Back to Journals » Drug Design, Development and Therapy » Volume 14
Synthesis, Characterization, and Pharmacodynamics Study of Enrofloxacin Mesylate
Authors Pei L, Yang W, Fu J, Liu M, Zhang T, Li D, Huang R, Zhang L, Peng G, Shu G, Yuan Z , Lin J, Zhang W, Zhong Z, Zhao L, Fu H
Received 19 November 2019
Accepted for publication 10 February 2020
Published 24 February 2020 Volume 2020:14 Pages 715—730
DOI https://doi.org/10.2147/DDDT.S239307
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Lin-lin Pei,1,* Wen-zhu Yang,1,* Jing-yuan Fu,1,* Meng-xi Liu,1 Ting-ting Zhang,1 Dong-bo Li,1 Ruo-yue Huang,1 Li Zhang,1 Guang-neng Peng,1 Gang Shu,1 Zhi-xiang Yuan,2 Ju-chun Lin,1 Wei Zhang,1 Zhi-jun Zhong,1 Ling Zhao,1 Hua-lin Fu1
1Department of Pharmacy, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, Sichuan, People’s Republic of China; 2College of Pharmacy, Southwest Minzu University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua-lin Fu
Department of Pharmacy, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, Sichuan 611130, People’s Republic of China
Tel +86 028-86291162
Email [email protected]
Introduction: Enrofloxacin is used in the treatment of a wide variety of bacterial infections in mammals. However, its poor solubility limits the clinical use.
Methods: In order to improve the solubility of enrofloxacin, the enrofloxacin mesylate (EM) were obtained by a chemical synthesis method. The characterization of EM was carried out using ultraviolet scan (UV), synchronous thermal analysis (SDT), fourier transform infrared spectrometer (FTIR) and mass spectrometry (MS), nuclear magnetic resonance (NMR) and X-ray powder diffraction analysis (XRPD). Acute toxicity of EM in Kunming mice was studied. Besides, pharmacokinetic studies were performed in New Zealand rabbits at a single oral dose of 10 mg/kg, and the antibacterial activity of EM was also evaluated.
Results: EM was successfully synthesized and purified. The stoichiometric ratio of mesylate to enrofloxacin was 1:1 and the aqueous solubility of EM was 483.01± 4.06 mg/mL, the solubility of EM was about 2000 times higher than enrofloxacin. The oral lethal dose (LD50) of EM was 1168.364 mg/kg, and the pharmacokinetics indicated that the oral relative bioavailability of EM was about 1.79 times and 1.48 times higher than that of enrofloxacin and enrofloxacin hydrochloride, respectively. In addition, the in vitro antibacterial activity of EM was not significantly changed compared with enrofloxacin and enrofloxacin hydrochloride.
Conclusion: EM has higher solubility, low toxicity for oral use, and increases the oral bioavailability in rabbit. This study may be of benefit for the development of new enrofloxacin drugs.
Keywords: enrofloxacin mesylate, characterization, antibacterial effect, acute toxicity, pharmacokinetics
Introduction
Enrofloxacin, also known as ethyl ciprofloxacin, is a chemically synthesized third-generation fluoroquinolone.1 The United States Food and Drug Administration (FDA) also approved enrofloxacin as a quinolone antibiotic for livestock and aquatic products in October 1996. Enrofloxacin exhibits good antibacterial activity against a variety of Gram-positive (G+) bacteria and has special effects on mycoplasma. The advantage of enrofloxacin is that it has a broad spectrum of antibacterial, strong bactericidal power, rapid action, wide distribution in the body, what is more, enrofloxacin, as a fluoroquinolone drug for animals, kills bacteria by replicating DNA of tissue bacteria.2,3 Enrofloxacin can be used in combination with other antimicrobial agents to kill pathogenic microorganisms, and no cross-resistance between other antibiotics. In previous reports, fluoroquinolone antibiotics have also been used in combination with macrolides for treatment of Legionella pneumophila4 and in combination with beta-lactam antibiotics for treatment of bacteraemia caused by Gram-negative bacilli.5
The chemical structure of enrofloxacin is shown in Figure 1. The quinoline ring is an essential structure for the antibacterial action of fluoroquinolones including enrofloxacin.3,6-8 Fluorine atom is a characteristic substituent of fluoroquinolones, which is on the C6 position of the drug, that can enhance DNA gyrase affinity,9 increase the permeability of the bacterial cell wall, and enhance the antibacterial effect against of G + bacteria such as Staphylococcus. The introduction of piperazinyl in the C7 position of the drug, on the one hand, improved its antibacterial activity (such as anti-Pseudomonas aeruginosa activity) and its antibacterial spectrum, on the other hand, induced the production of side effects. The ethyl on the piperazine ring of the drugs, enhanced its lipophilicity and penetration ability. At the same time, it also reduces its toxic effects on the central nervous system. And the introduction of cyclopropyl at position N1 enhances the antibacterial effect of enrofloxacin. Because of its acidic carboxyl and alkaline nitrogen atoms, enrofloxacin is both acidic and alkaline, so its solubility greatly depending on the solvent and the pH value. The acid-base dissociation constants of enrofloxacin were pKa1=6.06 and pKa2=7.70, and the isoelectric point was 6.85. There is a study shown that enrofloxacin has the best solubility when the pH of the solution is 5.02.10 And the pH value of the solution does not have a significant effect on the hydrolysis rate.11
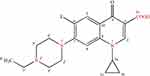 |
Figure 1 Chemical structure of enrofloxacin. |
Enrofloxacin is easily dissolved in methylene chloride and sodium hydroxide solution, dissolved in acetonitrile, slightly soluble in methanol and very slightly soluble in water. The lower water solubility is one of the major drawbacks of enrofloxacin. The literature shows that its solubility in water is only 0.23 g/L.12 The low solubility results in the low bioavailability and limits the study of enrofloxacin in vitro and in vivo. According to most of the literature on improving the solubility of drugs, when the solubility of the drug is less than 100 μg/mL usually shows dissolution-limited absorption.13 In this case, it is necessary to increase the dose of the drug to maintain the blood concentration, but it also leads to some side effects. So it is necessary to increase the solubility of enrofloxacin to meet clinical needs and research. To facilitate the clinical use of enrofloxacin, there are generally two major methods to increase the solubility of enrofloxacin: physical solubilization techniques and chemical solubilization techniques. Physical solubilization method is mainly used to achieve the solubilization effect by forming complex12,14 or a cyclodextrin inclusion compound15 between the active pharmaceutical ingredients (APIs) and excipients, while chemical method is through the APIs and acid or alkali form salt to increase solubility. Such as the synthesis of enrofloxacin citrate16 and enrofloxacin hydrochloride, etc. Salt formation can not only increase the dissolution rate and solubility of the drug, but also improve the bioavailability of the drug.17,18 In the pharmaceutical field, methanesulfonic acid is used to form salt with a chosen drug matter, salts of methanesulfonic acid are highly water-soluble and have no tendency to form hydrates.19 More than 30 registered drugs based on mesylate are known.20 This study uses the chemical solubilization technology to synthesize EM, and the synthesized EM was characterized by 1H-NMR,13C-NMR, MS, FTIR, XRPD, and DSC-TGA analyses to determine its chemical structure. Then the toxicity, in vitro antibacterial activity and pharmacokinetics of EM were studied.
Materials and Methods
Materials
Enrofloxacin (≥98% by enrofloxacin), enrofloxacin sodium (content 83.88% by enrofloxacin) and enrofloxacin hydrochloride (content was 90.67%) was purchased from Zhejiang Guobang Pharmaceutical Co., Ltd. methanesulfonic acid (content 98%) was purchased from Chengdu Huaxia Chemical Reagent Co., Ltd. enrofloxacin mesylate (self-made, content is 74.49% based on enrofloxacin). Mueller-Hinton Agar (MHA) and Mueller-Hinton Broth (MHB) medium were purchased from Hangzhou Microbial Reagent Co., Ltd. All strains were provided by Sichuan Provincial Key Laboratory of Animal Diseases and Human Health in Sichuan Agricultural University. Spectrum Two infrared spectrometer, PerkinElmer, USA; 6120B mass spectrometer, Agilent Technologies, Inc.; AVANCE III500M nuclear magnetic resonance spectrometer, Bruker Technology Co., Ltd. Vario EL cube elemental analyzer, Elementar, Germany; Q600 synchronous thermal analyzer Simultaneous DSC/TGA (SDT), American TA Instruments; D8 ADVANCE X-ray diffractometer, Brooke Technology Co., Ltd.; UV-2000 ultraviolet spectrophotometer, Shanghai Unico Instrument Co., Ltd.; SPX biochemical incubator, Ningbo Donghai Instrument Co., Ltd.; portable pressure steam sterilization pot, Shanghai Huaxian Medical Nuclear Instrument Co., Ltd; LC2010 high-performance liquid chromatography, Shimazu international trade (Shanghai) co., Ltd.
Kunming mice (20±2g) were used for the acute toxicity study. New Zealand white rabbits (2.5±0.3kg) were used for the pharmacokinetic study. All the experimental animals were provided by the Experimental Animal Center of Sichuan Agricultural University (Chengdu, China). Before the experiment, the animals were acclimatized at 25°C±2°C under natural light/dark conditions for 1 week with free access to food and water. Twelve hours before dosing, the animals were made to fast but were allowed free access to water. All animal studies were approved by the Animal Ethical Experimentation Committee of Sichuan Agricultural University (SYXK [Chuan] 2019–187), and were performed according to the requirements of the People’s Republic of China National Act on the use of experimental animals.
Synthesis of EM
EM was prepared by improved modified solvent method. At 25°C, 1 g of enrofloxacin was dispersed with 2 mL of water, and 6.2 mL of mesylate with a concentration of 0.45 mol/L was added (the molar ratio of mesylate to enrofloxacin was 1:1). The solution was stirred at the speed of 20–30 r/min until the salt solution was clarified, and the solution was filtered, the obtained filtrate slowly evaporates solvent under 45°C water bath to obtain kosher salt solid. Then, 0.5 g of EM kosher salt was dissolved in 0.5 mL of water, slowly add the dissolved EM kosher salt solution into 30mL isopropanol, stir for 1.5 h, filter the mixed solution, dry the filtrate in a 45°C oven to obtain the refined EM salt.
Determination of Solubility
Solubility of EM in water, hydrochloric acid solution (PH 1), and phosphate buffer (PH 7.6) solvents was determined by the equilibrium method21 and compared with enrofloxacin, enrofloxacin sodium, and enrofloxacin hydrochloride. Excess enrofloxacin, enrofloxacin sodium, enrofloxacin hydrochloride, and EM were added in conical flask containing 2 mL water, hydrochloric acid solution (PH 1), and phosphate buffer (PH 7.6), respectively. In triplicate, after vortexed for 5 min, the conical flask was put in a water bath at 25 ± 2°C and shaken at 100 r/min for 24 h, until the solution was equilibrated, then the suspension was filtered with 0.22 μm nylon filter. The filtrate was diluted and determined by ultraviolet spectrophotometry (UV) quantitative analysis.
Characterization
Ultraviolet Scan (UV)
Enrofloxacin and EM in aqueous solution were prepared using water as a solvent to ensure the concentrations of enrofloxacin was 2 μg/mL – 9 μg/mL and scan at 200 nm–400 nm. Check whether the chromophoric group changes after enrofloxacin and methanesulfonic acid were salted.
Fourier Transform Infrared Spectrometer (FTIR)
To obtain the infrared scanning pattern of enrofloxacin, enrofloxacin hydrochloride and EM, FTIR were carried out in air under normal atmospheric conditions, using a spectrum two infrared spectrometer (PerkinElmer, USA). Enrofloxacin, enrofloxacin hydrochloride, and EM were pressed into KBr tablets, and infrared scanning was performed within the range of 400 cm −1 to 4000 cm −1 to ensure that the resolution of the instrument was no less than 2 cm−1, in order to examine the differences of the chemical bond or functional group information in each drug molecule.22
Mass Spectrometry (MS)
The Mass Spectrometry was performed using a 6120B mass spectrometry (Agilent Technologies). The condition is EI source. Nitrogen was used as the collision gas, and the pseudomolecular ions of the analytic were decomposed by using the optimal collision activation dissociation (CAD) condition and the corresponding stable isotope marker internal standard. The instrument parameters were set according to the reported method23–25 to ensure that the information of the molecular structure of EM could be obtained.
Nuclear Magnetic Resonance (NMR)
The nuclear magnetic resonance was performed using an AVANCE III500M nuclear magnetic resonance spectrometer (Bruker Technology), and uniform experimental parameters were used in all sample tests. The main parameters in hydrogen spectrum test are as follows: spectrum width (SWH) 8012.820 HZ, sampling data point (TD) 65,536, scanning times (NS) 16, delay time (D1) 1.0s, and receiving gain (RG) 12. The main parameters in the carbon spectrum test: spectrum width (SWH) 24,038.461 HZ, sampling data point (TD) 65,536, scanning times (NS) 512, delay time (D1) 2.0s, and receiving gain (RG) 194.26. In triplicate, enrofloxacin was dissolved in CDCl3, TMS was used as internal standard and EM was dissolved in D2O. Hydrogen and carbon spectra of enrofloxacin and EM were recorded at 400M HZ and 100M HZ, respectively. Determination of the chemical shifts of hydrogen and carbon atoms by NMR.26,27
Elemental Analysis
The C, H, O and N elements of EM were analyzed, in order to detect the proportion of four elements and infer their molecular composition.
Synchronous Thermal Analysis (SDT)
SDT thermal analysis has the characteristics of differential thermal analysis (DTA) and thermogravimetric analysis (TGA). It can provide the signal of melting point, melting heat, crystallization temperature and thermal stability of the test sample. The DSC-TGA analyses were performed using TA instruments equipment, aluminum oxide was used as reference. The heating rate was set at 10°C/min and the temperature range was 25–550°C, in an inert atmosphere of N2.
X-Ray Powder Diffraction Analysis (XRPD)
The enrofloxacin and EM were scanned using an X-Ray powder diffractometer. The light source was Cu Kα radiation with a wavelength of 1.542 Å, and the DS (divergent slit) and SS (scattering slit) were 1°. The RS (receiving slit) was 0.2 mm. An operating voltage of 40 kV and current of 25 mA, the scanning angle was 5–45° with a speed of 0.06°/s.
The Antibacterial Activity in vitro
Microbroth dilution method28,29 was used to study in vitro antimicrobial activity of EM. Individual colonies grown on MHA medium for 18–24 h were placed in 5mL MHB medium, shaken for 16 h at 37°C and 100 r/min, and then diluted to 1.0×108 CFU/mL with MHB broth for later use. The minimum inhibitory concentration (MIC) test systems were created by diluting different drug solution in 96-well plates to a volume of 100 μL, and the enrofloxacin sodium, enrofloxacin hydrochloride and EM were double diluted28 to final concentrations ranging from 25 to 0.0488 μg/mL, and then 100 μL bacterial suspensions was added to each drug-containing well. The MIC was determined visually as the first dilution step with a complete growth inhibition30–32 and the minimum bactericidal concentration (MBC) was determined after the MIC. 10 μL mixtures were extracted from the wells in the 96-well plate that were visually free of bacterial growth, and then uniformly coated on the MHA medium after serial dilution, and incubated at 37°C for 18–24 h. The number of colonies on the plate was calculated, and the minimum concentration killing 99.9% of the bacteria was recorded as MBC.30–32 The MIC and MBC of standard strains and clinical strains of Escherichia coli, Salmonella and Staphylococcus aureus were determined.
Acute Toxicity Study
In accordance with the requirements of veterinary drug research technical guidelines, 5 dose groups were set up in this experiment, namely, 625 mg/kg, 884 mg/kg, 1250 mg/kg, 1767 mg/kg and 2500 mg/kg, respectively. The state and behavior of the mice were observed after administration, and the mortality was recorded. The improved Karber method33 was used to calculate the LD50 of EM.
Pharmacokinetic Study
The pharmacokinetic of enrofloxacin, enrofloxacin hydrochloride and EM was determined by high-performance liquid chromatography (HPLC). After oral administration with a single oral dose of 10 mg/kg, the rabbit ear vein blood samples were collected at 0 h, 0.083 h, 0.167 h, 0.333 h, 0.667 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 4 h, 6 h, 9 h, 12 h, 24 h, 36 h and 48 h, respectively. The peak concentration (Cmax) and the time at which Cmax was observed (Tmax) were calculated. Additionally, DAS software was used to process the data to obtain the pharmacokinetic parameters which were analyzed by SPSS19.0.
Results and Discussion
Determination of Solubility
The bioavailability of drugs is a vital concern in the pharmaceutical field, the low aqueous solubility of candidate drugs will lead to the increase of drug concentration, which will lead to some adverse reactions.34 EM has been synthesized successfully in this study, a white, slightly bitter, loose solid powder that can dissolve in water easily, and the EM reduces the bitterness of enrofloxacin. The yield of EM was 94.41% and the content of enrofloxacin in EM was 74.49%. The solubility results for enrofloxacin, EM, enrofloxacin sodium, and enrofloxacin hydrochloride are shown in Table 1. It can be seen that the solubility of EM is much higher than enrofloxacin hydrochloride and enrofloxacin sodium, so it has a good dissolution advantage. The higher solubility in water may have been associated with the smaller lipo-hydro partition coefficient of the drug, the larger the distribution coefficient of lipid-water, the drugs more soluble in fat, and vice versa. Except for, the lipo-hydro partition coefficient of EM is 0.019±0.002, which is smaller than enrofloxacin (3.32±0.22) and other enrofloxacin salts (enrofloxacin sodium, 0.047±0.01; enrofloxacin hydrochloride, 0.031±0.007). In addition, this may be related to the polar surface area of the drug molecule. With the increase of the polar surface area, the solubility becomes higher. Due to the introduction of multiple oxygen atoms, the polar surface area of the EM molecule may be the largest among all prepared enrofloxacin salts. EM is a new crystal that is different from enrofloxacin, it is possible that the crystal structure of EM is more conducive to its dissolution, but the crystal structure of EM needs further study. Thus, the solubility is greatly improved.
 |
Table 1 The Results of Solubility and Lip-Hydro Partition Coefficient |
Characterization
Ultraviolet Scan (UV)
The UV scanning results of the enrofloxacin and EM aqueous solutions are shown in Figure 2. The UV absorption curves of the enrofloxacin and the EM are generally the same. This shows that the UV-chromophoric groups were not destroyed after enrofloxacin was formed into salt with methanesulfonic acid. However, the peaks of enrofloxacin are shifted: λmax=271 nm to λmax=276 nm, that indicate that salt formation infects chromophoric groups.
 |
Figure 2 The UV spectrums of enrofloxacin and enrofloxacin mesylate. |
Fourier Transform Infrared Spectrometer (FTIR)
The infrared scanning results of the main bands of enrofloxacin and EM are shown in Figure 3. The infrared spectrum of EM is basically the same as the enrofloxacin, such as at 3446 cm−1, O-H stretching vibration of the carboxyl; stretching vibration peak of carbonyl C=O at 1729 cm−1; stretching vibration peak of C=C bond at 1629 cm−1 and 1506 cm−1; bending vibration of C–H bond at 1475 cm−1; the characteristic absorption peak of the C–F bond at 1289 cm−1. These prove that EM and enrofloxacin both contain the same structural groups such as F-Ph, -CH2, -COOH, C=O and so on. In addition, there are many new characteristic peaks on the infrared spectrum of EM, such as 3492 cm−1, methanesulfonic acid O-H absorption peak; 2457 cm−1–2655 cm−1 amine salt absorption peak; 1058 cm−1 and 1195 cm−1 methanesulfonic acid S = O absorption peak; 638 cm− 1 is the methanesulfonic acid S-O absorption peak. This shows that methanesulfonic acid may bind with the basic nitrogen atom of enrofloxacin and form an amine salt. The above hypothesis was further confirmed by comparing the infrared spectra of enrofloxacin hydrochloride with EM (Figure 4). It can be seen that both of them have similar amine salt peaks, and that EM has more absorption peaks of the methanesulfonic acid characteristic group than enrofloxacin hydrochloride.
 |
Figure 3 The FTIR spectrums of enrofloxacin and enrofloxacin mesylate. |
 |
Figure 4 The FTIR spectrums of enrofloxacin hydrochloride and enrofloxacin mesylate. |
Mass Spectrometry (MS)
Figures 5 and 6 show the mass spectra of enrofloxacin and EM, respectively. The molecular ion peak ([M+H]+) in the mass spectrum of Figure 6 is 360.1728, which is in agreement with the molecular weight of enrofloxacin C19H22FN3O3, indicating that enrofloxacin is the base portion of EM.
 |
Figure 5 The MS spectrum of enrofloxacin. |
 |
Figure 6 The MS spectrum of enrofloxacin mesylate. |
Nuclear Magnetic Resonance (NMR)
The 1H-NMR spectrum of enrofloxacin is shown in Figure 7. The spectrum shows 21 hydrogens (excluding -COOH active hydrogen) except the solvent, which is consistent with the molecular structure of enrofloxacin. According to the enrofloxacin hydrogen spectrum data reference35 enrofloxacin is now assigned to Table 2. The 1H-NMR spectrum of EM is shown in Figure 8. The spectrum shows 24 hydrogens (one –SO3H and one –COOH may exchange with D2O). According to the attribution of enrofloxacin and the chemical environment of hydrogen atoms, the hydrogen atom of EM will be assigned to Table 3. Among them, δ 2.73 (S, 3H, CH3) is exactly one molecule of three hydrogens on the methanesulfonic acid methyl group. The rest is hydrogen on the base of enrofloxacin. Except for the piperazine ring and ethyl, the chemical shift values of other hydrogen atoms are not changed much. Two hydrogens at the 3ʹ position on the piperazine ring (δ 3.86, d, J = 8.4 HZ, 2H, CH2) and two hydrogens at the 5ʹ position (δ 3.7, d, J = 8.0 HZ, 2H, CH2) shift to low field; two hydrogens at the 7ʹ position on the ethyl (δ 3.3, m) and three hydrogens at the 8ʹ position (δ 1.36, m) all move to the lower field. This may be due to the fact that the formation of a quaternary ammonium salt at the N4ʹ position introduces a positive charge, giving the surrounding H a different degree of shielding effect.36
 |
Table 2 The 1H-NMR (400 M) Data of Enrofloxacin (CDCl3) |
 |
Table 3 The 1H-NMR (400M HZ) Data of Enrofloxacin Mesylate (D2O) |
 |
Figure 7 The 1H-NMR spectrum of enrofloxacin. |
 |
Figure 8 The 1H-NMR spectrum of enrofloxacin mesylate. |
The 13C-NMR spectrum of enrofloxacin is shown in Figure 9, which shows the resonance of 19 carbon atoms, corresponding to 19 carbon atoms in the enrofloxacin molecule. Its attribution is shown in Table 4. The 13C-NMR spectrum of EM is shown in Figure 10, which shows the resonance of 20 carbon atoms, corresponding to 20 carbon atoms in the EM molecule. Its attribution is shown in Table 5. Of these, δ 38.46 (Q, CH3) is just methyl sulfonate on methanesulfonic acid. The rest is carbon on the base of the enrofloxacin. Except for the piperazine ring and the ethyl group, the chemical shift values of other carbon atoms are not much changed. Both C3′ and C5′carbons (δ46.41, T, CH2) on the piperazine ring move towards the high field; C7′ (δ50.86, T, CH2) and C8′ (δ 7.46, Q on the ethyl group, CH3) all move to the high field to varying degrees. This may be due to the fact that after N4′ forms a quaternary ammonium salt, the positive charge is concentrated on the hydrogen ion connected to the nitrogen atom. The C atom around the N4′ atom is not directly connected with the positive charge, the induction effect is weakened, and the electric field effect makes each C shifts to the high field.36
 |
Table 4 The 13C-NMR (100M HZ) Data of Enrofloxacin (CDCl3) |
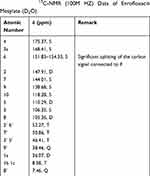 |
Table 5 The 13C-NMR (100M HZ) Data of Enrofloxacin Mesylate (D2O) |
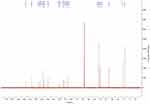 |
Figure 9 The 13C-NMR spectrum of enrofloxacin. |
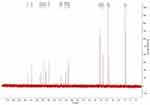 |
Figure 10 The 13C-NMR spectrum of enrofloxacin mesylate. |
A comprehensive analysis of the hydrogen spectrum indicated that methanesulfonic acid combined with enrofloxacin N4ʹ to form a salt. The theoretical formula of the formed EM salt may be C19H22FN3O3⋅ CH4O3S.
Elemental Analysis
The elemental analysis result of EM is Table 6. The proportion of N, C, H and S elements of EM is similar to the theoretical formula C19H22FN3O3⋅ CH4O3S. Combined with other characterization methods, the molecular formula of EM is C19H22FN3O3⋅ CH4O3S.
 |
Table 6 The Elemental Analysis of Enrofloxacin Mesylate |
Synchronous Thermal Analysis (SDT)
The DSC-TGA curve of enrofloxacin and EM is shown in Figure 11. The melting point of enrofloxacin and EM is 225.5°C and 298.5 °C, respectively. The heat absorption peak on the DSC curve and the significant mass loss on the TGA curve after 298.5°C indicate that EM dissolves during melting and it has better thermal stability. Beyond that, the mass loss of EM between 30°C and 75°C is due to the volatilization of adsorbed solvent. After 75°C and before decomposition temperature, there was no significant mass loss in the TGA curve, indicating that EM molecules did not contain crystal water.
 |
Figure 11 The DSC-TGA analysis of enrofloxacin and enrofloxacin mesylate. |
X-Ray Powder Diffraction (XRPD)
The powder diffraction pattern of enrofloxacin and EM is shown in Figure 12. X-ray powder diffraction characterization showed that EM has a distinct diffraction peak, compared with enrofloxacin, EM has the characteristics of diffraction peak in 2θ were 7.93, 9.61, 11.89, 22.07, 22.95, 23.68, 24.36, 24.97, 35.29, 35.97, respectively, indicating that it is a crystal.37 In addition, the numbers of diffraction peaks of EM were greatly different from enrofloxacin, and the energetic peak of EM is less than that of enrofloxacin, indicating that EM crystals may have small particle size, which facilitates their rapid dissolution. The angular positions, and relative intensity, as well as the shape of the diffraction peaks of EM, are greatly different from enrofloxacin, indicating that mesylate and enrofloxacin are not a simple physical mixture, but mesylate reacts with enrofloxacin to form a crystal different from enrofloxacin. Under crystal state, the molecule maintains its stable arrangement in space with hydrogen bond, due to the introduction of salt bond, the interaction force of molecules inside the crystal cell is increased, which is conducive to the stability of the crystal.
 |
Figure 12 The X-ray powder diffraction analysis of enrofloxacin and enrofloxacin mesylate. |
Antibacterial Activity in vitro
The MIC and MBC determination of enrofloxacin and each enrofloxacin salt are based on enrofloxacin. The inhibitory effects of enrofloxacin and its salts on six strains of bacteria are shown in Table 7. The MIC values of EM which prepared in this experiment, enrofloxacin, enrofloxacin hydrochloride in Chinese Veterinary Pharmacopoeia, and enrofloxacin sodium commonly used in clinical trials were both in the range of 0.0727–1.5625 μg/mL. The experimental results show that the bacteriostatic effect of EM synthesized by ourselves is basically the same as that of enrofloxacin, which has not changed its bacteriostatic effect, but has greatly increased the solubility of enrofloxacin, solved the problem that enrofloxacin is insoluble in water, and enriched the use of enrofloxacin. Enrofloxacin exhibits antibacterial activity mainly through the binding of antibacterial active sites with bacterial DNA gyrase to prevent the replication of bacterial DNA.2,3,9 The salt formation may not change its antibacterial active groups, so the bactericidal effect does not change significantly.
 |
Table 7 The MIC and MBC Determination Results of Enrofloxacin and Its Salts |
Acute Toxicity Study
After administration, the mice showed: dumbness, less movement, glassy eyes, depressed spirit, sluggish breathing and slow movement, some mice even showed severe ataxia; after a short period of depression, poisoned mice suddenly became excited and restless, jumping, some performance for the whole body tremor, rolling or running, eventually collapse and die, death began at 5 min, and the peak of death was 13–19 min in the low and medium dose group, and 6–9 min in the high dose group. The death statistics of mice in each group are shown in Table 8. After SPSS 19.0 analysis, the oral LD50 of EM was 1168.364 mg/kg, indicating that the oral drug safety of EM was high.
 |
Table 8 The Death Number Statistical Results of Mice in Every Groups |
Pharmacokinetic Study
After administration of enrofloxacin, EM and enrofloxacin hydrochloride, according to the blood concentration of each group, the pharmacokinetic parameters are shown in Table 9. The Cmax of EM was 1.391±0.158 mg/L, which was significantly higher than enrofloxacin hydrochloride (0.989±0.195 mg/L) (P < 0.01) and enrofloxacin hydrochloride (0.877±0.155 mg/L) (P < 0.01). Because of its good solubility, EM could form a higher concentration of the drug in the gastrointestinal tract, to promote the drug absorption. There was no significant difference in the peak time (Tmax) of enrofloxacin, EM and enrofloxacin hydrochloride. The area under the curve (AUC0-t) of EM was significantly higher than that of enrofloxacin and enrofloxacin hydrochloride (P < 0.05). Using enrofloxacin as a reference drug, the relative bioavailability of EM and enrofloxacin hydrochloride can be calculated according to the following equation:38,39
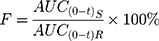
 |
Table 9 The Pharmacokinetic Parameters of Enrofloxacin, Enrofloxacin Mesylate and Enrofloxacin Hydrochloride (n=6) |
AUC(0-t)R is the area under the curve of the reference drug; AUC(0-t)S is the area under the curve of the drug under test.
The relative bioavailability of EM and enrofloxacin hydrochloride was F=179.78±28.91%, 121.02±18.85%, respectively. The data showed that the relative bioavailability of EM was the best. Indicating that salt formation using enrofloxacin and mesylate improved the bioavailability of enrofloxacin.
Conclusion
New salt formation of enrofloxacin was successfully synthesized in this study by the chemical methods. And its structure was analyzed by ultraviolet and infrared spectroscopy, mass spectrometry and nuclear magnetic resonance, etc. the molecular formula is determined as C19H22FN3O3·CH4O3S. The in vitro antibacterial test showed that the antibacterial effect of EM was not significantly different from that of enrofloxacin, enrofloxacin hydrochloride and enrofloxacin sodium. The acute toxicity test showed that EM was safe to take orally, and the pharmacokinetic results showed that EM significantly improved the bioavailability of enrofloxacin, which ensured the efficacy of enrofloxacin in clinical use. Furthermore, the EM has higher solubility and thermal stability, and more conducive to clinical use. It is hope that the outcome of this study may help to the development of new enrofloxacin drugs.
Acknowledgments
First of all, I (Linlin Pei) would like to express my gratitude to all those who helped me during the writing of this article. I gratefully acknowledge the help of my tutor, Professor. FU, who has offered me valuable suggestions in the academic studies. Second, I also owe a special debt of gratitude to all the students in the lab, because they help me in the process of the experiment. I have benefited a lot. Last, I should finally like to express my gratitude to my beloved parents who have always been helping me out of difficulties and supporting without a word of complaint.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Attili AR, Preziuso S, Ngu Ngwa V, et al. Clinical evaluation of the use of enrofloxacin against Staphylococcus aureus clinical mastitis in sheep. Small Ruminant Res. 2016;136:72–77. doi:10.1016/j.smallrumres.2016.01.004
2. Zordok WA, Sadeek SA. Synthesis, thermal analyses, characterization and biological evaluation of new enrofloxacin vanadium(V) solvates(L) (L = An, DMF, Py, Et3N and o-Tol). J Mol Struct. 2016;1120:50–61. doi:10.1016/j.molstruc.2016.05.011
3. Efthimiadou EK, Karaliota A, Psomas G. Mononuclear metal complexes of the second-generation quinolone antibacterial agent enrofloxacin: synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron. 2008;27(6):1729–1738. doi:10.1016/j.poly.2008.02.006
4. Kumar GP, Phani AR, Prasad RGSV, et al. Polyvinylpyrrolidone oral films of enrofloxacin: film characterization and drug release. Int J Pharm. 2014;471(1–2):146–152. doi:10.1016/j.ijpharm.2014.05.033
5. Majdi NA, John WW, Brian DL, et al. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Chin J Inf Chemother. 2010;53(4):1386.
6. Dönmez F, Yardım Y, Şentürk Z. Electroanalytical determination of enrofloxacin based on the enhancement effect of the anionic surfactant at anodically pretreated boron-doped diamond electrode. Diam Relat Mater. 2018;84:95–102. doi:10.1016/j.diamond.2018.03.013
7. López-Gresa MP, Ortiz R, Perelló L, et al. Interactions of metal ions with two quinolone antimicrobial agents (cinoxacin and ciprofloxacin): spectroscopic and X-ray structural characterization. Antibacterial studies. J Inorg Biochem. 2002;92(1):65–74. doi:10.1016/S0162-0134(02)00487-7
8. Turel I. The interactions of metal ions with quinolone antibacterial agents. Coord Chem Rev. 2002;232(1–2):27–47. doi:10.1016/S0010-8545(02)00027-9
9. Ftouni H, Sayen S, Boudesocque S, et al. Structural study of the copper(II)–enrofloxacin metallo-antibiotic. Inorganica Chim Acta. 2012;382:186–190. doi:10.1016/j.ica.2011.12.012
10. Lizondo M, Pons M, Gallardo M, et al. Physicochemical properties of enrofloxacin. J Pharm Biomed Anal. 1997;15(12):1845–1849. doi:10.1016/S0731-7085(96)02033-X
11. Wu Y, Liao X, Wang Z, et al. [Hydrolysis characteristics of enrofloxacin. Chin J Appl Ecol. 2006;17(6):1086.
12. Soliman MH, Mohamed GG, Hindy AMM. Biological activity, spectral and thermal characterization of mixed ligand complexes of enrofloxacin and glycine: in vitro antibacterial and antifungal activity studies. Monatshefte Fuer Chemie/Chem Monthly. 2015;146(2):259–273. doi:10.1007/s00706-014-1315-5
13. Kawabata Y, Wada K, Nakatani M, et al. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10. doi:10.1016/j.ijpharm.2011.08.032
14. Xiong X, Zhang M, Hou Q, et al. Solid dispersions of telaprevir with improved solubility prepared by co-milling: formulation, physicochemical characterization, and cytotoxicity evaluation. Mater Sci Eng C. 2019;105:110012. doi:10.1016/j.msec.2019.110012
15. Calsavara LPV, Moraes FFD, de Moraes FF. Enrofloxacin inclusion complexes with cyclodextrins. J Inclusion Phenom Macrocyclic Chem. 2012;73(1–4):219–224. doi:10.1007/s10847-011-0045-0
16. Golovnev NN, Vasiliev AD, Kirik SD. Enrofloxacinium citrate monohydrate: preparation, crystal structure, thermal stability and IR-characterization. J Mol Struct. 2012;1021(31):112–117. doi:10.1016/j.molstruc.2012.04.059
17. Wu W, Lobmann K, Rades T, et al. On the role of salt formation and structural similarity of co-formers in co-amorphous drug delivery systems. Int J Pharm. 2018;535(1–2):86–94. doi:10.1016/j.ijpharm.2017.10.057
18. Diniz LF, Carvalho PS, de Melo CC, et al. Development of a salt drug with improved solubility: ethionamide nitrate. J Mol Struct. 2017;1137:119–125. doi:10.1016/j.molstruc.2017.02.036
19. Meissner T, Niess M. Isotachophoretic separation of alkylsulfonates and determination of methanesulfonic acid as main component and as trace component in pharmaceutical drug substances. J Chromatogr A. 2004;1035(2):271–275. doi:10.1016/j.chroma.2004.02.061
20. Guillory KJ, Handbook of Pharmaceutical Salts:? Properties, Selection, and Use. In: Heinrich Stahl P, Wermuth CG, Verlag Helvetica VHCA, editors. Chimica Acta, Zürich, Switzerland. Weinheim: Wiley-VCH; 2002:
21. Hampson JW, Maxwell RJ, Li S, et al. Solubility of Three Veterinary Sulfonamides in Supercritical Carbon Dioxide by a Recirculating Equilibrium Method. J Chem Eng Data. 1999;44(6):1222–1225. doi:10.1021/je990075m
22. Tabatabaee M, Mahmoodikhah H, Ahadiat G, et al. Synthesis, crystal structure, and spectroscopic characterization of two new binuclear complexes of manganese(II) and vanadium(V) with dipicolinate ligands containing 2-aminopyrimidinium as a counter cation. Monatshefte Für Chemie - Chemical Monthly. 2013;144(5):621–626. doi:10.1007/s00706-012-0878-2
23. Smyth WF, Mcclean S, Massaro CF, Smyth TJ, Brooks P, Robledo VR.
24. Junza A, Saurina J, Barron D, et al. Metabolic profile modifications in milk after enrofloxacin administration studied by liquid chromatography coupled with high resolution mass spectrometry. J Chromatogr A. 2016;1460:92–99. doi:10.1016/j.chroma.2016.07.016
25. Krebber R, Hoffend F, Ruttmann F. Simple and rapid determination of enrofloxacin and ciprofloxacin in edible tissues by turbulent flow chromatography/tandem mass spectrometry (TFC–MS/MS). Anal Chim Acta. 2009;637(1):208–213. doi:10.1016/j.aca.2008.11.006
26. Asakura T, Isobe K, Kametani S, et al. Characterization of water in hydrated Bombyx mori silk fibroin fiber and films by 2 H NMR relaxation and 13 C solid state NMR. Acta Biomater. 2017;50:322–333. doi:10.1016/j.actbio.2016.12.052
27. Sarker AK, Cashin PJ, Balakrishnan VK, et al. Binding of Sulfonamide Antibiotics to CTABr Micelles Characterized Using 1 H NMR Spectroscopy. Langmuir ACS J Surf Colloids. 2016;32(31):7814. doi:10.1021/acs.langmuir.6b00947
28. Liu B, Liu C, Huang H, et al. Antibacterial and antibiotic synergistic activities of the extract from Pithecellobium clypearia against clinically important multidrug-resistant gram-negative bacteria. Eur J Integr Med. 2019;32:100999. doi:10.1016/j.eujim.2019.100999
29. Wang JL, Wang JT, Sheng WH, et al. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in Taiwan: mortality analyses and the impact of vancomycin, MIC = 2 mg/L, by the broth microdilution method. BMC Infect Dis. 2010;10(1):159. doi:10.1186/1471-2334-10-159
30. Knaack D, Idelevich EA, Schleimer N, et al. Bactericidal activity of bacteriophage endolysin HY-133 against Staphylococcus aureus in comparison to other antibiotics as determined by minimum bactericidal concentrations and time-kill analysis. Diagn Microbiol Inf Dis. 2019;93(4):362–368. doi:10.1016/j.diagmicrobio.2018.11.005
31. Kanayama S, Okamoto K, Ikeda F, et al. Bactericidal activity and post-antibiotic effect of ozenoxacin against Propionibacterium acnes. J Inf Chemother. 2017;23(6):374–380. doi:10.1016/j.jiac.2017.03.004
32. Chraibi M, Farah A, Lebrazi S, et al. Antimycobacterial natural products from Moroccan medicinal plants: Chemical composition, bacteriostatic and bactericidal profile of Thymus satureioides and Mentha pulegium essential oils. Asian Pacific Journal of Tropical Biomedicine. 2016;6(10):836–840. doi:10.1016/j.apjtb.2016.08.002
33. Wang AZ, Ma QX, Zhao HJ, et al. A comparative study of the mortality rate of rats receiving a half lethal dose of fat intravenously: under general anaesthesia versus under spinal anaesthesia. Injury. 2012;43(3):311–314. doi:10.1016/j.injury.2011.08.022
34. de Almeida AC, Torquetti C, Ferreira PO, et al. Cocrystals of ciprofloxacin with nicotinic and isonicotinic acids: mechanochemical synthesis, characterization, thermal and solubility study. Thermochim Acta. 2019;685:178346.
35. Lin L, Zhai Y, Wang D, et al. Preparation, characterization and spectroscopic properties of difluoroboron complexes with some fluoroquinolones. J Fluor Chem. 2016;182:7–11. doi:10.1016/j.jfluchem.2015.11.010
36. Psota L, Franzen Sieveking M, Turnier J, et al. Nitrogen nuclear magnetic resonance spectroscopy. Nitrogen‐15 and proton chemical shifts of methylanilines and methylanilinium ions. Org Magn Res. 1978;11(8):401–405. doi:10.1002/mrc.1270110808
37. Rakhmatullin A, Polovov IB, Maltsev D, et al. Combined Approach for the Structural Characterization of Alkali Fluoroscandates: solid-State NMR, Powder X-ray Diffraction, and Density Functional Theory Calculations. Inorg Chem. 2018;57(3):1184–1195. doi:10.1021/acs.inorgchem.7b02617
38. Abbas RP, Palumbo DP, Walters FP, et al. Single-dose Pharmacokinetic Properties and Relative Bioavailability of a Novel Methylphenidate Extended-release Chewable Tablet Compared With Immediate-release Methylphenidate Chewable Tablet. Clin Ther. 2016;38(5):1151–1157. doi:10.1016/j.clinthera.2016.02.026
39. Ullah MA, Al Maruf A, Azad MA, et al. Relative bioavailability and pharmacokinetic properties of two different enteric formulations of esomeprazole in healthy bangladeshi male volunteers: an open-label, single-dose, randomized-sequence, two-way crossover study. Clin Ther. 2010;32(7):1419–1426. doi:10.1016/j.clinthera.2010.07.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
