Back to Journals » Patient Preference and Adherence » Volume 9
Systematic review and meta-analysis of prospective studies for ECP treatment in patients with steroid-refractory acute GVHD
Authors Zhang H, Chen R, Cheng J, Jin N, Chen B
Received 29 October 2014
Accepted for publication 18 November 2014
Published 17 January 2015 Volume 2015:9 Pages 105—111
DOI https://doi.org/10.2147/PPA.S76563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Hongming Zhang, Runzhe Chen, Jian Cheng, Nan Jin, Baoan Chen
Department of Hematology and Oncology (Key Department of Jiangsu Medicine), Zhongda Hospital, Medical School, Southeast University, Nanjing, Jiangsu Province, People’s Republic of China
Purpose: The aim of this systematic review was to evaluate the efficacy and safety of extracorporeal photopheresis (ECP) treatment in patients with steroid-refractory acute graft-versus-host disease (SR-aGVHD).
Methods: An electronic search was carried out on the MEDLINE, EMBASE, Science Citation Index (SCI), and Cochrane Library databases. We included prospective clinical trials in SR-aGVHD treated by ECP. The main endpoints consisted of mortality, exacerbation, or response.
Results: Only seven studies involving 121 patients met the inclusion criteria for further review. Our analysis showed positive results of ECP for aGVHD. The overall response rate (ORR) was 0.71 and the complete response rate (CRR) was 0.71. The efficacy of ECP for skin aGVHD, liver aGVHD, and gut aGVHD were 0.86, 0.60, and 0.68, respectively. However, no sufficient evidence verifies the exact benefit in this review, because the number of patients enrolled in trials is limited and publish bias exists.
Conclusion: ECP is an effective therapy for skin, liver, and gut aGVHD, and large double-blind clinical trials are required to prove the outcome of this meta-analysis.
Keywords: extracorporeal photopheresis, steroid-refractory acute graft-versus-host disease, allogeneic hematopoietic stem cell transplantation
Introduction
Acute graft-versus-host disease (aGVHD) after allogeneic hematological stem cell transplantation (allo-HSCT) remains the leading cause for early morbidity and mortality.1,2 Despite prophylaxis, International Bone Marrow Transplantation Registry severity index grade B–D acute GVHD still occurs in 39%–59% of patients undergoing T-cell-replete related or unrelated donor allo-HSCT.3,4 Corticosteroids are the cornerstone of initial therapy effective in 25% to 69% of patients; however, if patients do not respond to steroids, they will have an unfavorable prognosis, with poor survival.4,5
Extracorporeal photopheresis (ECP) is currently being used for the treatment of cutaneous T-cell lymphoma, selected autoimmune diseases, and rejection after solid organ transplantation.6–8 It is based on the infusion of autologous peripheral blood mononuclear cells collected by apheresis, incubated with the photoactive drug 8-methoxypsoralen (8-MOP) and ultraviolet (UV)-A irradiation.9 These years, ECP has been confirmed to be an effective therapy for acute GVHD in patients who are unresponsive to first-line treatment with corticosteroids and calcineurin inhibitors, though the definition of steroid-refractory aGVHD (SR-aGVHD) has not been systemically defined. At present, the results of ECP treatment have been reported only in a small number of patients with SR-aGVHD and the effect of ECP treatment has been contradictory for the published studies. Herein, we performed a systematic review of the literature and meta-analysis of all known prospective trials to test if ECP provides advantages in achievement of the SR-aGVHD.
Materials and methods
Evidence retrieval
Prospective studies examining the role of ECP in the treatment of aGVHD were reviewed. We searched the following databases: MEDLINE, EMBASE, Science Citation Index (SCI), and the Cochrane Library on 25 October, 2014 according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.10 The keywords of our search were “extracorporeal photopheresis”, “extracorporeal photochemotherapy”, “extracorporeal photoimmunotherapy”, “photopheresis”, “ECP”, or “PUVA therapy” pairing independently with “graft-versus-host disease” or “GVHD”. In addition, we searched again for possible included studies. Languages were not restricted to prevent publication bias.
Study selection
Two independent investigators executed the trial selection independently. Disagreements were settled by consensus or by seeking an independent third viewpoint. Studies of ECP with a minimum of five patients were included, and for those studies that included both aGVHD and cGVHD (chronic GVHD), only the studies with enough patients with aGVHD were analyzed. Case reports, review articles, and studies with fewer than five patients were also excluded (Figure 1).
Validity assessment and data extraction
Two reviewers independently selected studies by examining titles and abstracts to determine those potentially relevant to our study question. Reported results of these identified studies were further analyzed for inclusion. Disagreement was settled by discussion and review of the articles. The quality of included noncomparative cohort studies was assessed by the Newcastle–Ottawa scale modified for single-arm cohorts.11
Statistical analysis
All statistical calculations were implemented with STATA software (v12.0; Stata Corporation, College Station, TX, USA). We used pooled relative risk (RR) to assess the efficacy of ECP therapy with 95% confidence intervals (CIs); P<0.05 was considered statistically significant. We estimated odds ratios with their 95% CIs using the standardized mean difference (SMD). Heterogeneity was evaluated with I2 values. Random-effects models were used to evaluate the included studies regardless of heterogeneity.
Results
Study screening, essential characteristics, and methodological qualities in enrolled trials
Our search yielded 518 studies that described ECP in the treatment of GVHD (Figure 1). After their titles and abstracts were scanned, 272 trials were not eligible for this present meta-analysis. One hundred and eighty-two studies were excluded based on the following criteria: not a clinical study, not involving ECP for aGVHD, and not being full articles. Finally, seven studies involving 121 patients met our inclusion criteria for further review; the design features and participant characteristics of these studies are presented in Table 1. The overall quality of these nine studies was moderate according to the Newcastle–Ottawa scale11 as shown in Table 2.
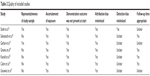  | Table 2 Quality of included studies |
Overall response rate and complete response rate
Overall response rate (ORR, partial response rate plus complete response rate [CRR]) data were extracted from six studies (62 patients).12–17 High heterogeneity was not found between these studies (I2=44.1%). The pooled proportion of ORR was 0.71 (95% CI: 0.54–0.89, P=0.147; Figure 2). Data on the CRR were extracted from five studies (101 patients).12,13,15,16,18 The heterogeneity between the studies was not high (I2=38.5%). The pooled proportion of CR was 0.71 (95% CI: 0.58–0.84, P=0.181; Figure 3).
Efficacy of ECP for skin SR-aGVHD
RRs were available for six studies13–18 (104 patients) with existing relevant data. The pooled RR was 0.86 (95% CI: 0.79–0.93, P=0.716; Figure 4A). The funnel plot was extremely asymmetrical, which means that publication bias of the included studies exists (Figure 4B).
Efficacy of ECP for liver SR-aGVHD
RRs were available for six studies13–18 (43 patients) with existing relevant data. The pooled RR was 0.60 (95% CI: 0.44–0.76, P=0.563; Figure 5A). The funnel plot was not very symmetrical, which means that publication bias of the included studies exists (Figure 5B).
Efficacy of ECP for gut SR-aGVHD
RRs were available for six studies13–18 (52 patients) with existing relevant data. The pooled RR was 0.68 (95% CI: 0.55–0.82, P=0.780; Figure 6A). The funnel plot was asymmetrical, which means that publication bias of the included studies exists (Figure 6B).
Highlight of this meta-analysis
Many studies including some meta-analyses19,20 have been conducted to evaluate its effect for GVHD. However, this was the first meta-analysis of prospective studies to date only analyzing the role of ECP in the treatment of SR-aGVHD. Though the same patients were already reported in other review studies, we only include those patients (n=121) with aGVHD compared with other studies.
Discussion
ECP is a therapy widely used for T-cell lymphoma, mycosis fungoides, Sézary syndrome, GVHD, and other diseases.21,22 Acute GVHD is defined by GVHD starting within the first 100 days after transplantation, which is a complex interplay of donor T-cells and host antigen-presenting cells and B-cells.4 Acute GVHD remains the leading cause for early morbidity and mortality with symptoms that include skin rash and desquamation, liver dysfunction, and diarrhea.2 Treatment of steroid-refractory GVHD, especially SR-aGVHD, has been a challenge over the past 20 years.23 In this comprehensive meta-analysis, we evaluate the efficacy of ECP treatment in SR-aGVHD. Our analysis indicated that though some side effects exist, ECP is a suitable option for patients with SR-aGVHD, and is effective in a remarkable proportion of patients. For organ-specific response, the response of skin (0.86) was the highest, followed by gut (0.68), and liver (0.60).
It was clear that the reports we included had many deficiencies, so limitations associated with this meta-analysis and our selected studies must be noted, with the most important being the absence of uniform criteria for assessment of SR-aGVHD: the definition of SR-aGVHD varies according to each study. As a result, no general recommendation can be made on ECP treatment schedule; this meant that almost every study we included had different ECP starting criteria, treating regimens, and protocols. Because of the different definitions of SR-aGVHD, the criteria differ for treating with ECP in the included seven studies. Additionally, the precision of pooled effect size is affected by the small sample size of the included studies, so we had to use a random-effects instead of fixed-effects model for all the studies to increase power and precision regardless of heterogeneity. No randomized controlled trials were identified during our literature search, so our evidence of the efficacy of ECP remains insufficient.
In summary, the beneficial effect of ECP in the treatment of SR-aGVHD should be further studied with uniform treating criteria and under the context of large multicenter randomized trials to document its effect.
Acknowledgments
This work was supported by the National Natural Science Foundation of the People’s Republic of China (Grant no 81170492, 81370673), National High Technology Research and Development Program 863 of the People’s Republic of China (Grant no 2012AA022703), National Key Basic Research Program 973 of the People’s Republic of China (Grant no 2010CB732404), Key Medical Projects of Jiangsu Province (Grant no BL2014078), and Key Discipline of Jiangsu Province (2011–2015).
Disclosure
The authors report no conflicts of interest in this work.
References
Bolaños-Meade J, Vogelsang GB. Novel strategies for steroid-refractory acute graft-versus-host disease. Curr Opin Hematol. 2005;12(1):40–44. | ||
Wolff D, Ayuk F, Elmaagacli A, et al. Current Practice in diagnosis and treatment of acute graft-versus-host disease: results from a survey among German-Austrian-Swiss hematopoietic stem cell transplant centers. Biol Blood Marrow Transplant. 2013;19(5):767–776. | ||
Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. | ||
Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. | ||
Jagasia M, Greinix H, Robin M, et al. Extracorporeal photopheresis versus anticytokine therapy as a second-line treatment for steroid-refractory acute GVHD: a multicenter comparative analysis. Biol Blood Marrow Transplant. 2013;19(7):1129–1133. | ||
Greinix HT, Volc-Platzer B, Kalhs P, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96(7):2426–2431. | ||
Bladon J, Taylor PC. Extracorporeal photopheresis in cutaneous T-cell lymphoma and graft-versus-host disease induces both immediate and progressive apoptotic processes. Br J Dermatol. 2002;146(1):59–68. | ||
Chiesa-Fuxench ZC, González-Chávez J. Extracorporeal photopheresis: a review on the immunological aspects and clinical applications. P R Health Sci J. 2010;29(4):337–347. | ||
Heshmati F. Mechanisms of action of extracorporeal photochemotherapy. Transfus Apher Sci. 2003;29(1):61–70. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. | ||
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Proceedings of the Third Symposium on Systematic Reviews Beyond the Basics: Improving Quality and Impact. 2010;7:3–5. | ||
Smith EP, Sniecinski I, Dagis AC, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs-host disease. Biol Blood Marrow Transplant. 1998;4(1):27–37. | ||
Salvaneschi L, Perotti C, Zecca M, et al. Extracorporeal photochemotherapy for treatment of acute and chronic GVHD in childhood. Transfusion. 2001;41(10):1299–1305. | ||
Garban F, Drillat P, Makowski C, et al. Extracorporeal chemophototherapy for the treatment of graft-versus-host disease: hematologic consequences of short-term, intensive courses. Haematologica. 2005;90(8):1096–1101. | ||
Kanold J, Merlin E, Halle P, et al. Photopheresis in pediatric graft-versus-host disease after allogeneic marrow transplantation: clinical practice guidelines based on field experience and review of the literature. Transfusion. 2007;47(12):2276–2289. | ||
Calore E, Calo A, Tridello G, et al. Extracorporeal photochemotherapy may improve outcome in children with acute GVHD. Bone Marrow Transplant. 2008;42(6):421–425. | ||
Ussowicz M, Musiał J, Mielcarek M, et al. Steroid-sparing effect of extracorporeal photopheresis in the therapy of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplant Proc. 2013;45(9):3375–3380. | ||
Greinix HT, Knobler RM, Worel N, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91(3):405–408. | ||
Abu-Dalle I, Reljic T, Nishihori T, et al. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies. Biol Blood Marrow Transplant. 2014;20(11):1677–1686. | ||
Malik MI, Litzow M, Hogan W, et al. Extracorporeal photopheresis for chronic graft-versus-host disease: a systematic review and meta-analysis. Blood Res. 2014;49(2):100–106. | ||
Taverna F, Coluccia P, Arienti F, et al. Biological quality control for extracorporeal photochemotherapy: assessing mononuclear cell apoptosis levels in ECP bags of chronic GvHD patients. J Clin Apher. Epub 2014 Sep 16. | ||
Garban F, Carras S, Drillat P, et al. Extracorporeal photopheresis as a curative treatment strategy in non epidermotropic T-cell lymphoma and large granular lymphocyte leukemia. Ann Oncol. 2012;23(9):2386–2390. | ||
Perfetti P, Carlier P, Strada P, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42(9):609–617. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.