin Decoction, consists of four herbal ingredients (Glycyrrhiza uralensis Fisch, Paeonia lactiflora Pall, Scutellaria baicalensis Georgi, and Ziziphus jujuba Mill).276 In phase I/II clinical trials, capecitabine combined with YIV-906 potentiated its anti-hepatoma activity, resulting in a mean OS of 9.2 months. Of note, the incidence of severe gastrointestinal toxicity associated with capecitabine was significantly reduced compared with capecitabine treatment alone.276,277 In addition, YIV-906 was confirmed to enhance the antitumor activity of sorafenib mediated by stimulating tumor autophagy and apoptosis as well as immune response from TME.278 A phase II clinical trial (NCT04000737) is undergoing to compare YIV-906 plus sorafenib versus sorafenib alone regarding of efficacy and safety as a first-line treatment for aHCC patients with HBV. Moreover, Yang and his team reported that the anti-tumor activity of anti-PD1 was markedly enhanced in combination of YIV-906 via promoting the adaptive and innate immune responses.279 In terms of single herbs, Kanglaite as an extract from Coix seeds also enhanced the suppressive effects of cisplatin on HCC cells, exhibiting synergistic effects in the combination with conventional treatments.280 Of significant mention is icaritin, a prenylflavonoid derivative from epimedium. A single-arm phase I study preliminarily confirmed that icaritin at an optimized dose conferred the safety profile and durable survival benefits to aHCC patients through modulating host immune activities, accompanied by an OS of 192 days, but did not induce immune-related AEs (like interstitial lung disease and thyroid dysfunction).281 Subsequently, the underlying mechanisms were established that the immunomodulating activities induced by icaritin involving the crosstalk of various cytokines, immune cells, and immune checkpoints via IL-6/JAK/STAT3 pathways rather than a single target, which is superior over conventional targeted therapies and ICIs for aHCC treatment.282 Based on these premises, at 2021 CSCO, Qin shared the results of a multicenter, randomized, double-blind phase III clinical trial (NCT03236636) comparing the safety profile and efficacy of icaritin with cinobufotalin in first-line Treatment of aHCC subjects. Patients receiving icaritin treatment showed a significantly prolonged mean OS compared with the control arm (13.45 vs 6.87 months, HR = 0.43, P = 0.0092), with a lower incidence of AEs (12.6% vs 26.2%). The final results of this trial will bring outstanding breakthroughs in immunotherapies for aHCC. Moreover, numerous TCMs, such as Huaier Granule and Jinlong Capsule, are widely used for HCC treatment,283 while other clinical trials (NCT03851471, NCT02399033) assessing the efficacy of TCMs for HCC are underway. Several limitations of TCMs still remain, including their poor absorption, low bioavailability, and unknown molecular mechanisms, despite the dramatic emergence of novel drug delivery systems, TCM analogs, and high-throughput and omics technologies.274,284 Of course, this may be associated with the concept of “holism” during HCC treatment. There is still a long way to go to make full use of TCMs for HCC in not only the East but also the West.
Conclusion and Outlook
Recent decades have witnessed remarkable progress in systemic therapy for aHCC, with the approvals of first-line drugs, including lenvatinib beyond sorafenib, and second-line drugs, including molecular targeted inhibitors (MTIs) (regorafenib, cabozantinib and ramucirumab) and ICIs (pembrolizumab and nivolumab). Subsequently, the advent of combination modalities of ICIs and MTIs enable the clinical efficacy of systemic therapy to reach a record high for aHCC. Atezolizumab-bevacizumab combination achieved a high OS of 19.2 months in the first-line setting for aHCC with tolerable toxicity and nivolumab-ipilimumab combination has been also established the one of top options for aHCC patients with the prior treatment of a first-line TKI (sorafenib or lenvatinib) (Table 3). Additionally, systemic therapy, especially ICIs, has been confirmed to potently reduce tumor burden to facilitate TACE and surgical resection, which may improve clinical outcomes and reduce the recurrence after operation.
 |
Table 3 Summary of Efficacy and Safety of the Approved Systemic Therapies for aHCC |
Despite this, some challenges deserve cautious consideration in the development of systemic therapy for aHCC. The triplet combination of nivolumab + ipilimumab + cabozantinib failed to report satisfactory survival benefits compared to the nivolumab + cabozantinib arm (PFS, 6.8 vs 5.5 months; ORR, 26% vs 17%), but with a terribly higher incidence of AEs (71% vs 42%).285 Thus, greater advances in HCC will rely on the elucidation of its complex biological mechanisms from multiple perspectives, such as the interactions of molecules and cells, and the role of TME. The current boom in multi-omics analysis and high-throughput sequencing may help identify novel therapeutic targets for HCC treatment.286,287 Moreover, synergistic rather than additive combinations, particularly in immune-based regimens, should be extensively explored beyond atezolizumab-bevacizumab combination on the basis of the immunomodulatory mechanisms.288 Immune-based combination therapy will usher in a new first-line treatment landscape in the next few years.289 Regarding treatment efficacy for HCC, delayed responses or pseudo-progressions in HCC are worthy of note that patients initially receive the progressive disease assessed after treatment, but later obtain a decrease in tumor burden or a treatment benefit.290 An instance is the nivolumab treatment with which aHCC patients show delay clinical benefits.291 The mechanism behind the pseudo-progressions remains uncertain, which makes discriminating it from hyperprogression tricky. Moreover, a portion of aHCC patients fail to obtain clinical benefit or just develop resistance to systemic therapy. Therefore, some predictive biomarkers seem to be pivotal to assess treatment efficacy and select HCC patients likely to benefit more from treatment. AFP is an only valuable biomarker to guide treatment and predict prognosis for HCC, while considerable effort has been made to pursuit potential biomarkers for HCC.68,292,293 Of note, several emerging biomarkers, such as prothrombin induced by vitamin K absence-II (PIVKA-II), featuring a higher sensitivity and specificity than AFP have not yet been widely deployed in preclinical and clinical settings.294 The limitation of geographic populations and underlying liver disease, and the uncertain biomechanisms to some extent contribute to the existing disappointing situation. Further well-designed, large-scale, and international multicenter prospective clinical studies as well as in-depth mechanistic studies are warranted to validate the clinical power of these biomarkers. In addition, the shortage of effective biomarkers for HCC may result from the limited cancerous tissue available for molecular testing and the molecular heterogeneity of HCC.295 Stringent collection and analysis of blood samples from HCC patients should be implemented to overcome this barrier. New serum biomarkers and the DNA sequencing of circulating tumor cells may be promising biomarkers useful to decision-making for HCC.296,297 Interestingly, multiple biomarkers in concert may also exhibit superiority over biomarkers used alone.298 This is exemplified by the multi-marker panels consisting of AFP, PIVKA-II, and other biomarkers.299 AEs or toxicities during HCC treatment also need to be considered with caution owing to their undesirable impact on the efficacy of systemic therapy and quality of life. An optimal dosing schedule, therapy duration, and rational combination regimens are warranted in future clinical trials to ameliorate AEs or toxicities. Furthermore, a better understanding of underlying mechanisms is crucial for improving the management of AEs as well as choosing the next treatment.300 However, toxicities or AEs may represent clinical benefits in such cases as TKI-induced AEs53,301–303 and immune-mediated AEs associated with ICIs.304 Last but not least, the integration of multidisciplinary treatments, including the combination of systemic and LRTs as well as adjuvant and neoadjuvant therapy, should be implemented to establish an optimal treatment strategy in terms of OS of aHCC patients and their quality of life.
Overall, the optimal combined combination regimens based on a better understanding of biological mechanisms of HCC, personalized treatments depending on accurate, user-friendly biomarkers, and rational multiple disciplinary intervention are the direction of effort in the near future, which will achieve an excellent prognosis and a better quality of life for aHCC patients.
Acknowledgments
The present study was supported by Award for Liaoning Distinguished Professor, Natural Science Foundation of Hebei Province (202130821010436) and National Natural Scientific Foundation of China (81672700).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):7.
3. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73(Suppl 1):S4–S13.
4. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249.
5. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462.
6. Vogel A, Martinelli E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32(6):801–805.
7. Maida M, Orlando E, Camma C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20(15):4141–4150.
8. Faria SC, Szklaruk J, Kaseb AO, Hassabo HM, Elsayes KM. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: concepts, perspectives, and radiologic implications. Abdom Imaging. 2014;39(5):1070–1087.
9. Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–491.e1.
10. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
11. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
12. Galle PR, Dufour JF, Peck-Radosavljevic M, Trojan J, Vogel A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021;17(10):1237–1251. doi:10.2217/fon-2020-0758
13. Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16(10):617–630. doi:10.1038/s41575-019-0179-x
14. Roderburg C, Ozdirik B, Wree A, Demir M, Tacke F. Systemic treatment of hepatocellular carcinoma: from sorafenib to combination therapies. Hepat Oncol. 2020;7(2):HEP20. doi:10.2217/hep-2020-0004
15. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
16. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
17. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
18. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
19. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
20. Flynn MJ, Sayed AA, Sharma R, Siddique A, Pinato DJ. Challenges and Opportunities in the Clinical Development of Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. Hepatology. 2019;69(5):2258–2270. doi:10.1002/hep.30337
21. Cheng H, Sun G, Chen H, et al. Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res. 2019;9(8):1536–1545.
22. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
23. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
24. Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13(1):1–14. doi:10.1007/s10456-009-9160-6
25. Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 2018;17(1):36. doi:10.1186/s12943-018-0801-5
26. Regad T. Targeting RTK Signaling Pathways in Cancer. Cancers. 2015;7(3):1758–1784. doi:10.3390/cancers7030860
27. Ghouri YA, Mian I, Rowe JH, Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi:10.4103/jcar.JCar_9_16
28. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12(1):27. doi:10.1186/s13045-019-0718-5
29. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
30. Ren Z, Zhu K, Kang H, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894–900.
31. Branco F, Alencar RS, Volt F, et al. The Impact of Early Dermatologic Events in the Survival of Patients with Hepatocellular Carcinoma Treated with Sorafenib. Ann Hepatol. 2017;16(2):263–268.
32. Abdel-Rahman O, Lamarca A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic-review and meta-analysis of the impact on survival. Expert Rev Gastroenterol Hepatol. 2017;11(1):75–83.
33. Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(26):4293–4300.
34. Abou-Alfa GK. Selection of patients with hepatocellular carcinoma for sorafenib. J Natl Compr Canc Netw. 2009;7(4):397–403.
35. Huitzil FD, Saltz SL, Song J, et al. Retrospective analysis of outcome in hepatocellular carcinoma (HCC) patients (pts) with hepatitis C (C+) versus B (B+) treated with sorafenib (S) [abstract]. J Clin Oncol. 2011;29:e14636.
36. Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: a Meta-Analysis of Randomized Phase III Trials. J Clin Oncol. 2017;35(6):622–628.
37. Kolamunnage-Dona R, Berhane S, Potts H, et al. Sorafenib is associated with a reduced rate of tumour growth and liver function deterioration in HCV-induced hepatocellular carcinoma. J Hepatol. 2021;75(4):879–887.
38. Giambartolomei S, Covone F, Levrero M, Balsano C. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C Virus (HCV) core protein. Oncogene. 2001;20(20):2606–2610.
39. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008.
40. Chiu J, Tang YF, Yao TJ, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: a retrospective analysis of efficacy, safety, and survival benefits. Cancer. 2012;118(21):5293–5301.
41. Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. 2020;2(82):101946.
42. Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–1147.
43. Kudo M. Lenvatinib in Advanced Hepatocellular Carcinoma. Liver Cancer. 2017;6(4):253–263.
44. Kudo M, Finn RS, Qin S, et al. Analysis of survival and objective response (OR) in patients with hepatocellular carcinoma in a phase III study of lenvatinib (REFLECT). J Clin Oncol. 2019;37(Suppl 4):S186–S186.
45. Vogel A, Frenette C, Sung M, et al. Baseline Liver Function and Subsequent Outcomes in the Phase 3 REFLECT Study of Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer. 2021;10(5):510–521.
46. Vogel A, Qin S, Kudo M, et al. 618O - Health-related quality of Life (HRQOL) and disease symptoms in patients with unresectable hepatocellular carcinoma (HCC) treated with lenvatinib (LEN) or sorafenib (SOR). Annals of Oncology. 2017;1(28):v210.
47. Vogel A, Qin S, Kudo M, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(8):649–658.
48. Kim JJ, McFarlane T, Tully S, Wong WWL. Lenvatinib Versus Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma: a Cost-Utility Analysis. Oncologist. 2020;25(3):e512–e519.
49. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255.
50. Kudo M, New A. Era of Systemic Therapy for Hepatocellular Carcinoma with Regorafenib and Lenvatinib. Liver Cancer. 2017;6(3):177–184.
51. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353–358.
52. Kim HD, Bang Y, Lee MA, et al. Regorafenib in patients with advanced Child-Pugh B hepatocellular carcinoma: a multicentre retrospective study. Liver Int. 2020;40(10):2544–2552.
53. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36(Suppl 4):S412–S412.
54. Bruix J, Reig M, Merle P, et al. 755P - Alpha-fetoprotein (AFP) response in patients with unresectable hepatocellular carcinoma (HCC) in the phase III RESORCE trial. Annals of Oncology. 2019;30:v291.
55. Teufel M, Seidel H, Kochert K, et al. Biomarkers Associated With Response to Regorafenib in Patients With Hepatocellular Carcinoma. Gastroenterology. 2019;156(6):1731–1741.
56. Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20(11):2959–2970.
57. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
58. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308.
59. Miksad R, Cicin I, Chen Y, et al. O-022 - Outcomes based on Albumin‐Bilirubin (ALBI) grade in the phase 3 CELESTIAL trial of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma (HCC). Annals of Oncology. 2019;30:iv134.
60. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36(Suppl 4):S207–S207.
61. Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–693.
62. Kelley RK, Mollon P, Blanc JF, et al. Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma. Adv Ther. 2020;37(6):2678–2695.
63. Soto-perez-de-celis E, Aguiar PN, Cordon ML, Chavarri-Guerra Y, Lopes GL. Cost-Effectiveness of Cabozantinib in the Second-Line Treatment of Advanced Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2019;17(6):669–675.
64. Rimassa L, Kelley RK, Meyer T, et al. 678PD - Outcomes based on plasma biomarkers for the phase III CELESTIAL trial of cabozantinib (C) versus placebo (P) in advanced hepatocellular carcinoma (aHCC). Annals of Oncology. 2019;1(30):v257–v258.
65. Syed YY. Ramucirumab: a Review in Hepatocellular Carcinoma. Drugs. 2020;80(3):315–322.
66. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870.
67. Gilabert M, Raoul JL. Potential of ramucirumab in treating hepatocellular carcinoma patients with elevated baseline alpha-fetoprotein. J Hepatocell Carcinoma. 2018;5:91–98.
68. Zhu AX, Kang Y-K, Yen C-J, et al. REACH-2: a randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J Clin Oncol. 2018;36(Suppl 15):S4003–S4003.
69. Zhu A, Finn R, Galle P, et al. LBA-001 - Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: pooled efficacy and safety across two global randomized Phase 3 studies (REACH-2 and REACH). Annals of Oncology. 2018;29:v122.
70. Kok B, Abraldes JG. Child-Pugh Classification: time to Abandon? Semin Liver Dis. 2019;39(1):96–103.
71. Brandi GKM, Kang YK, et al. OP-07 Ramucirumab for patients with hepatocellular carcinoma and elevated alpha-fetoprotein following sorafenib treatment: exploratory analysis of REACH-2 trial results by albumin-bilirubin grade and Child-Pugh score [abstract].
72. Kudo M, Ikeda M, Galle PR, et al. Ramucirumab in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: outcomes by treatment-emergent ascites. Hepatol Res. 2021;51(6):715–721.
73. Demir T, Lee SS, Kaseb AO. Systemic therapy of liver cancer. Adv Cancer Res. 2021;2(149):257–294.
74. Kudo M. Ramucirumab as Second-Line Systemic Therapy in Hepatocellular Carcinoma. Liver Cancer. 2018;7(4):305–311.
75. Li X, Qiu M, Wang S, Zhu H, Feng B, Zheng L. A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2020;85(3):593–604.
76. Qin S, Bi F, Gu S, et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: a Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39(27):3002–3011.
77. Yen CJ, Kim TY, Feng YH, et al. A Phase I/Randomized Phase II Study to Evaluate the Safety, Pharmacokinetics, and Efficacy of Nintedanib versus Sorafenib in Asian Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2018;7(2):165–178.
78. Cheng AL, Thongprasert S, Lim HY, et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2016;64(3):774–784.
79. Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516.
80. Hsu C, Yang TS, Huo TI, et al. Vandetanib in patients with inoperable hepatocellular carcinoma: a Phase II, randomized, double-blind, placebo-controlled study. J Hepatol. 2012;56(5):1097–1103.
81. Philip PA, Mahoney MR, Allmer C, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23(27):6657–6663.
82. Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179.
83. Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10(8):794–800.
84. Turpin A, de Baere T, Heurgue A, et al. Liver transarterial chemoembolization and sunitinib for unresectable hepatocellular carcinoma: results of the PRODIGE 16 study. Clin Res Hepatol Gastroenterol. 2021;45(2):101464.
85. Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630.
86. Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13(11):788–799.
87. Faivre S, Santoro A, Kelley RK, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39(8):1468–1477.
88. Kasprzak A, Adamek A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int J Mol Sci. 2018;19(12):3887.
89. Duffy AG, Ma C, Ulahannan SV, et al. Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma. Clin Cancer Res. 2017;23(16):4633–4641.
90. Alqahtani A, Khan Z, Alloghbi A, Said ahmed TS, Ashraf M, Hammouda DM. Hepatocellular Carcinoma: molecular Mechanisms and Targeted Therapies. Medicina. 2019;55(9):526.
91. Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38(5):614–622.
92. Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68(1):206–215.
93. Singh AR, Joshi S, Burgoyne AM, et al. Single Agent and Synergistic Activity of the “First-in-Class” Dual PI3K/BRD4 Inhibitor SF1126 with Sorafenib in Hepatocellular Carcinoma. Mol Cancer Ther. 2016;15(11):2553–2562.
94. Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67.
95. Koeberle D, Dufour JF, Demeter G, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann Oncol. 2016;27(5):856–861.
96. Hwang YH, Choi JY, Kim S, et al. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol Res. 2004;29(2):113–121.
97. Chen L, Shi Y, Jiang CY, Wei LX, Wang YL, Dai GH. Expression and prognostic role of pan-Ras, Raf-1, pMEK1 and pERK1/2 in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2011;37(6):513–520.
98. Tai WM, Yong WP, Lim C, et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann Oncol. 2016;27(12):2210–2215.
99. Lim HY, Merle P, Weiss KH, et al. Phase II Studies with Refametinib or Refametinib plus Sorafenib in Patients with RAS-Mutated Hepatocellular Carcinoma. Clin Cancer Res. 2018;24(19):4650–4661.
100. Bitzer M, Horger M, Giannini EG, et al. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma - The SHELTER study. J Hepatol. 2016;65(2):280–288. doi:10.1016/j.jhep.2016.02.043
101. Bollard J, Miguela V, Ruiz de Galarreta M, et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut. 2017;66(7):1286–1296. doi:10.1136/gutjnl-2016-312268
102. Reiter FP, Denk G, Ziesch A, et al. Predictors of ribociclib-mediated antitumour effects in native and sorafenib-resistant human hepatocellular carcinoma cells. Cell Oncol. 2019;42(5):705–715. doi:10.1007/s13402-019-00458-8
103. Yoo C, Kang J, Lim HY, et al. Phase I Dose-Finding Study of OPB-111077, a Novel STAT3 Inhibitor, in Patients with Advanced Hepatocellular Carcinoma. Cancer Res Treat. 2019;51(2):510–518. doi:10.4143/crt.2018.226
104. Zhu H, Wang DD, Yuan T, et al. Multikinase Inhibitor CT-707 Targets Liver Cancer by Interrupting the Hypoxia-Activated IGF-1R-YAP Axis. Cancer Res. 2018;78(14):3995–4006. doi:10.1158/0008-5472.CAN-17-1548
105. Eatrides J, Wang E, Kothari N, Kim R. Role of Systemic Therapy and Future Directions for Hepatocellular Carcinoma. Cancer Control. 2017;24(3):1073274817729243. doi:10.1177/1073274817729243
106. Johnson PJ. Are there indications for chemotherapy in hepatocellular carcinoma? Surg Oncol Clin N Am. 2003;12(1):127–134. doi:10.1016/S1055-3207(02)00075-3
107. Huang CC, Wu MC, Xu GW, et al. Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst. 1992;84(4):262–264. doi:10.1093/jnci/84.4.262
108. Chou YY, Cheng AL, Hsu HC. Expression of P-glycoprotein and p53 in advanced hepatocellular carcinoma treated by single agent chemotherapy: clinical correlation. J Gastroenterol Hepatol. 1997;12(8):569–575. doi:10.1111/j.1440-1746.1997.tb00487.x
109. Abouelezz K, Khanapara D, Batiha GE, Ahmed EA, Hetta HF, Cytotoxic Chemotherapy as an Alternative for Systemic Treatment of Advanced Hepatocellular Carcinoma in Developing Countries. Cancer Manag Res. 2020;12:12239–12248. doi:10.2147/CMAR.S280631
110. Johnson PJ, Dobbs N, Kalayci C, et al. Clinical efficacy and toxicity of standard dose Adriamycin in hyperbilirubinaemic patients with hepatocellular carcinoma: relation to liver tests and pharmacokinetic parameters. Br J Cancer. 1992;65(5):751–755. doi:10.1038/bjc.1992.158
111. Chlebowski RT, Brzechwa-Adjukiewicz A, Cowden A, Block JB, Tong M, Chan KK. Doxorubicin (75 mg/m2) for hepatocellular carcinoma: clinical and pharmacokinetic results. Cancer Treat Rep. 1984;68(3):487–491.
112. Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62(3):479–483. doi:10.1002/1097-0142(19880801)62:3<479::AID-CNCR2820620306>3.0.CO;2-L
113. Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101(3):578–586. doi:10.1002/cncr.20368
114. Brandi G, de Rosa F, Agostini V, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013;18(12):1256–1257. doi:10.1634/theoncologist.2013-0093
115. Trevisani F, Brandi G, Garuti F, et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol. 2018;144(2):403–414. doi:10.1007/s00432-017-2556-6
116. Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17(12):3137–3144. doi:10.1245/s10434-010-1148-3
117. Ravaioli M, Cucchetti A, Pinna AD, et al. The role of metronomic capecitabine for treatment of recurrent hepatocellular carcinoma after liver transplantation. Sci Rep. 2017;7(1):11305. doi:10.1038/s41598-017-11810-z
118. Yang TS, Lin YC, Chen JS, Wang HM, Wang CH. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2000;89(4):750–756. doi:10.1002/1097-0142(20000815)89:4<750::AID-CNCR5>3.0.CO;2-R
119. Yen Y, Lim DW, Chung V, et al. Phase II study of oxaliplatin in patients with unresectable, metastatic, or recurrent hepatocellular cancer: a California Cancer Consortium Trial. Am J Clin Oncol. 2008;31(4):317–322. doi:10.1097/COC.0b013e318162f57d
120. Boige V, Taieb J, Hebbar M, et al. Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: a multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur J Cancer. 2006;42(4):456–459. doi:10.1016/j.ejca.2005.09.034
121. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–3508. doi:10.1200/JCO.2012.44.5643
122. Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist. 2014;19(11):1169–1178. doi:10.1634/theoncologist.2014-0190
123. Dhooge M, Coriat R, Mir O, et al. Feasibility of gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma patients with Child-Pugh B cirrhosis. Oncology. 2013;84(1):32–38. doi:10.1159/000342763
124. Patrikidou A, Sinapi I, Regnault H, et al. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti-angiogenic therapies. Invest New Drugs. 2014;32(5):1028–1035. doi:10.1007/s10637-014-0100-y
125. Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58(1):81–88. doi:10.1016/j.jhep.2012.09.006
126. Boige V, Raoul JL, Pignon JP, et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer. 2007;97(7):862–867. doi:10.1038/sj.bjc.6603956
127. Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532–1538. doi:10.1093/jnci/dji315
128. Chia WK, Ong S, Toh HC, et al. Phase II trial of gemcitabine in combination with cisplatin in inoperable or advanced hepatocellular carcinoma. Ann Acad Med Singap. 2008;37(7):554–558.
129. Lee JO, Lee KW, Oh DY, et al. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol. 2009;20(8):1402–1407. doi:10.1093/annonc/mdp010
130. Petrelli F, Coinu A, Borgonovo K, et al. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma. a systematic review and pooled analysis. Clin Oncol. 2014;26(8):488–496. doi:10.1016/j.clon.2014.04.031
131. Qin LX. Inflammatory immune responses in tumor microenvironment and metastasis of hepatocellular carcinoma. Cancer Microenviron. 2012;5(3):203–209. doi:10.1007/s12307-012-0111-1
132. Daniyan AF, Brentjens RJ. Immunotherapy: hiding in plain sight: immune escape in the era of targeted T-cell-based immunotherapies. Nat Rev Clin Oncol. 2017;14(6):333–334. doi:10.1038/nrclinonc.2017.49
133. Khan AA, Liu ZK, Xu X. Recent advances in immunotherapy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2021;20(6):511–520. doi:10.1016/j.hbpd.2021.06.010
134. Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi:10.1016/j.ccr.2006.06.016
135. Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi:10.1053/j.gastro.2007.03.102
136. Meirow Y, Kanterman J, Baniyash M, Paving the Road to Tumor Development and Spreading: myeloid-Derived Suppressor Cells are Ruling the Fate. Front Immunol. 2015;6:523. doi:10.3389/fimmu.2015.00523
137. Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6(9):e24671. doi:10.1371/journal.pone.0024671
138. Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L. Preclinical and Clinical Therapeutic Strategies Affecting Tumor-Associated Macrophages in Hepatocellular Carcinoma. J Immunol Res. 2018;2018:7819520. doi:10.1155/2018/7819520
139. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–543. doi:10.1038/s41575-021-00438-0
140. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
141. Yau T, Park JW, Finn RS, et al. LBA38_PR - CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Annals of Oncology. 2019;1(30):v874–v875. doi:10.1093/annonc/mdz394.029
142. Lleo A, Rimassa L, Colombo M. Hepatotoxicity of immune check point inhibitors: approach and management. Dig Liver Dis. 2019;51(8):1074–1078. doi:10.1016/j.dld.2019.06.017
143. Kuo YH, Yen YH, Chen YY, et al. Nivolumab Versus Regorafenib in Patients With Hepatocellular Carcinoma After Sorafenib Failure. Front Oncol. 2021;11:683341. doi:10.3389/fonc.2021.683341
144. Lee CH, Lee YB, Kim MA, et al. Effectiveness of nivolumab versus regorafenib in hepatocellular carcinoma patients who failed sorafenib treatment. Clin Mol Hepatol. 2020;26(3):328–339. doi:10.3350/cmh.2019.0049n
145. Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75(3):600–609. doi:10.1016/j.jhep.2021.04.047
146. Fessas P, Kaseb A, Wang Y, et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer. 2020;8(2):e001033. doi:10.1136/jitc-2020-001033
147. Choi WM, Lee D, Shim JH, et al. Effectiveness and Safety of Nivolumab in Child-Pugh B Patients with Hepatocellular Carcinoma: a Real-World Cohort Study. Cancers. 2020;12(7):1968. doi:10.3390/cancers12071968
148. El-Khoueiry AB, Melero I, Yau TC, et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): subanalyses of CheckMate-040. J Clin Oncol. 2018;36(Suppl 4):S475–S475. doi:10.1200/JCO.2018.36.4_suppl.475
149. Sangro B, Melero I, Wadhawan S, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. 2020;73(6):1460–1469. doi:10.1016/j.jhep.2020.07.026
150. Teng W, Lin CC, Ho MM, et al. Alpha-fetoprotein response at different time-points is associated with efficacy of nivolumab monotherapy for unresectable hepatocellular carcinoma. Am J Cancer Res. 2021;11(5):2319–2330.
151. Rapposelli IG, De Matteis S, Lanuti P, et al. Heterogeneity of Response and Immune System Activity during Treatment with Nivolumab in Hepatocellular Carcinoma: results from a Single-Institution Retrospective Analysis. Cancers. 2021;13(2):213. doi:10.3390/cancers13020213
152. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
153. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: a Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
154. Qin S, Chen Z, Fang W, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40(Suppl 4):S383–S383. doi:10.1200/JCO.2022.40.4_suppl.383
155. Vogel A, Merle P, Verslype C, et al. ALBI score and outcomes in patients with hepatocellular carcinoma: post hoc analysis of the randomized controlled trial KEYNOTE-240. Ther Adv Med Oncol. 2021;13:17588359211039928. doi:10.1177/17588359211039928
156. Ryoo BY, Merle P, Kulkarni AS, et al. Health-related quality-of-life impact of pembrolizumab versus best supportive care in previously systemically treated patients with advanced hepatocellular carcinoma: KEYNOTE-240. Cancer. 2021;127(6):865–874. doi:10.1002/cncr.33317
157. Chiang CL, Chan SK, Lee SF, Wong IO, Choi HC. Cost-effectiveness of Pembrolizumab as a Second-Line Therapy for Hepatocellular Carcinoma. JAMA Netw Open. 2021;4(1):e2033761. doi:10.1001/jamanetworkopen.2020.33761
158. Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020;470:8–17. doi:10.1016/j.canlet.2019.12.002
159. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
160. Qin S, Finn RS, Kudo M, et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15(16):1811–1822. doi:10.2217/fon-2019-0097
161. Wainberg ZA, Segal NH, Jaeger D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol. 2017;35(Suppl 15):4071. doi:10.1200/JCO.2017.35.15_suppl.4071
162. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. doi:10.1038/nrgastro.2015.173
163. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. doi:10.1016/j.jhep.2013.02.022
164. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–551. doi:10.1016/j.jhep.2016.10.029
165. Yau T, Kang Y-K, Kim T-Y, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37(Suppl 15):S4012–S4012. doi:10.1200/JCO.2019.37.15_suppl.4012
166. Lai E, Astara G, Ziranu P, et al. Introducing immunotherapy for advanced hepatocellular carcinoma patients: too early or too fast? Crit Rev Oncol Hematol. 2021;2(157):103167. doi:10.1016/j.critrevonc.2020.103167
167. Dovedi SJ, Elder MJ, Yang C, et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1(+) Activated T Cells. Cancer Discov. 2021;11(5):1100–1117. doi:10.1158/2159-8290.CD-20-1445
168. Zhang R, Zhang Z, Liu Z, et al. Adoptive cell transfer therapy for hepatocellular carcinoma. Front Med. 2019;13(1):3–11. doi:10.1007/s11684-019-0684-x
169. Mizukoshi E, Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol. 2019;12(1):52. doi:10.1186/s13045-019-0742-5
170. Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–541. doi:10.1038/nrc3565
171. Rochigneux P, Chanez B, De Rauglaudre B, Mitry E, Chabannon C, Gilabert M. Adoptive Cell Therapy in Hepatocellular Carcinoma: biological Rationale and First Results in Early Phase Clinical Trials. Cancers. 2021;13:2. doi:10.3390/cancers13020271
172. Ping Y, Liu C, Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell. 2018;9(3):254–266. doi:10.1007/s13238-016-0367-1
173. Tan AT, Yang N, Lee Krishnamoorthy T, et al. Use of Expression Profiles of HBV-DNA Integrated Into Genomes of Hepatocellular Carcinoma Cells to Select T Cells for Immunotherapy. Gastroenterology. 2019;156(6):1862–1876.e9. doi:10.1053/j.gastro.2019.01.251
174. Qasim W, Brunetto M, Gehring AJ, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62(2):486–491. doi:10.1016/j.jhep.2014.10.001
175. Sun L, Guo H, Jiang R, Lu L, Liu T, He X. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumour Biol. 2016;37(1):799–806. doi:10.1007/s13277-015-3845-9
176. Dargel C, Bassani-Sternberg M, Hasreiter J, et al. T Cells Engineered to Express a T-Cell Receptor Specific for Glypican-3 to Recognize and Kill Hepatoma Cells In Vitro and in Mice. Gastroenterology. 2015;149(4):1042–1052. doi:10.1053/j.gastro.2015.05.055
177. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi:10.1126/science.aar6711
178. Shi D, Shi Y, Kaseb AO, et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: results of Phase I Trials. Clin Cancer Res. 2020;26(15):3979–3989. doi:10.1158/1078-0432.CCR-19-3259
179. Dai H, Tong C, Shi D, et al. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology. 2020;9(1):1846926. doi:10.1080/2162402X.2020.1846926
180. Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11(83). doi:10.1186/1479-5876-11-83
181. Gustafsson K, Ingelsten M, Bergqvist L, Nystrom J, Andersson B, Karlsson-Parra A. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 2008;68(14):5965–5971. doi:10.1158/0008-5472.CAN-07-6494
182. Palmer DH, Midgley RS, Mirza N, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–132. doi:10.1002/hep.22626
183. Tada F, Abe M, Hirooka M, et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41(5):1601–1609. doi:10.3892/ijo.2012.1626
184. Nakagawa H, Mizukoshi E, Kobayashi E, et al. Association Between High-Avidity T-Cell Receptors, Induced by alpha-Fetoprotein-Derived Peptides, and Anti-Tumor Effects in Patients With Hepatocellular Carcinoma. Gastroenterology. 2017;152(6):1395–1406.e10. doi:10.1053/j.gastro.2017.02.001
185. Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5):e1129483. doi:10.1080/2162402X.2015.1129483
186. Mizukoshi E, Nakagawa H, Kitahara M, et al. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015;369(1):242–249. doi:10.1016/j.canlet.2015.08.020
187. Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6(1):140. doi:10.1186/s40425-018-0458-z
188. Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi:10.1038/nbt.2287
189. Moehler M, Heo J, Lee HC, et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology. 2019;8(8):1615817. doi:10.1080/2162402X.2019.1615817
190. Genetic Engineering & Biotechnology News. Pexa-Vec/Nexavar Combination Fails Phase III Trial in Liver Cancer; 2019. Available from: https://www.genengnews.com/topics/genome-editing/pexa-vec-nexavar-combination-fails-phase-iii-trial-in-liver-cancer/.
191. LaRocca CJ, Warner SG. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin Transl Med. 2018;7(1):35. doi:10.1186/s40169-018-0214-5
192. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15(5):310–324. doi:10.1038/nrclinonc.2018.9
193. Finn RS, Qin S, Ikeda M, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(Suppl 3):S267–S267. doi:10.1200/JCO.2021.39.3_suppl.267
194. Qin S, Ren Z, Feng Y, et al. OP02-03 Efficacy and safety of atezolizumab + bevacizumab vs sorafenib in Chinese patients with unresectable HCC in the phase III IMbrave150 study[abstract].
195. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/JCO.20.02672
196. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75(4):960–974. doi:10.1016/j.jhep.2021.07.004
197. Qin S. Clinical practice of hepatocellular carcinoma: guideline of CSCO. CSCO. 2020;1:88.
198. Eso Y, Takeda H, Taura K, Takai A, Takahashi K, Seno H. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma. Curr Oncol. 2021;28(5):4157–4166. doi:10.3390/curroncol28050352
199. Wang Y, Lu LC, Guan Y, et al. Atezolizumab plus bevacizumab combination enables an unresectable hepatocellular carcinoma resectable and links immune exclusion and tumor dedifferentiation to acquired resistance. Exp Hematol Oncol. 2021;10(1):45. doi:10.1186/s40164-021-00237-y
200. Yamada T, Minami T, Tateishi R, Koike K. Limited efficacy of atezolizumab and bevacizumab for hepatocellular carcinoma previously treated with tyrosine kinase inhibitor. Liver Int. 2021;41(9):2233–2234. doi:10.1111/liv.15010
201. Zhang X, Wang J, Shi J, Jia X, Dang S, Wang W. Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib for Patients With Unresectable or Metastatic Hepatocellular Carcinoma. JAMA Netw Open. 2021;4(4):e214846. doi:10.1001/jamanetworkopen.2021.4846
202. Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi:10.1111/cas.13806
203. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
204. Rizzo A, Dadduzio V, Ricci AD, et al. Lenvatinib plus pembrolizumab: the next frontier for the treatment of hepatocellular carcinoma? Expert Opin Investig Drugs. 2021;6(6):1–8.
205. Kudo M, Ikeda M, Motomura K, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol. 2020;38(Suppl 4):S513–S513. doi:10.1200/JCO.2020.38.4_suppl.513
206. Kudo M, Motomura K, Wada Y, et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: results from a phase 1b trial (VEGF Liver 100). J Clin Oncol. 2019;37(Suppl 15):S4072–S4072. doi:10.1200/JCO.2019.37.15_suppl.4072
207. Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: an Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25(2):515–523. doi:10.1158/1078-0432.CCR-18-2484
208. Xu J, Shen J, Gu S, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): a Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
209. Bai L, Sun M, Xu A, et al. Phase 2 study of AK104 (PD-1/CTLA-4 bispecific antibody) plus lenvatinib as first-line treatment of unresectable hepatocellular carcinoma. J Clin Oncol. 2021;39(Suppl 15):S4101–S4101. doi:10.1200/JCO.2021.39.15_suppl.4101
210. Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;4(5):16. doi:10.1186/s40425-017-0218-5
211. Kudo M. Combination Cancer Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2018;7(1):20–27. doi:10.1159/000486487
212. Yau T, Kang YK, Kim TY, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: the CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6(11):e204564. doi:10.1001/jamaoncol.2020.4564
213. El-Khoueiry AB, Yau T, Kang Y-K, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): long-term results from CheckMate 040. J Clin Oncol. 2021;39(Suppl 3):S269–S269. doi:10.1200/JCO.2021.39.3_suppl.269
214. Saung MT, Pelosof L, Casak S, et al. FDA Approval Summary: nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist. 2021;26(9):797–806. doi:10.1002/onco.13819
215. Parikh ND, Marshall A, Betts KA, et al. Network meta-analysis of nivolumab plus ipilimumab in the second-line setting for advanced hepatocellular carcinoma. J Comp Eff Res. 2021;10(5):343–352. doi:10.2217/cer-2020-0236
216. Wong JSL, Kwok GGW, Tang V, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9(2):e001945. doi:10.1136/jitc-2020-001945
217. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol. 2017;35(Suppl 15):S4073–S4073. doi:10.1200/JCO.2017.35.15_suppl.4073
218. Kelley RK, Sangro B, Harris W, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39(27):2991–3001. doi:10.1200/JCO.20.03555
219. Kelley RK, Sangro B, Harris WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Oncol. 2020;38(Suppl 15):S4508–S4508. doi:10.1200/JCO.2020.38.15_suppl.4508
220. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(Suppl 4):S379–S379. doi:10.1200/JCO.2022.40.4_suppl.379
221. Goyal L, Zheng H, Abrams TA, et al. A Phase II and Biomarker Study of Sorafenib Combined with Modified FOLFOX in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res. 2019;25(1):80–89. doi:10.1158/1078-0432.CCR-18-0847
222. Yau TC, Cheung FY, Lee F, et al. A multicenter phase II study of sorafenib, capecitabine, and oxaliplatin (SECOX) in patients with advanced hepatocellular carcinoma: final results of Hong Kong-Singapore Hepatocellular Carcinoma Research Collaborative Group study [abstract]. J Clin Oncol. 2013;31(Suppl 4):S4117. doi:10.1200/jco.2013.31.15_suppl.4117
223. Assenat E, et al. Sorafenib (S) alone versus S combined with gemcitabine and oxaliplatin (GEMOX) in first-line treatment of advanced hepatocellular carcinoma (HCC): final analysis of the randomized phase II; GONEXT trial (a UNICANCER/FFCD PRODIGE 10 trial)[abstract]. J Clin Oncol. 2013;31(Suppl 4):S4028. doi:10.1200/jco.2013.31.15_suppl.4028
224. Assenat E, Pageaux GP, Thezenas S, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1(st)-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer. 2019;120(9):896–902. doi:10.1038/s41416-019-0443-4
225. Yau TC, Tang V, Leung RC-Y, et al. Randomized phase II trial of sorafenib, capecitabine and oxaliplatin (SECOX) versus single agent sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2019;37(Suppl 4):S365–S365. doi:10.1200/JCO.2019.37.4_suppl.365
226. Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304(19):2154–2160. doi:10.1001/jama.2010.1672
227. Abou-Alfa GK, Shi Q, Knox JJ, et al. Assessment of Treatment With Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol. 2019;5(11):1582–1588. doi:10.1001/jamaoncol.2019.2792
228. Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44(2):343–354. doi:10.1016/j.immuni.2015.11.024
229. Garg AD, More S, Rufo N, et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6(12):e1386829. doi:10.1080/2162402X.2017.1386829
230. Qin S, Chen Z, Liu Y, et al. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol. 2019;37(Suppl 15):S4074–S4074. doi:10.1200/JCO.2019.37.15_suppl.4074
231. Garg AD, Agostinis P. Editorial: immunogenic Cell Death in Cancer: from Benchside Research to Bedside Reality. Front Immunol. 2016;1(7):110.
232. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
233. Varghese J, Kedarisetty C, Venkataraman J, et al. Combination of TACE and Sorafenib Improves Outcomes in BCLC Stages B/C of Hepatocellular Carcinoma: a Single Centre Experience. Ann Hepatol. 2017;16(2):247–254. doi:10.5604/16652681.1231585
234. Palmer DH, Malagari K, Kulik LM. Role of locoregional therapies in the wake of systemic therapy. J Hepatol. 2020;72(2):277–287. doi:10.1016/j.jhep.2019.09.023
235. Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi:10.1016/j.jhep.2018.11.029
236. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi:10.1016/S2468-1253(17)30156-5
237. Li D, Pang Y, Xu L, Xu X. Efficacy and safety of sorafenib combined with TACE in the treatment of advanced hepatocellular carcinoma: a meta-analysis. J BUON. 2021;26(4):1355–1364.
238. Xie Y, Tian H, Xiang H. Is transcatheter arterial chemoembolization plus sorafenib better than chemoembolization plus placebo in the treatment of hepatocellular carcinoma? Tumori. 2021;107(4):292–303. doi:10.1177/0300891620945029
239. Zhao RC, Zhou J, Wei YG, et al. Cost-effectiveness analysis of transcatheter arterial chemoembolization with or without sorafenib for the treatment of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2017;16(5):493–498. doi:10.1016/S1499-3872(17)60009-2
240. He M, Li Q, Zou R, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: a Randomized Clinical Trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
241. Kondo M, Morimoto M, Kobayashi S, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19(1):954. doi:10.1186/s12885-019-6198-8
242. Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–432. doi:10.1016/S2468-1253(18)30078-5
243. Wada Y, Takami Y, Matsushima H, et al. The Safety and Efficacy of Combination Therapy of Sorafenib and Radiotherapy for Advanced Hepatocellular Carcinoma: a Retrospective Study. Intern Med. 2018;57(10):1345–1353. doi:10.2169/internalmedicine.9826-17
244. Munoz-Schuffenegger P, Barry A, Atenafu EG, et al. Stereotactic body radiation therapy for hepatocellular carcinoma with Macrovascular invasion. Radiother Oncol. 2021;156:120–126. doi:10.1016/j.radonc.2020.11.033
245. Mahvash A, Murthy R, Odisio BC, et al. Yttrium-90 resin microspheres as an adjunct to sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2016;4(3):1–7. doi:10.2147/JHC.S96085
246. Inarrairaegui M, Melero I, Sangro B. Immunotherapy of Hepatocellular Carcinoma: facts and Hopes. Clin Cancer Res. 2018;24(7):1518–1524. doi:10.1158/1078-0432.CCR-17-0289
247. Jung SM, Kim JM, Choi GS, et al. Characteristics of Early Recurrence After Curative Liver Resection for Solitary Hepatocellular Carcinoma. J Gastrointest Surg. 2019;23(2):304–311. doi:10.1007/s11605-018-3927-2
248. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. doi:10.1097/01.sla.0000197706.21803.a1
249. Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin N Am. 2003;12(1):51–63. doi:10.1016/S1055-3207(02)00090-X
250. Su YY, Li CC, Lin YJ, et al. Adjuvant versus Neoadjuvant Immunotherapy for Hepatocellular Carcinoma: clinical and Immunologic Perspectives. Semin Liver Dis. 2021;41(3):263–276. doi:10.1055/s-0041-1730949
251. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
252. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv255. doi:10.1093/annonc/mdy308
253. Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer. 2012;1(3–4):226–237. doi:10.1159/000343837
254. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–1391.e6. doi:10.1053/j.gastro.2015.02.055
255. Lee JH, Lee JH, Lim YS, et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68(1):23–32. doi:10.1007/s00262-018-2247-4
256. Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology. 2016;5(3):e1083671. doi:10.1080/2162402X.2015.1083671
257. Yuan BH, Li RH, Yuan WP, et al. Harms and benefits of adoptive immunotherapy for postoperative hepatocellular carcinoma: an updated review. Oncotarget. 2017;8(11):18537–18549. doi:10.18632/oncotarget.14507
258. Lee JH, Tak WY, Lee Y, et al. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6(7):e1328335. doi:10.1080/2162402X.2017.1328335
259. Shimizu K, Kotera Y, Aruga A, et al. Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum Vaccin Immunother. 2014;10(4):970–976. doi:10.4161/hv.27678
260. Dawkins J, Webster RM. The hepatocellular carcinoma market. Nat Rev Drug Discov. 2019;18(1):13–14. doi:10.1038/nrd.2018.146
261. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi:10.1016/S1470-2045(15)00198-9
262. Huang Y, Zhang Z, Zhou Y, Yang J, Hu K, Wang Z. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy? Onco Targets Ther. 2019;2(12):541–548. doi:10.2147/OTT.S187357
263. Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol. 2012;38(4):286–295. doi:10.1016/j.ejso.2012.01.006
264. Cheng Y, Ni S, Chen Y, et al. Erzhu Qinggan Jiedu Recipe improves the clinical outcome of hepatocellular cancer after surgical resection: a case-control retrospective study. Intern Med J. 2021;51(6):853–860. doi:10.1111/imj.14844
265. Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi:10.1136/gutjnl-2018-315983
266. Zhai XF, Liu XL, Shen F, et al. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: a randomized controlled study. Cancer. 2018;124(10):2161–2168. doi:10.1002/cncr.30915
267. Bouattour M, Fartoux L, Rosmorduc O, et al. BIOSHARE multicenter neoadjuvant phase 2 study: results of pre-operative sorafenib in patients with resectable hepatocellular carcinoma (HCC)—From GERCOR IRC. J Clin Oncol. 2016;34(Suppl 4):S252–S252. doi:10.1200/jco.2016.34.4_suppl.252
268. Ye SL, Chen X, Yang J, et al. Evaluation of sorafenib in Chinese unresectable hepatocellular carcinoma patients with prior surgery and portal vein tumor thrombosis: a subset analysis of GIDEON study data. Tumour Biol. 2017;39(3):1010428317695030. doi:10.1177/1010428317695030
269. Kaseb AO, Pestana RC, Vence LM, et al. Randomized, open-label, perioperative phase II study evaluating nivolumab alone versus nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol. 2019;37(Suppl 4):S185–S185. doi:10.1200/JCO.2019.37.4_suppl.185
270. Yarchoan M, Zhu Q, Durham JN, et al. Feasibility and efficacy of neoadjuvant cabozantinib and nivolumab in patients with borderline resectable or locally advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl):335. doi:10.1200/JCO.2021.39.3_suppl.335
271. Chen X, Zhang Y, Zhang N, et al. Lenvatinib combined nivolumab injection followed by extended right hepatectomy is a feasible treatment for patients with massive hepatocellular carcinoma: a case report. Onco Targets Ther. 2019;3(12):7355–7359. doi:10.2147/OTT.S217123
272. Cheng RF, Wu ZL. Discussion of the pathogenesis theory of primary liver cancer: deficiency, depression, phlegm, blood stasis, and toxin. Shandong Zhong Yi Za Zhi. 2014;33(10):804–806.
273. Hu B, Ping SK. Yin deficiency of liver and kidney is the basis of liver cancer. Chin J Information Traditional Chin Med. 2009;16(2):93.
274. Hu Y, Wang S, Wu X, et al. Chinese herbal medicine-derived compounds for cancer therapy: a focus on hepatocellular carcinoma. J Ethnopharmacol. 2013;149(3):601–612. doi:10.1016/j.jep.2013.07.030
275. Xi SY, Minuk GY. Role of traditional Chinese medicine in the management of patients with hepatocellular carcinoma. World J Hepatol. 2018;10(11):799–806. doi:10.4254/wjh.v10.i11.799
276. Yen Y, So S, Rose M, et al. Phase I/II study of PHY906/capecitabine in advanced hepatocellular carcinoma. Anticancer Res. 2009;29(10):4083–4092.
277. Changou CA, Shiah HS, Chen LT, et al. A Phase II Clinical Trial on the Combination Therapy of PHY906 Plus Capecitabine in Hepatocellular Carcinoma. Oncologist. 2021;26(3):e367–e373. doi:10.1002/onco.13582
278. Lam W, Jiang Z, Guan F, et al. PHY906(KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of Sorafenib by changing the tumor microenvironment. Sci Rep. 2015;3(5):9384. doi:10.1038/srep09384
279. Yang X, Lam W, Jiang Z, et al. YIV-906 potentiated anti-PD1 action against hepatocellular carcinoma by enhancing adaptive and innate immunity in the tumor microenvironment. Sci Rep. 2021;11(1):13482. doi:10.1038/s41598-021-91623-3
280. Chen C, Ai QD, Wei YH. Kanglaite enhances the efficacy of cisplatin in suppression of hepatocellular carcinoma via inhibiting CKLF1 mediated NF-kappaB pathway and regulating transporter mediated drug efflux. J Ethnopharmacol. 2021;2(264):113388. doi:10.1016/j.jep.2020.113388
281. Fan Y, Li S, Ding X, et al. First-in-class immune-modulating small molecule Icaritin in advanced hepatocellular carcinoma: preliminary results of safety, durable survival and immune biomarkers. BMC Cancer. 2019;19(1):279. doi:10.1186/s12885-019-5471-1
282. Qin SK, Li Q, Ming xu J, et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: immunodynamic biomarkers and overall survival. Cancer Sci. 2020;111(11):4218–4231. doi:10.1111/cas.14641
283. Li JJ, Liang Q, Sun GC. Traditional Chinese medicine for prevention and treatment of hepatocellular carcinoma: a focus on epithelial-mesenchymal transition. J Integr Med. 2021;19(6):469–477. doi:10.1016/j.joim.2021.08.004
284. Wang X, Wang N, Cheung F, et al. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med. 2015;13(3):142–164. doi:10.1016/S2095-4964(15)60171-6
285. Yau T, Zagonel V, Santoro A, et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2020;38(Suppl 4):S478–S478. doi:10.1200/JCO.2020.38.4_suppl.478
286. Liu F, Qin L, Liao Z, et al. Microenvironment characterization and multi-omics signatures related to prognosis and immunotherapy response of hepatocellular carcinoma. Exp Hematol Oncol. 2020;1(9):10. doi:10.1186/s40164-020-00165-3
287. Angelbello AJ, Chen JL, Childs-Disney JL, et al. Using Genome Sequence to Enable the Design of Medicines and Chemical Probes. Chem Rev. 2018;118(4):1599–1663. doi:10.1021/acs.chemrev.7b00504
288. Rizzo A, Ricci AD, Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Future Oncol. 2021;17(7):755–757. doi:10.2217/fon-2020-0986
289. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
290. Rimola J, Da Fonseca LG, Sapena V, et al. Radiological response to nivolumab in patients with hepatocellular carcinoma: a multicenter analysis of real-life practice. Eur J Radiol. 2021;3(135):109484. doi:10.1016/j.ejrad.2020.109484
291. Reig M, Rimola J, Torres F, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58(6):2023–2031. doi:10.1002/hep.26586
292. Gu Y, Li X, Bi Y, et al. CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging. 2020;12(1):784–807. doi:10.18632/aging.102656
293. Huang B, Tian ZF, Li LF, et al. LHX3 is an advanced-stage prognostic biomarker and metastatic oncogene in hepatocellular carcinoma. Cancer Biomark. 2019;26(1):31–39. doi:10.3233/CBM-182257
294. Yang Y, Li G, Lu Z, et al. Progression of Prothrombin Induced by Vitamin K Absence-II in Hepatocellular Carcinoma. Front Oncol. 2021;1(11):726213. doi:10.3389/fonc.2021.726213
295. Lu LC, Hsu CH, Hsu C, Cheng AL. Tumor Heterogeneity in Hepatocellular Carcinoma: facing the Challenges. Liver Cancer. 2016;5(2):128–138. doi:10.1159/000367754
296. von Felden J, Craig AJ, Villanueva A. Role of circulating tumor DNA to help decision-making in hepatocellular carcinoma. Oncoscience. 2018;5(7–8):209–211. doi:10.18632/oncoscience.446
297. Qu C, Wang Y, Wang P, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A. 2019;116(13):6308–6312. doi:10.1073/pnas.1819799116
298. Rizzo A, Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat Res Commun. 2021;1(27):100328. doi:10.1016/j.ctarc.2021.100328
299. Piratvisuth T, Tanwandee T, Thongsawat S, et al. Multimarker Panels for Detection of Early Stage Hepatocellular Carcinoma: a Prospective, Multicenter, Case-Control Study. Hepatol Commun. 2021;1(4):1847.
300. Fuca G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457.
301. Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61(2):318–324.
302. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Association of adverse events (AEs) with efficacy outcomes for cabozantinib (C) in patients (pts) with advanced hepatocellular carcinoma (aHCC) in the phase III CELESTIAL trial. J Clin Oncol. 2019;37(Suppl 15):S4088–S4088.
303. Sung MW, Finn RS, Qin S, et al. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT). J Clin Oncol. 2019;37(Suppl 4):S317–S317.
304. Pinato DJ, Marron TU, Mishra-Kalyani PS, et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer. 2021;1(157):140–152.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
