Back to Journals » Drug Design, Development and Therapy » Volume 18
Synthesis and Characterization of a New Carbon-11 Labeled Positron Emission Tomography Radiotracer for Orexin 2 Receptors Neuroimaging
Authors Wang Y, Wang Y, Liu Y , Cheng H, Dagnew TM , Xu Y, Wang C
Received 23 September 2023
Accepted for publication 13 December 2023
Published 31 January 2024 Volume 2024:18 Pages 215—222
DOI https://doi.org/10.2147/DDDT.S404992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yanli Wang,1 Yongle Wang,1,2 Yan Liu,1 Hua Cheng,1 Tewodros Mulugeta Dagnew,1 Yulong Xu,1 Changning Wang1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, 02129, USA; 2School of Pharmacy, Minzu University of China, Beijing, 100081, People’s Republic of China
Correspondence: Changning Wang; Yulong Xu, Email [email protected]; [email protected]
Purpose: Orexin receptors (OXRs) play a crucial role in modulating various physiological and neuropsychiatric functions within the central nervous system (CNS). Despite their significance, the precise role of OXRs in the brain remains elusive. Positron emission tomography (PET) imaging is instrumental in unraveling CNS functions, and the development of specific PET tracers for OXRs is a current research focus.
Methods: The study investigated MDK-5220, an OX2R-selective agonist with promising binding properties (EC50 on OX2R: 0.023 μM, Ki on hOX2R: 0.14 μM). Synthesized and characterized as an OX2R PET probe, [11C]MDK-5220 was evaluated for its potential as a tracer. Biodistribution studies in mice were conducted to assess OX2R binding selectivity, with particular attention to its interaction with P-glycoprotein (P-gp) on the blood-brain barrier.
Results: [11C]MDK-5220 exhibited promising attributes as an OX2R PET probe, demonstrating robust OX2R binding selectivity in biodistribution studies. However, an observed interaction with P-gp impacted its brain uptake. Despite this limitation, [11C]MDK-5220 presents itself as a potential candidate for further development.
Discussion: The study provides insights into the functionality of the OX system and the potential of [11C]MDK-5220 as an OX2R PET probe. The observed interaction with P-gp highlights a consideration for future modifications to enhance brain uptake. The findings pave the way for innovative tracer development and propel ongoing research on OX systems, contributing to a deeper understanding of their role in the CNS.
Conclusion: [11C]MDK-5220 emerges as a promising OX2R PET probe, despite challenges related to P-gp interaction. This study lays the foundation for further exploration and development of PET probes targeting OXRs, opening avenues for advancing our understanding of OX system functionality within the brain.
Keywords: synthesis and characterization, orexin-2 receptor, positron emission tomography, carbon-11 labeling, neuroimaging
Introduction
Orexins (also known as hypocretins) are endogenous neuropeptides secreted by the hypothalamus, consisting of two subtypes: orexin-A and orexin-B.1,2 These neuropeptides interact with G protein-coupled receptors, orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R),3,4 respectively, to regulate various physiological functions, including energy homeostasis, the sleep-wake cycle, stress response, and brain reward mechanisms.5–8 Studies have highlighted the close association of orexin-signaling abnormalities with numerous diseases, especially sleep disorders. Further investigations have revealed that OX1R and OX2R exhibit distinct expressions and distributions in the brain, indicating specific physiological functions.9 While OX1Rs are predominantly situated in the limbic system, paraventricular thalamic nucleus, and locus coeruleus, overseeing emotions, rewards, and autonomic regulation,10,11 OX2Rs exhibit a more exclusive expression pattern, predominantly found in regions crucial for the control of arousal, thereby playing a pivotal role in the regulation of sleep and wakefulness.12,13
Agonism of orexin receptors has emerged as a promising therapeutic approach for various diseases, particularly in the treatment of narcolepsy.14,15 The development of OXR agonists, including dual OXR agonists and selective OXR agonists, has sparked significant interest in this field.16 However, despite notable progress in the synthesis of OXR PET probes, the development of specific OX2R PET radioligands remains a challenging endeavor.17 Despite these developments, the biological mechanisms of OX1R and OX2R in CNS-related diseases have not been fully elucidated to date.18 Further research is essential to unravel the precise roles and functions of these receptors in the context of central nervous system diseases. By gaining a comprehensive understanding of the OX1R and OX2R pathways, researchers can potentially advance the development of targeted therapies using selective OXR agonists and antagonists, leading to improved treatment strategies for various medical conditions.19
Molecular imaging, specifically positron emission tomography (PET), is a nuclear medicine imaging technology known for its noninvasive nature, high sensitivity, and capacity for functional imaging, offering valuable biological insights at the molecular level.20–23 PET imaging utilizing appropriate OXR radioligands holds tremendous potential to shed light on the biological functions of the orexin system and facilitate the discovery of novel selective OXR agonists.24,25 Notably, significant efforts have been dedicated to validating promising orexin-2 receptor PET tracers, as illustrated in Figure 1. Despite substantial progress in this area, the development of PET tracers targeting the orexin-2 receptor is still in its early stages. Consequently, the therapeutic potential of orexin-2 receptor-modulating pharmacotherapy, coupled with the unmet needs for clinical orexin-2 receptor PET tracers, serves as a powerful driving force for further advancements in PET tracers directed toward this target. However, it is worth noting that existing OXR PET probes have encountered limitations such as limited brain uptake or non-specific binding, which impedes their translation for effective OX2R imaging in preclinical or clinical applications. For instance, Watanabe et al reported F18-labeled tetrahydroisoquinoline derivatives as OX1R PET tracers with low brain uptake in mice.26 Similarly, C11 and 18F-labeled OX2R PET radioligands, including [11C]CW4,27 [11C]EMPA,28 [18F]Seltorexant29 and [18F]DAN-1,30 have shown limited brain uptake or inactivation in studies involving rodents and nonhuman primates (NHPs). The unexpected nonspecific binding observed in these studies has hindered their further translation to OX2R imaging. While we recently reported [11C]CW24,31 a nonselective PET radioligand for OXRs, which exhibited good brain uptake in rodents and NHPs, its moderate binding affinity and specificity for OXRs required optimization. Additionally, we explored [18F]Seltorexant29 as a potential brain OX2R PET imaging probe, providing some groundwork for the development of new OX2R PET probes and further research on the OX system. However, the exploration of more PET radioligands for OXRs is essential to comprehensively understand their roles in various physiological and neuropsychiatric processes. As of now, there are no PET radioligands specifically targeting OX2R for neuroimaging in either preclinical or clinical applications. Therefore, the development of highly selective and efficient PET radioligands targeting OX2R remains a key goal to advance research in this field and holds the potential for future clinical applications. Advancements in this area could lead to significant breakthroughs in the understanding and treatment of various disorders associated with OX2R signaling.
 |
Figure 1 Structures of orexin receptor radiotracers. |
Radiolabeled potent known OX agonists and antagonists with short half-lives isotopes, such as carbon-11 (t1/2 = 20 min) and fluorine-18 (t1/2 = 109 min),32–34 offer a practical and efficient approach for the specific development of PET imaging probes targeting OXRs, including [11C]CW4, [11C]EMPA, and [18F]Seltorexant. As part of our ongoing efforts in the development of OXR PET radioligands, we conducted a screening process to identify selective OXR agonists that could be labeled with radioisotopes. Our selection criteria focused on two key aspects. First, selected OXR agonist candidates must exhibit excellent binding affinity and selectivity for OXR to ensure accurate target measurement in vivo. Second, since the brain is our main region of interest, candidates should possess appropriate physicochemical properties to effectively penetrate the blood-brain barrier (BBB).35,36 One of the exceptional drug candidates meeting stringent criteria is MDK-5220, an Orexin 2 Receptor (OX2R)-selective agonist discovered by Takashi Nagahara et al. With a potent EC50 on OX2R at 23 nM and an OX1R/OX2R EC50 ratio of 70, MDK-5220 demonstrates remarkable specificity and activity. The compound’s IC50 value (23 nM) further underscores its potency, specifically targeting the Orexin 2 Receptor. In both CHO cells overexpressing human OX1R and HEK-293 cells overexpressing human OX2R, MDK-5220 displaces orexin-A in a concentration-dependent manner. The compound exhibits a high affinity for hOX2R with a Ki of 0.14 μM, emphasizing its selectivity for OX2R over OX1R, where the Ki is 0.77 μM.37 These findings underscore MDK-5220’s dual strength in potency and selectivity, positioning it as a promising candidate for further exploration and development in the realm of OX2R-targeted therapeutics. Previous studies have demonstrated that MDK-5220 exhibits favorable properties, including good binding affinity and selectivity for OX2R, suitable brain uptake, and favorable pharmacokinetic characteristics, making it an ideal candidate for radiolabeling and OX2R PET imaging in the brain. By utilizing MDK-5220 as a radioligand, our aim is to deepen our understanding of the OX system and potentially advance the development of novel PET imaging probes. This advancement will further enhance research in this field and open new possibilities for potential clinical applications. The combination of radiolabeling strategies and the use of promising candidates like MDK-5220 holds great potential in advancing our knowledge of OXRs and their role in various physiological and neuropsychiatric processes. This progress may lead to the development of more effective therapeutic strategies and the discovery of novel treatments for disorders involving the orexin system.
Materials and Methods
The animal studies described in this paper were conducted at Massachusetts General Hospital under the oversight of PHS Assurance of Compliance No. A3596-01. The Institutional Animal Care and Use Committee (IACUC) for Massachusetts General Hospital (MGH), represented by the Subcommittee on Research Animal Care (SRAC), diligently reviewed and granted approval for all procedures detailed in this paper, ensuring the highest standards of ethical conduct in our research. For this study, a total of eight male C57BL6 mice, aged 5 months, were employed.
For this study, we sourced all reagents and solvents from reputable commercial suppliers, including Sigma-Aldrich (St. Louis, MO) and Acros Organics, to ensure the highest quality and purity standards. Analytical separation was carried out using an Agilent 1100 series High-Performance Liquid Chromatography (HPLC) system equipped with a diode-array detector, quaternary pump, vacuum degasser, and autosampler. Analytical thin-layer chromatography (TLC) was performed using silica gel GF254 as the stationary phase. Mass spectrometry data were meticulously recorded using an Agilent 6310 ion trap mass spectrometer (ESI source) connected to an Agilent 1200 series HPLC system with a quaternary pump, vacuum degasser, diode-array detector, and autosampler. These state-of-the-art analytical techniques were employed to ensure the accuracy and reliability of our experimental results.
Results and Discussion
In this study, we present the synthesis of the precursor for [11C]MDK-5220 preparation. Comprehensive evaluations of [11C]MDK-5220, including binding specificity and brain permeability, were conducted using and in vivo dynamic PET imaging in rodents, providing essential information for the future development of OX2R PET radiotracers. These efforts hold promise for advancing our understanding of OXR-related diseases and facilitating the development of targeted therapies using OX2R agonists.
As presented in Scheme 1, straightforward method was performed for the preparation of precursor 2. The MDK-5220 was demethylated to form precursor 2 in 92% yield by using BBr3 at −78 °C.38 The purity of precursor 2 was >99% (see Supporting Information and Figure S1). No traces of MDK-5220 or reagents used in the synthesis were found in precursor 2. Then, the radiosynthesis of [11C]MDK-5220 was implemented by a standard methylation method. The prepared [11C]CH3I was trapped in anhydrous DMF (300 μL) involving precursor 2 (2.0 mg) and K2CO3 (18.0 mg). The reaction bulb was heated at 80 °C and kept for 5 min. HPLC mobile phase (0.5 mL) was used to quench the radioactive mixture containing [11C]MDK-5220 and then applied to a reverse phase semipreparative HPLC to afford [11C] MDK-5220 in 10% yield (n = 3). [11C]MDK-5220 was collected in a flask with the retention time of 11.5 min and diluted in water (30 mL). [11C]MDK-5220 was loaded onto a solid-phase exchange (SPE) C-18 cartridge, rinsed with water (4 × 5 mL), and eluted with EtOH (0.3 mL). The radiochemical purity was >98% (n = 3, see Supporting Information and Figure S2), and the molar activity at the time of injection was 6.3 Ci/μmol on average. The identity of [11C]MDK-5220 was confirmed by co-injection with MDK-5220 as a reference standard. The obtained [11C]MDK-5220 was formulated into sterile saline (2.7 mL) and testified stability competent for succedent in vivo studies.
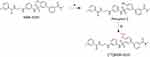 |
Scheme 1 Radiosynthesis of [11C] MDK-5220. Reagents and conditions: (a) BBr3, DCM, −78 °C to RT, 12 h; (b) [11C]CH3I, DMF, K2CO3, 80 °C, 5 min. |
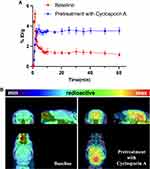
Following encouraging radiosynthesis results, we conducted PET-CT imaging in 6-month-old male C57BL/6 mice to evaluate the in vivo properties of [11C]MDK-5220 as an OX2R PET probe. [11C]MDK-5220 was administered as an intravenous bolus at a dose of 100–150 μCi (0.1–0.15 mL), followed by a 60-minute dynamic PET imaging scan and a 10-minute Magnetic Resonance Imaging (MRI) scan. The brain permeability of [11C]MDK-5220 was first examined. In vivo PET imaging of [11C]MDK-5220 in mice revealed proper BBB permeability, reaching a maximum %ID/g of 5.1 at 2 minutes after whole brain injection (Figure 2). Time-activity curves (TAC) demonstrated rapid brain penetration, binding to OX2R, and gradual clearance, suggesting plausible brain kinetics.
The modest radiotracer uptake of [11C]-5220 in the brain could be attributed to its interaction with efflux transporters such as P-glycoprotein (P-gp) located at the blood-brain barrier. To investigate this theory, we conducted PET imaging experiments in mice that were pre-treated with [11C]MDK-5220 along with the competitive P-gp inhibitor Cyclosporin A (CsA, 0.5 mg/kg). Both brain biodistribution analysis and blocking studies confirmed the robust binding selectivity and specificity of this probe for OX2R. Notably, however, pretreatment with unlabeled MDK-5220 and the P-gp competitor CsA led to a significant increase in the brain uptake of [11C]MDK-5220. This discovery implies a potential interaction between [11C]MDK-5220 and P-gp at the blood-brain barrier (Figure 2). Nonetheless, our study underscores the promise of [11C]MDK-5220 as a PET imaging probe for brain OX2R, thereby laying the groundwork for the creation of novel OX2R PET probes and the advancement of research on OX systems.
Blocking studies were performed to confirm the specificity of [11C]MDK-5220 by preadministering unlabeled MDK-5220 (1.0 mg/kg) and MDK-5220 (0.1 mg/kg) 5 minutes prior to radiotracer administration. TAC showed a significant increase in brain uptake of [11C]MDK-5220 when pretreated with the blocking agent (Figure 3A). To account for potential baseline differences, brain uptake was normalized based on the highest radioactivity in the blood at each time point. Normalized TAC curves revealed a blocking effect in the MDK-5220-pretreated group of mice (Figure 3B), supporting the binding specificity of [11C]MDK-5220 to OX2R.
Subsequently, we investigated the biodistribution of [11C]MDK-5220 in major organs of mice using in vivo PET/MR imaging. Figure 4 illustrates the radioactive absorption in the brain and major organs at different time points (5, 15, 30, 60 min). Among the peripheral organs examined, the kidney uptake was observed to be low, while the heart, liver, and lung exhibited high initial uptakes. The blood exhibited a high initial uptake with 2.82% ID/g at 5 min, followed by rapid clearance with 1.83% ID/g at 30 min and further reduced to 1.03% ID/g at 60 min. Remarkably, the liver displayed a relatively delayed clearance with 3.41% ID/g at 60 min. This high liver uptake and slow clearance collectively indicate that [11C] MDK-5220 is primarily excreted by the liver.
 |
Figure 4 Biodistribution of [11C] MDK-5220 in selected organs of mice at 5, 15, 30, and 60 min after intravenous administration of radioligand (n = 3 for each time point). |
Conclusion
In this study, our primary aim was to screen known selective OX2R agonists and, among them, identify MDK-5220 as a highly promising drug candidate for OX2R imaging. With the successful synthesis of the labeled precursor, we successfully produced [11C]MDK-5220. In vivo, PET imaging in rodents revealed that [11C]MDK-5220 exhibits suitable blood-brain barrier penetration for OX2R imaging in the brain. The probe’s excellent binding selectivity and specificity were further corroborated through regional brain biodistribution analysis and blocking studies. An intriguing observation emerged from our investigations as pretreatment with the competitive P-gp inhibitor CsA led to an increase in brain uptake of [11C]MDK-5220. This finding strongly suggests that the probe might interact with efflux transporters, warranting further investigation in future studies. Furthermore, in vivo PET/MR imaging studies exploring the biodistribution of [11C]MDK-5220 in major organs of mice demonstrated that [11C]MDK-5220 is primarily excreted by the liver, providing valuable insights into its metabolism. Moreover, this research lays the groundwork for advancements in the development of OX2R PET probes, furthering our understanding of neuropsychiatric functions and physiological processes.
Acknowledgments
The National Institute of Health DA048123 supported this research. The imaging studies were performed at the Athinoula A. Martinos Center for Biomedical Imaging of the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Regional Resource supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved using instruments supported by the NIH Shared Instrument Grant Program and/or High-End Instrument Grant Program; extraordinary grant numbers: S10RR017208, S10RR026666, S10RR022976, S10RR019933, S10RR023401. The authors are thankful to the Martinos Center radiopharmacy and imaging staff for the help with rodent experiments and radioisotope production.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev. 2006;58(1):46–57. PubMed PMID: 16507882. doi:10.1124/pr.58.1.4
2. Kukkonen JP. Orexin/hypocretin signaling. Curr Top Behav Neurosci. 2017;33:17–50. PubMed PMID: 27909990. doi:10.1007/7854_2016_49
3. Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M. Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci. 2003;60(1):72–87. PubMed PMID: 12613659. doi:10.1007/s000180300005
4. Smart D, Jerman J. The physiology and pharmacology of the orexins. Pharmacol Ther. 2002;94(1–2):51–61. PubMed PMID: 12191593. doi:10.1016/s0163-7258(02)00171-7
5. Milbank E, López M. Orexins/hypocretins: key regulators of energy homeostasis. Front Endocrinol. 2019;10:830. PubMed PMID: 31920958; PMCID: PMC6918865. doi:10.3389/fendo.2019.00830
6. Scammell TE. Overview of sleep: the neurologic processes of the sleep-wake cycle. J Clin Psychiatry. 2015;76(5):e13. PubMed PMID: 26035194. doi:10.4088/JCP.14046tx1c
7. Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol Psychiatry. 2017;81(8):683–692. PubMed PMID: 27955897; PMCID: PMC5359079. doi:10.1016/j.biopsych.2016.10.013
8. Baimel C, Bartlett SE, Chiou LC, et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172(2):334–348. PubMed PMID: 24641197; PMCID: PMC4292951. doi:10.1111/bph.12639
9. Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37(4):335–344. PubMed PMID: 10860677. doi:10.1006/hbeh.2000.1584
10. Abbas MG, Shoji H, Soya S, Hondo M, Miyakawa T, Sakurai T. Comprehensive behavioral analysis of male ox1r (-/-) mice showed implication of orexin receptor-1 in mood, anxiety, and social behavior. Front Behav Neurosci. 2015;9:324. PubMed PMID: 26696848; PMCID: PMC4674555. doi:10.3389/fnbeh.2015.00324
11. Vraka K, Mytilinaios D, Katsenos AP, et al. Cellular localization of orexin 1 receptor in human hypothalamus and morphological analysis of neurons expressing the receptor. Biomolecules. 2023;13(4):592. PubMed PMID: 37189339; PMCID: PMC10135972. doi:10.3390/biom13040592
12. Inutsuka A, Yamanaka A. The regulation of sleep and wakefulness by the hypothalamic neuropeptide orexin/hypocretin. Nagoya J Med Sci. 2013;75(1–2):29–36. PubMed PMID: 23544265; PMCID: PMC4345701.
13. Oh J, Petersen C, Walsh CM, Bittencourt JC, Neylan TC, Grinberg LT. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2019;24(9):1284–1295. PubMed PMID: 30377299; PMCID: PMC6491268. doi:10.1038/s41380-018-0291-2
14. Han Y, Yuan K, Zheng Y, Lu L. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull. 2020;36(4):432–448. PubMed PMID: 31782044; PMCID: PMC7142186. doi:10.1007/s12264-019-00447-9
15. Mogavero MP, Silvani A, Lanza G, DelRosso LM, Ferini-Strambi L, Ferri R. Targeting orexin receptors for the treatment of insomnia: from physiological mechanisms to current clinical evidence and recommendations. Nat Sci Sleep. 2023;15:17–38. PubMed PMID: 36713640; PMCID: PMC9879039. doi:10.2147/nss.S201994
16. Pardon M, Claes P, Druwé S, et al. Modulation of sleep behavior in zebrafish larvae by pharmacological targeting of the orexin receptor. Front Pharmacol. 2022;13:1012622. PubMed PMID: 36339591; PMCID: PMC9632972. doi:10.3389/fphar.2022.1012622
17. Lau J, Rousseau E, Kwon D, Lin KS, Bénard F, Chen X. Insight into the development of PET radiopharmaceuticals for oncology. Cancers (Basel). 2020;12(5):1312. PubMed PMID: 32455729; PMCID: PMC7281377. doi:10.3390/cancers12051312
18. Ten-Blanco M, Flores Á, Cristino L, Pereda-Pérez I, Berrendero F. Targeting the orexin/hypocretin system for the treatment of neuropsychiatric and neurodegenerative diseases: from animal to clinical studies. Front Neuroendocrinol. 2023;69:101066. doi:10.1016/j.yfrne.2023.101066
19. Couvineau A, Dayot S, Nicole P, et al. The anti-tumoral properties of orexin/hypocretin hypothalamic neuropeptides: an unexpected therapeutic role. Front Endocrinol. 2018;9:573. PubMed PMID: 30319552; PMCID: PMC6170602. doi:10.3389/fendo.2018.00573
20. Jin C, Luo X, Li X, et al. Positron emission tomography molecular imaging-based cancer phenotyping. Cancer. 2022;128(14):2704–2716. PubMed PMID: 35417604; PMCID: PMC9324101. doi:10.1002/cncr.34228
21. Crișan G, Moldovean-Cioroianu NS, Timaru DG, Andrieș G, Căinap C, Chiș V. Radiopharmaceuticals for PET and SPECT imaging: a literature review over the last decade. Int J Mol Sci. 2022;23(9):5023. PubMed PMID: 35563414; PMCID: PMC9103893. doi:10.3390/ijms23095023
22. Liu Y, Chen Z, Wang Y, et al. Noninvasive positron emission tomography imaging of SIRT1 in a model of early-stage alcoholic liver disease. Mol Pharm. 2023;20(4):1990–1995. PubMed PMID: 36827644. doi:10.1021/acs.molpharmaceut.2c00904
23. Xu Y, Wang Y, Wang H, Wang C. Synthesis and characterization of carbon-11 labeled iloperidone for imaging of α1-Adrenoceptor in brain. Front Mol Biosci. 2020;7:586327. PubMed PMID: 33195432; PMCID: PMC7542234. doi:10.3389/fmolb.2020.586327
24. Dale NC, Hoyer D, Jacobson LH, Pfleger KDG, Johnstone EKM. Orexin signaling: a complex, multifaceted process. Front Cell Neurosci. 2022;16:812359. PubMed PMID: 35496914; PMCID: PMC9044999. doi:10.3389/fncel.2022.812359
25. Rong J, Haider A, Jeppesen TE, Josephson L, Liang SH. Radiochemistry for positron emission tomography. Nat Commun. 2023;14(1). doi:10.1038/s41467-023-36377-4
26. Watanabe H, Fukui K, Shimizu Y, et al. Synthesis and biological evaluation of F-18 labeled tetrahydroisoquinoline derivatives targeting orexin 1 receptor. Bioorg Med Chem Lett. 2019;29(13):1620–1623. PubMed PMID: 31056243. doi:10.1016/j.bmcl.2019.04.044
27. Wang C, Wilson CM, Moseley CK, et al. Evaluation of potential PET imaging probes for the orexin 2 receptors. Nucl Med Biol. 2013;40(8):1000–1005. PubMed PMID: 23953751; PMCID: PMC3812298. doi:10.1016/j.nucmedbio.2013.07.001
28. Wang C, Moseley CK, Carlin SM, Wilson CM, Neelamegam R, Hooker JM. Radiosynthesis and evaluation of [11C]EMPA as a potential PET tracer for orexin 2 receptors. Bioorg Med Chem Lett. 2013;23(11):3389–3392. PubMed PMID: 23601709; PMCID: PMC3664928. doi:10.1016/j.bmcl.2013.03.079
29. Bai P, Liu Y, Xu Y, et al. Synthesis and characterization of a new Positron emission tomography probe for orexin 2 receptors neuroimaging. Bioorg Chem. 2022;123:105779. PubMed PMID: 35397430; PMCID: PMC9050936. doi:10.1016/j.bioorg.2022.105779
30. Watanabe H, Matsushita N, Shimizu Y, et al. Synthesis and characterization of a novel (18)F-labeled 2,5-diarylnicotinamide derivative targeting orexin 2 receptor. Medchemcomm. 2019;10(12):2126–2130. PubMed PMID: 32904113; PMCID: PMC7451066. doi:10.1039/c9md00397e
31. Bai P, Bai S, Placzek MS, et al. A new positron emission tomography probe for orexin receptors neuroimaging. Molecules. 2020;25(5):1018. PubMed PMID: 32106419; PMCID: PMC7179119. doi:10.3390/molecules25051018
32. Nerella SG, Singh P, Sanam T, Digwal CS. PET molecular imaging in drug development: the imaging and chemistry perspective. Front Med Lausanne. 2022;9:812270. PubMed PMID: 35295604; PMCID: PMC8919964. doi:10.3389/fmed.2022.812270
33. Cole EL, Stewart MN, Littich R, Hoareau R, Scott PJ. Radiosyntheses using fluorine-18: the art and science of late stage fluorination. Curr Top Med Chem. 2014;14(7):875–900. PubMed PMID: 24484425; PMCID: PMC4140448. doi:10.2174/1568026614666140202205035
34. Pretze M, Grosse-Gehling P, Mamat C. Cross-coupling reactions as valuable tool for the preparation of PET radiotracers. Molecules. 2011;16(2):1129–1165. PubMed PMID: 21270732; PMCID: PMC6259626. doi:10.3390/molecules16021129
35. Geldenhuys WJ, Mohammad AS, Adkins CE, Lockman PR. Molecular determinants of blood-brain barrier permeation. Ther Deliv. 2015;6(8):961–971. PubMed PMID: 26305616; PMCID: PMC4675962. doi:10.4155/tde.15.32
36. He Q, Liu J, Liang J, et al. Towards improvements for penetrating the blood-brain barrier-recent progress from a material and pharmaceutical perspective. Cells. 2018;7(4):24. PubMed PMID: 29570659; PMCID: PMC5946101. doi:10.3390/cells7040024
37. Nagahara T, Saitoh T, Kutsumura N, et al. Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J Med Chem. 2015;58(20):7931–7937. PubMed PMID: 26267383. doi:10.1021/acs.jmedchem.5b00988
38. Wang Y, Yao Y, Liu J, et al. Synthesis and biological activity of piperine derivatives as potential PPARγ agonists. Drug Des Devel Ther. 2020;14:2069–2078. PubMed PMID: 32546971; PMCID: PMC7266110. doi:10.2147/dddt.S238245
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.