Back to Journals » Local and Regional Anesthesia » Volume 16
Systemic Analgesia versus Continuous Erector Spinae Plane Block (ESPB) Infusion During Paediatric Nephrectomy: A Randomized, Controlled Trial
Authors Adlan S, Abd El-Rahman A , Mohamed SAB, Thabet AM , Hamada EM , Farouk BR, El Sherif FA
Received 14 March 2023
Accepted for publication 1 June 2023
Published 5 June 2023 Volume 2023:16 Pages 59—69
DOI https://doi.org/10.2147/LRA.S401980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stefan Wirz
Suzan Adlan,1 Ahmad Abd El-Rahman,2 Sahar Abdel-Baky Mohamed,2 Ahmed M Thabet,3 Eman Maghawry Hamada,1 Basma Rezk Farouk,2 Fatma Adel El Sherif2
1National Cancer Institute, Cairo University, Cairo, Egypt; 2South Egypt Cancer Institute, Assiut University, Assiut, Egypt; 3Faculty of Medicine, Assiut University, Assiut, Egypt
Correspondence: Ahmad Abd El-Rahman, South Egypt Cancer Institute, Assiut University, Assiut, Egypt, Tel +2001149606060, Fax +2 088 2348609, Email [email protected]
Purpose: A subcostal flank incision is required for open radical nephrectomy, which is a surgical procedure used to remove tumors of the kidney that are malignant. The erector spinae plane block (ESPB) and continuous catheter use in children are receiving more and more support by paediatric regional anaesthesiologists. Our objective was to compare systemic analgesic to continuous ESPB for pain relief in paediatric patients undergoing open radical nephrectomy.
Methods: Sixty children with cancer ASA I or II and undergoing open radical nephrectomy between the ages of two and seven participated in this prospective, randomized, controlled, and open label study. The cases were divided into two equal groups (E and T groups); Group E received ipsilateral continuous ultrasound-guided ESPB at T9 (thoracic vertebrae), with a bolus of 0.4 mL/kg bupivacaine 0.25%. Immediately postoperatively, Group E (ESPB group) received continuous ESPB with a PCA (patient controlled analgesia) pump at a rate of 0.2 mL/kg/hour bupivacaine 0.125%. Group T (Tramadol group), Tramadol hydrochloride was administered intravenously at a dose of 2 mg/kg/8hour, which could be increased to 2 mg/kg/6hours. Then, we followed up on patients’ total analgesic consumption for 48 hours following surgery, as well as the time it took for them to request rescue analgesic, their FLACC and sedation scores, and their hemodynamics and side effects immediately following surgery as well as at 2, 4, 6, 8, 12, 18, 24, 36, and 48 hours.
Results: A highly significant difference in total tramadol consumed in group T 11.97 ± 1.13 mg/kg while group E was 2.07± 1.54 mg/kg (p < 0.001). 100% patients in group T requested analgesia compared to 46.7% patients in group E (p < 0.001). From 2 to 48 hour, FLACC significantly decreased in E compared to T group (p≤ 0.006) at all-time points.
Conclusion: Ultrasound-guided continuous ESPB significantly provided better postoperative pain relief, reduced postoperative tramadol consumption and reduced pain scores compared with the use of tramadol alone, in paediatric cancer patients undergoing nephrectomy.
Keywords: acute pain, continuous infusion, paediatric nephrectomy, systemic analgesia, ultrasound guided erector spinae plane block, Wilms tumors
Introduction
Radical nephrectomy is a surgical procedure that uses a subcostal flank incision to remove malignant kidney tumors. In terms of perioperative analgesia, thoracic epidural injection or infusion has long been the standard method, and these patients frequently experience mild to severe pain following surgery.1 Proper analgesia is essential for early ambulation and shorter hospital stays.1
Thoracic epidural injection or infusion has been used as perioperative analgesia, for long as a standard technique. Thoracic paravertebral block (TPVB) has also been claimed to be safe and equally effective,1 but has fewer side effects, such as hypotension, urinary retention, nausea, and vomiting, compared to epidural anesthesia.2
A newly described ultrasound-guided block in the adult population is the erector spinae plane block (ESPB),3 which involves injecting local anesthetic between the erector spinae muscle and the underlying transverse process. It was initially described at the T5 level for pain in the thoracic region following thoracic surgery.4
While performing ESB technique in pediatric population there is a technical difficulty due to thinner layers of muscles and sliding fascial planes.5 But, the advancements of ultrasound technology have led to an escalation in conduct of such techniques.
ESPB for postoperative analgesia after nephrectomy for Wilms tumor was reported in two paediatric cases.5 On the other hand, there is growing evidence for ESPBs and continuous catheters usage in children.2,4,6–8 In this study, we applied this relatively new technique to the paediatric population to provide postoperative analgesia following nephrectomy conducted to manage malignant kidney tumors in children. We suggest that a continuous ultrasound-guided (US)-ESPB could enhance and prolong postoperative analgesia duration while avoiding or at least sparing opioids. Our objective was to compare the analgesic effectiveness of systemic analgesia and continuous ESPB in paediatric cancer patients undergoing open radical nephrectomy.
Patients and Methods
Enrolment and Eligibility
This prospective, randomized, open-label study was approved by the South Egypt Cancer Institute’s local ethics committee (IRB no. 515). It was carried out in the departments of paediatric oncology and anaesthesiology, and it was prospectively recorded in the trial registry on ClinicalTrials.gov (identifier: NCT04613830) between November 2020 and November 2022. It strictly adhered to the Helsinki Declaration’s regulations and amendments. Sixty-eight children with cancer ASA I or II with malignant kidney tumors scheduled for open radical nephrectomy were enrolled. Before participating in the study, written informed consent was signed by the parents or authorized legal guardian. This study adhered to the CONSORT guidelines that were applicable.
The trial did not include any patients with known sensitivities or contraindications to local anesthetics, significant organ dysfunction, cardiac dysrhythmia, congenital heart disease, mental retardation, coagulopathy, skin lesions or infections at or near the planned needle insertion site, or parents who refused to participate. On November 20th, 2020, enrollment for new patients began.
Blinding and Randomization
- Patients were divided into two groups using the random number table method, Ultrasound-guided ESPB continuous infusion group (group E, n = 30) and a tramadol group (group T, n = 30) in a 1:1 ratio.
- The anaesthesiologist received a sealed envelope containing the code number of the patient as well as the anaesthesia regimens of the two groups from a separate research investigator who was not involved in the investigation. The attending anaesthesiologist, physician and data collection personnel were blinded to the patient group assignment. Before beginning surgery, patients were divided randomly into two equal groups that are described below, following the induction of a balanced general anaesthesia and intubation.
- Ultrasound-guided ESPB continuous infusion group (Group E): After a negative aspiration, the children underwent ipsilateral ultrasound-guided ESPB at the T9 level with 0.3 mL/kg of plain bupivacaine 0.25% injected. The space was then filled with a peripheral nerve block catheter of 20 gauge size. The catheter was then adhered with an adhesive after the needle was removed. A PCA pump was used to initiate continuous local anaesthetic infusion into the ESP following surgery, with an infusion rate of 0.2 mL/kg/hour bupivacaine 0.125%, and a maximum dose of 2 mg/kg bupivacaine 0.25%, all of 1.5 mL rescue boluses and a 30 minute lockout interval were programmed. Along with the scheduled intravenous tramadol dose of up to 1 mg/kg/8 hour which could be increased to 2 mg/kg/6 hour according to the needs of the patient as a rescue analgesic.
- Tramadol group (Group T): Children received intravenous tramadol hydrochloride at a dose of 2 mg/kg/8 hours, which could be increased to 2 mg/kg/6 hours as a rescue analgesic following surgery according to the needs of the patient.
Study Protocol
- As part of a preoperative visit one day before surgery, patients were examined to assess their medical condition, conduct laboratory tests, and determine whether or not they met the aforementioned inclusion criteria.
- During the preoperative counseling, the parents were made aware of the study’s procedures.
- The FLACC score, where 0 indicates “no pain” and 10 indicate “worst possible pain”, was used to instruct the patients on how to report pain. It was requested of the parents that their children fast for two hours from water and six hours from solid food. As a sedative, all children received oral midazolam at 0.5 mg/kg 30 minutes prior to admission to the operating room.
- After the standard monitoring probes were used, an intravenous access was established by inserting a peripheral venous cannula of 22 gauge and beginning an intravenous infusion of dextrose at a concentration of 5% in 1/2 normal saline (7 mL/kg over one hour).9 Intravenous (IV) administration of propofol (2 mg/kg) and fentanyl (1 µg/kg) produced general anaesthesia. Endotracheal intubation was achieved following the administration of 0.5 mg/kg IV atracurium. In group E, ultrasound-guided ESPB was carried out, and the skin incision was carried out 15 minutes after performing the block.
- The same surgical team carried out all surgeries. As a result, all incisions were made using the same method. A combination of oxygen-air (50–50%) and sevoflurane (2%) was used to maintain anaesthesia. When the heart rate accelerated more than 20% from the original values during the procedure, the MAC of sevoflurane was increased to maintain hypnosis and prevent awareness. Analgesia was maintained with fentanyl (0.5µg/kg). Also, muscle relaxation was maintained by additional dose of atracurium (0.1 mg/kg). Adjustments were made to the ventilator settings to keep the end-tidal carbon dioxide at 36–40 mmHg.
- All patients received an intravenous infusion of 15 mg/kg paracetamol toward the end of the surgery. At the conclusion of the surgery, the inhalational sevoflurane was turned off, and a mixture of neostigmine 0.05 mg/kg and atropine 0.01 mg/kg was used to stop muscle relaxation. Full awake extubation was carried out to all patients, and they were then taken to the post-anesthesia care unit for continuation of post-anaesthesia observation.
Ultrasound-Guided Erector Spinae Plane Block (US-ESPB) Technique
- An experienced anaesthetist used a sonosite edge (Sonosite Inc., USA®) machine and high frequency (13–16 MHZ) linear transducer (Sonosite Inc. ®) to perform the ultrasound ESP block under complete aseptic conditions while the patient was sitting and under general anaesthesia. The ultrasound probe was attached to a cover sheath, lubricated with a sufficient amount of gel, and positioned in a longitudinal orientation 3 centimeters lateral to the T9 spinous process. This brought to light the trapezius, rhomboid major, and erector spinae, three muscles that were located close to the shadow of the hyperechoic transverse process. 3 mL of 2% lidocaine were used to anesthetize the skin. To insert the tip of an 18G 50-mm Tuohy needle (Pajunk, Geisinger, Germany®) into the fascial plane on the deep (anterior) aspect of the erector spinae muscle, it was inserted in-plane in a superior-to-inferior direction. After that, 2 mL of 0.9% NaCl were injected to confirm the location of the needle tip and the correct injection plane by hydro-dissection. When the needle tip made contact with the transverse process, there was visible fluid spread, lifting the erector spinae muscle off the transverse process’s bony shadow (Figure 1). After that, 0.3 mL/kg of 0.25% bupivacaine (maximum 20 mL) was injected following a negative aspiration. After that, a 20G peripheral nerve catheter was easily inserted into the space four centimeters distal to the needle tip under real-time ultrasound guidance. After the needle was taken out, the catheter was secured to the patient’s back and a Luer lock connector with an antibacterial filter. During surgery, no local analgesics were used.
- After the surgery was finished, an ultrasound-guided bolus of local anesthetic was injected through the catheter, emphasizing that its distribution covered at least two supero-inferior segments above and below the incision site.
- A PCA pump containing 0.125% bupivacaine at a rate of 0.2 mL/kg/hour and all of 1.5 mL rescue boluses and a 30 minute lockout interval were used to begin the continuous infusion of ESPB immediately after the end of surgery with a maximum dose of 2 mg/kg bupivacaine 0.25%.
Postoperative Follow-Up
Following recovery, patients were moved to the post anesthesia care unit (PACU) and were kept there for 48 hours for additional follow-up, in accordance with the following guidelines:
- The behavioral pain assessment scale known as the FLACC10 (Face, Legs, Activity, Cry, Consolability) was used to assess postoperative pain immediately following the procedure as well as at 2, 4, 6, 8, 12, 18, 24, 36, and 48 hours. If the FLACC scale score was more than or equal to 4 (≥4), rescue analgesia was administered in the form of intravenous tramadol at a dose of 2 mg/kg/8 hours, which could be increased to 2 mg/kg/6 hours depending on the needs. 15 mg/kg of paracetamol was administered additionally; if the patient’s FLACC score remained more than or equal 4 (≥4) to keep their FLACC scores less than 4.
- The duration to the initial request for rescue analgesic and the total amount of intravenous tramadol taken during the first 48 hours after surgery was observed and documented.
- The patient’s vital signs—heart rate, mean noninvasive arterial blood pressure, oxygen saturation, and respiratory rate—were obtained immediately following surgery in addition to at 2, 4, 6, 8, 12, 18, 24, 36, and 48 hours.
- A Ramsay sedation scale ranging from 1 to 6 was used to measure the level of sedation at the aforementioned time points. Anxious, agitated, or both; 2: tranquil, focused, and cooperative; 3: only in response to commands; 4: responds quickly to a light glabellar tap or an audible stimulus; 5: slow response to a light glabellar tap or a loud sound; 6: no response to a light glabellar tap or a loud auditory stimulus).11
- Postoperative complications such as bradycardia (HR 60 bpm), hypotension (MAP 20% of original values), respiratory depression, and constipation were observed and recorded.
Statistical Analysis
Power of the Study
Our primary outcome was how much tramadol was needed for 48 hours after surgery to keep the FLACC score below 4, and our secondary outcomes were time to first request analgesia, number of patients requesting rescue analgesic, FLACC score, postoperative mean arterial blood pressure, heart rate, respiratory rate, oxygen saturation, sedation score, and potential untoward effects like nausea and vomiting after surgery, allergic reactions, arrhythmias, and respiratory depression.
The G*power software version 3.1.9.2 (Kiel University, Kiel, Germany),12 was used to calculate the sample size. A pilot study was conducted with 10 patients in each group because there were no previously published data on this topic. No patient in group E required any rescue analgesia. The mean total amount of tramadol consumption in the first 48 hours was (11.90 ± 1.29 mg/kg) in group T versus (2.30 ±1.77 mg/kg) in group E with 80% power at an alpha error value of 0.05 and a 95% confidence interval, four patients per group were sufficient to reject the null hypothesis (to guarantee normal distribution, 30 patients were included in each arm).
Statistical Tests
SPSS (the statistical package for the social sciences) was used for all of the statistical analysis, version 22 (SPSS Inc., Chicago, IL, USA®). Quantitative data were statistically described in terms of mean ± SD and median (range) when not normally distributed. Qualitative data were statistically described in terms of frequencies (number of cases) and relative frequencies (percentages) when appropriate. Comparison of quantitative variables was done using Student’s t-test for normally distributed data and Mann Whitney U-test for non-normally distributed data. For comparing categorical data, Chi square (χ2) test was performed. Fisher Exact test was used instead when the expected frequency is less than 5. P-value is always 2 tailed set significant < 0.05 level.
Results
Participant Flow
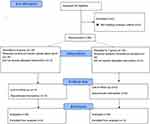
The eligibility of 68 paediatric cancer patients who underwent radical nephrectomy for malignant kidney tumors was evaluated; five patients’ parents refused to participate, and three patients did not meet the inclusion criteria. At the end, the study included 60 patients who were divided into two groups of 30 patients each. Figure 2 depicts the participants’ flowchart for the study.
Baseline and Clinical Data
Age, sex, weight, height, BMI, ASA, duration of surgery, anesthesia, and recovery revealed no significant differences between the two groups (P > 0.05) (Table 1).
 |
Table 1 Demographic and Clinical Data of the Studied Participants (n=60) |
Primary Outcome
On kilogram body weight-bases; the mean (±SD) total tramadol consumption in group T was (11.97 ± 1.13 mg/kg), while the ESPB group’s consumption was (2.07 ± 1.54 mg/kg), with a highly significant difference (P <0.001). Thus, calculating the overall consumption of tramadol in both groups in the 48 hours study period, showed significantly higher consumption in group T (197.20 ± 47.29 mg) than in group E (31.19 ± 24.32 mg) (P <0.001).
Secondary Outcomes
The median (range) number of first requests for analgesia in group E was 4 (2–12) compared to 4 (0–8) in group T (P=0.252) and the number of patients who requested analgesia in group T was 30 (100%) compared to 14 (46.7%) patients in group E (P<0.001) (Table 2).
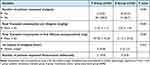 |
Table 2 Total Tramadol Consumption, 1st Request of Analgesia and Number of Patients Requested Analgesic Between Both Studied Groups (n=60) |
When the FLACC scores of the two groups were put into comparison, group E had a significant lower from 2 hours to 48 hours (P ≤ 0.006) (Figure 3), and moreover group T had three patients who required additional paracetamol as rescue analgesic, while group E had none (Table 2).
The sedation score did not differ significantly between the two groups studied (P > 0.05).
Hemodynamic variables like heart rate mean arterial blood pressure, respiratory rate, and oxygen saturation did not differ significantly between the two groups at any time (P > 0.05).
In terms of the postoperative side effects, eight patients in group T experienced nausea and vomiting, whereas one patient in group E did (P = 0.026). In addition, no patients developed any other side effects (hypotension, arrhythmias, or allergic reactions) during the intra- or post-operative period, and there were no complications associated with the block (Table 3).
 |
Table 3 Side Effects Observed During the Study Period in Both Studied Groups (n=60) |
Discussion
The highly significant difference in the need for postoperative rescue analgesia between the two groups in this randomized prospective trial on continuous ESPB in paediatric patients demonstrated that it resulted in sufficient postoperative analgesia for patients undergoing open radical nephrectomy. From 2 to 48 hours postoperatively, the FLACC score in group E remained significantly lower, indicating a longer duration of analgesia without the onset of serious untoward effects. During the course of the study, 16 patients in group E did not make a request for rescue analgesia. On the other hand, 30 patients in group T made requests for rescue analgesic doses, including three requests for paracetamol additionally. This is a strong indicator of the US-ESPB’s longer duration of analgesia.
To our knowledge, our study is the first prospective randomized trial to investigate the efficacy of ultrasound guided continuous ESP local anesthetic infusion compared to systemic analgesia alone for postoperative analgesia in children undergoing open radical nephrectomy. There are limited data published as randomized controlled trials applying continuous ESPB in paediatric patients for lower abdominal surgery.13
Due to the large subcostal flank cut wound that is typically used to provide a roomy surgical field, management of pain following open nephrectomy is a hard task. Because open nephrectomy necessitates extensive muscle cutting, this somatic pain constitutes 70–75% of the postoperative pain and lasts for 72 hours,14,15 in addition to visceral pain that is transmitted through sympathetic fibers (T8–T12 spinal segment), which is severe but usually lasts 24–36 hours.14,16
Poor postoperative analgesia leads to many consequences, including restricted ambulation and movement, painful coughing with an increased risk of developing pneumonia, thromboembolism, wound dehiscence, chronic pain development, and prolonged recovery time.17
Other local anesthetic implementing-techniques have been used in paediatric age groups for lower abdominal surgery, including the caudal block, subcostal approach of the transversus abdominis plane block, quadratus lumborum block, and isolated ilioinguinal nerve block.18 However, each method has advantages and disadvantages.18
One new block being tested for lower abdominal surgery is ESPB. A local anesthetic is placed in the plane between the transverse process and the erector spinae muscle during this inter-fascial plane block. Based on cadaveric and contrast studies, it is anticipated to act at the spinal nerve origin.19,20 The ESP block is performed with guidance from ultrasonography. The transverse process is the target because it is close to easily-identifiable nerves and vessels and the pleura and is easy to identify. It has the advantage of providing extensive analgesia with just one puncture. As a result, it is possible to carry out the block at upper or lower levels that are, to some extent, far from the surgical zone. This allows you to steer clear of local issues that might suggest that the puncture should be made at a particular location. The foreward spread of the injectate into the paravertebral and epidural space is thought to be a significant advantage of the ESP block over other inter-fascial blocks for abdominal procedures.21 This would block not only the spinal nerve roots but also rami communicants transmitting to sympathetic fibers, thus leading to relief from the associated visceral pain.21 In contrast, some clinical trials have shown that LA distribution after ESPB can cover 2–5 epidural and 5–9 intercostal levels.20 Paediatric regional anaesthesiologists have supported the use of ESP blocks due to their relative ease of performance and potential safety over neuraxial techniques.22 For instance, the thoracic paravertebral block is associated with more risk of pneumothorax.23,24 On the other hand, thoracic epidural analgesia is associated with a drop in blood pressure in many occasions due to cardio depressant effect as well as arterial and venous vasodilation. In either group of our study, hypotension did not affect any of our patients.
Many promising studies which concluded that ESPB provides better postoperative analgesia than placebo have been published.7 However, the majority of these studies applied the single-shot technique in different surgeries.25,26
ESPB is simple to perform, it also provides a chance of continued nerve blockade through a catheter placed in the same plane.27 Continued nerve blockades enforce postoperative analgesia, with faster recovery and more patient satisfaction.28 Recently, several case series with continued ESPB were performed.29,30 In these case series, improved analgesia was reported, but they did not compare these regimens with intravenous PCA in a randomized controlled trial.
The advantages of the multimodal analgesia approach include better analgesia with the use of lower doses of each drug, early return of organ functions, and thus earlier recovery and shorter hospital stay.31
It takes privilege of the additive effect created by the different medications used to provide better pain control. Considering multimodal approach, opioids are only used in small amounts to avoid side effects like constipation, itching, nausea, vomiting, slowed breathing, urinary retention, sedation, and drowsiness.32 We administered continuous infusion of 0.25% bupivacaine at a rate of 0.1 mL/kg/h via a catheter placed to provide continuous blockade for 48 hours. This continuous method is easier and safer than one-shot methods. The total systemic analgesic consumption and the associated opioid-related adverse events decreased significantly in the 48 hours- period when ESPB continuous infusion was compared to IV opioid. In addition, only 14 patients requested analgesia during this time, demonstrating the technique’s efficacy in these pediatric cancer patients by prolonging the time it took to first request of rescue analgesia, decreasing the number of rescue analgesics used, and reducing side effects without affecting hemodynamic stability.
Our findings are consistent with those of Aksu and Gurkan,5 who examined the efficacy of US-guided continued ESB for postoperative pain management in two children with Wilms tumor undergoing nephrectomy. In addition, the findings of this study could be compared to those of Piskin et al33 who came to the conclusion that as part of multimodal analgesia, US-guided continued ESPB provided adequate analgesia following video assisted thoracoscopies (VATS). When compared to the control group, continuous ESPB markedly reduced narcotic-analgesic consumption and its associated untoward effects.
The use of continued ESPB as part of opioid-saving postoperative pain management following VATS lobectomy was also reported by Scimia et al.34 The patients requested no additional opioids and experienced loss of cutaneous sensation in the T2-10 dermatomes. They also said that the continuous method was safe and easy to use. The use of this block for postoperative pain management in a patient who had surgery for multiple rib fractures was reported by Yayik et al.35 They reported a loss of cold sensation over the T2–9 dermatomes with an injection of 20 mL of 0.25% bupivacaine and continuous administration of 0.25% bupivacaine.
In two paediatric thoracotomy surgeries, Kumari et al36 came to a conclusion similar to ours that continuous ESP block supplementary to general anesthesia provided improved surgical analgesia and satisfactory postoperative pain control. In addition, Bhoi et al2 mentioned in a case report that this block was effective as an analgesic and might avoid the side effects of continuous neuraxial block. In addition, Bakshi et al6 found that in two pediatric patients undergoing thoracic surgery who had spread intraspinal disease, continuous ESP block was effective in preventing pulmonary complications.
In our study, no potential problems were found. Continuous ESPB, on the other hand, necessitates the infusion of local anesthetics into the paraspinal tissues, which are located away from the pleura and neuraxial structures. Despite the ESPB is considered as being safer than other similar fascial plane blocks, complications such as pneumothorax and hematoma may occur as a result of this technique.7 As a result, complications and tissue damage are unlikely.
Patient satisfaction regarding the presence of a catheter on their back for 48 hours, which may result in discomfort, was not evaluated in this study when the catheter was removed at the conclusion of the study. We will keep this in mind when conducting subsequent studies utilizing catheters for continuous postoperative infusions. This may be one of the limitations of our study. Because performing a block with no expected benefit other than the placebo effect is against ethical and regulatory protocols, this study also lacked blinding due to these concerns.
In conclusion, Ultrasound-guided continuous ESPB significantly provided better postoperative pain relief, reduced postoperative tramadol consumption and reduced pain scores compared with the use of tramadol alone, in paediatric cancer patients undergoing nephrectomy.
Data Sharing Information
Raw data (de-identified) used in this clinical trial are available from the corresponding author Dr. Ahmad Mohammad Abd El-Rahman. It will be available following publication and for a period of one year.
Acknowledgments
We are grateful to the operating theatre and SICU staff for their co-operation in data collection.
Disclosure
The authors declare no conflicts of interest.
References
1. Canturk M. Lumbar erector spinae plane block for postoperative analgesia after nephrectomy followed by emergent complication surgery. Minerva Anestesiol. 2019;85:1032–1033. doi:10.23736/S0375-9393.19.13663-2
2. Bhoi D, Acahrya P, Talawar P, Malviya A. Continuous erector spinae plane local anesthetic infusion for perioperative analgesia in pediatric thoracic surgery. Saudi J Anaesth. 2018;12:502–503. doi:10.4103/sja.SJA_243_18
3. Polaner DM, Taenzer AH, Walker BJ, et al. Pediatric Regional Anesthesia Network (PRAN): a multi-institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesth Analg. 2012;115:1353–1364. doi:10.1213/ANE.0b013e31825d9f4b
4. De la Cuadra-Fontaine JC, Concha M, Vuletin F, Arancibia H. Continuous erector spinae plane block for thoracic surgery in a pediatric patient. Paediatr Anaesth. 2018;28:74–75. doi:10.1111/pan.13277
5. Aksu C, Gürkan Y. Ultrasound guided erector spinae block for postoperative analgesia in pediatric nephrectomy surgeries. J Clin Anesth. 2018;45:35–36. doi:10.1016/j.jclinane.2017.12.021
6. Bakshi SG, Awaskar S, Qureshi SS, Gala K. Continuous erector spinae plane block in pediatric patients with intraspinal tumors – case reports. J Anaesthesiol Clin Pharmacol. 2020;36:558–560. doi:10.4103/joacp.JOACP_14_20
7. Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53:29–34. doi:10.1016/j.jclinane.2018.09.036
8. Munshey F, Rodriguez S, Diaz E, Tsui B. Continuous erector spinae plane block for an open pyeloplasty in an infant. J Clin Anesth. 2018;47:47–49. doi:10.1016/j.jclinane.2018.03.015
9. Abduallah MA, Al-Ahwal LA, Ahmed SA. Effect of erector spinae plane block on postoperative analgesia after pediatric Hip surgery: randomized controlled study. Pain Pract. 2022;22:440–446. doi:10.1111/papr.13099
10. Willis MH, Merkel SI, Voepel-Lewis T, Malviya S. FLACC behavioral pain assessment scale: a comparison with the child’s self-report. Pediatr Nurs. 2003;29:195–198.
11. Sessler CN, Grap MJ, Ramsay MAE. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(Suppl 3):S2. doi:10.1186/cc6148
12. Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/BF03193146
13. Aksu C, Şen MC, Akay MA, Baydemir C, Gürkan Y. Erector spinae plane block vs quadratus lumborum block for pediatric lower abdominal surgery: a double blinded, prospective, and randomized trial. J Clin Anesth. 2019;57:24–28. doi:10.1016/j.jclinane.2019.03.006
14. Niraj G, Tariq Z. Continuous erector spinae plane (ESP) analgesia in different open abdominal surgical procedures: a case series. J Anesth Surg. 2018;5(1):57–60. doi:10.15436/2377-1364.18.1853
15. Chapman E, Pichel AC. Anesthesia for nephrectomy. BJA Educ. 2016;16(3):98–101. doi:10.1093/bjaceaccp/mkv022
16. Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi:10.1016/0304-3959(90)90021-5
17. Alper I, Yuksel E. Comparison of acute and chronic pain after open nephrectomy versus laparoscopic nephrectomy: a prospective clinical trial. Medicine. 2016;95(16):e3433. doi:10.1097/MD.0000000000003433
18. Ponde V. Recent trends in pediatric regional anesthesia. Indian J Anaesth. 2019;63:746–753. doi:10.4103/ija.IJA_502_19
19. Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43:567–571. doi:10.1097/AAP.0000000000000789
20. Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical study. Reg Anesth Pain Med. 2018;43:756–762. doi:10.1097/AAP.0000000000000798
21. Singh S, Jha RK, Sharma M. The analgesic effect of bilateral ultrasound-guided erector spinae plane block in paediatric lower abdominal surgeries: a randomized, prospective trial. Indian J Anaesth. 2020;64:762–767. doi:10.4103/ija.IJA_630_20
22. Munshey F, Caruso TJ, Wang EY, Tsui BCH. Programmed intermittent bolus regimen for erector spinae plane blocks in children: a retrospective review of a single-institution experience. Anesth Analg. 2020;130(3):e63–e66. PMID: 30252704. doi:10.1213/ANE.0000000000003817
23. Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56:
24. Naja Z, Ziade MF, Lönnqvist PA. Bilateral paravertebral somatic nerve block for ventral hernia repair. Eur J Anaesthesiol. 2002;19:
25. Tulgar S, Aydin ME, Ahiskalioglu A, De Cassai A, Gurkan Y. Anesthetic techniques: focus on lumbar erector spinae plane block. Local Reg Anesth. 2020;2020(13):121–133. doi:10.2147/LRA.S233274
26. Aksu C, Gurkan Y. Opioid sparing effect of erector spinae plane block for pediatric bilateral inguinal hernia surgeries. J Clin Anesth. 2018;50:62–63. doi:10.1016/j.jclinane.2018.06.048
27. Ciftci B, Ekinci M, Celik EC, Tukac IC, Bayrak Y, Atalay YO. Efficacy of an ultrasound-guided erector spinae plane block for postoperative analgesia management after video-assisted thoracic surgery: a prospective randomized study. J Cardiothorac Vasc Anesth. 2020;34(2):444–449. doi:10.1053/j.jvca.2019.04.026
28. Liu SS, Salinas FV. Continuous plexus and peripheral nerve blocks for postoperative analgesia. Anesth Analg. 2003;96(1):263–272. doi:10.1097/00000539-200301000-00053
29. Leyva FM, Mendiola WE, Bonilla AJ, Cubillos J, Moreno DA, Chin KJ. Continuous erector spinae plane (ESP) block for postoperative analgesia after minimally invasive mitral valve surgery. J Cardiothorac Vasc Anesth. 2018;32(5):2271–2274. doi:10.1053/j.jvca.2017.12.020
30. Nath S, Bhoi D, Mohan VK, Talawar P. USG-guided continuous erector spinae block as a primary mode of perioperative analgesia in open posterolateral thoracotomy: a report of two cases. Saudi J Anaesth. 2018;12(3):471–474. doi:10.4103/sja.SJA_755_17
31. Baytar MS, Yılmaz C, Karasu D, Ç B. Comparison of ultrasonography guided serratus anterior plane block and thoracic paravertebral block in video-assisted thoracoscopic surgery: a prospective randomized double-blind study. Korean J Pain. 2021;34(2):234–240. doi:10.3344/kjp.2021.34.2.234
32. Horn A, Kaneshiro K, Tsui BCH. Preemptive and preventive pain psychoeducation and its potential application as a multimodal perioperative pain control option: a systematic review. Anesth Analg. 2020;130(3):559–573. doi:10.1213/ANE.0000000000004319
33. Pişkin Ö, Gökçe M, Altınsoy B, et al. Effects of continuous erector spinae plane block on postoperative pain in video-assisted thoracoscopic surgery: a randomized controlled study. Gen Thorac Cardiovasc Surg. 2022;70:64–71. doi:10.1007/s11748-021-01687-1
34. Scimia P, Basso Ricci E, Droghetti A, Fusco P. The ultrasound guided continuous erector spinae plane block for postoperative analgesia in video-assisted thoracoscopic lobectomy. Reg Anesth Pain Med. 2017;42(4):537. doi:10.1097/AAP.0000000000000616
35. Yayik AM, Ahiskalioglu A, Celik EC, Ay A, Ozenoglu A. Continuous erector spinae plane block for postoperative analgesia of multiple rib fracture surgery: case report. Rev Bras Anestesiol. 2019;69(1):91–94. doi:10.1016/j.bjan.2018.08.001
36. Kumari P, Kumar A, Sinha C, Kumar A, Ghosh S. Continuous Erector Spinae plane block in paediatric patient undergoing thoracotomy surgery. Saudi J Anaesth. 2022;16:253–254. doi:10.4103/sja.sja_820_21
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.