Back to Journals » International Journal of Nanomedicine » Volume 17
Synthesis and Characterization of a Cationic Thiomer Based on Ethyl Cellulose for Realization of Mucoadhesive Tablets and Nanoparticles
Received 27 May 2021
Accepted for publication 8 April 2022
Published 20 May 2022 Volume 2022:17 Pages 2321—2334
DOI https://doi.org/10.2147/IJN.S321467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Phong A Tran
Deni Rahmat, Clairine Devina
Faculty of Pharmacy, Pancasila University, Srengseng Sawah, Jagakarsa, South Jakarta, 12640, Indonesia
Correspondence: Deni Rahmat, Faculty of Pharmacy, Pancasila University, Srengseng Sawah, Jagakarsa, South Jakarta, 12640, Indonesia, Tel +6221786472728, Fax +62217864723, Email [email protected]
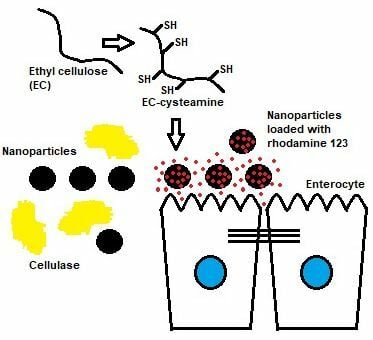
Purpose: Ethyl cellulose (EC) based nanoparticles are being extensively studied for their ability to achieve prolonged drug release and improve drug stability. Within this study, the thiolation of unmodified EC using cysteamine as a ligand was carried out to design nanoparticles with mucoadhesive properties and comparatively strong lipophilic properties.
Methods: The thiolation was performed via oxidation and reductive amination, whereas the nanoparticles were generated via the ionic gelation followed by the precipitation method.
Results: The number of free thiol groups on EC-cysteamine was in the range of 210– 261 μmol per gram of polymer. Tablets based on EC-cysteamine demonstrated mucoadhesive properties 16.7-fold improved compared with those comprising unmodified EC. The mean diameter of the particles was in the range of 94– 123 nm and the zeta potential was determined to be − 7.97 to − 14.70 mV. The nanoparticles remained attached to porcine intestinal mucosa for up to 36% after 3 h of incubation. The formation of nanoparticles improved the stability of EC-cysteamine conjugate against cellulase and provided a zero-order release. Moreover, both EC-cysteamine and the nanoparticles did not show any pronounced cytotoxicity.
Conclusion: Accordingly, EC-cysteamine nanoparticles could be a specific type of nanoparticulate delivery system with mucoadhesive properties. The amount of free thiol groups within EC-cysteamine nanoparticles together with their lipophilic properties could be further modified and modulated for a desired release behavior.
Keywords: ethyl cellulose, mucoadhesion, nanoparticle, stability, thiomer
Graphical Abstract:
Introduction
Nanoencapsulation of drugs can enhance their bioavailability and improve their biological activities. The methods of nanoencapsulation can impact on the release of drugs, thus changing their presence in systemic circulation. The design of the nanoencapsulation with respect to the route of administration can confer better therapeutic outcomes.1,2 Therefore, the effect of polymers on nanoencapsulation has been intensively reported. The physicochemical characteristics of nanoencapsulation depend essentially upon the nature of the polymeric excipients. The polymers should be stable and inert to the drugs. On the one hand, the nanoencapsulation used unmodified polymers to delay the release of drugs from nanoparticles. On the other hand, attempts have been made to develop optimized thiolation of polymeric excipients. The thiolation of polymeric excipients can form disulfide bonds to enable their controlled release properties. The polymers immobilized by free thiol groups are expressed as thiomers.3–6,7,8,9
One of the polymers used in nanoencapsulation is the cellulose family. The physicochemical characteristics of various nanoencapsulations based on cellulose derivatives have been investigated under in vitro and in vivo conditions. Recently, thiolations have been applied to the nanofabrication of polymeric particles based on cellulose derivatives. Previous studies showed that hydroxyethyl cellulose (HEC) and hydroxypropyl cellulose (HPC) were successfully modified by thiolation to generate positively charged thiomers and their nanoparticles with mucoadhesive properties. The nanoparticles were formed by the ionic gelation method with sodium tripolyphosphate (NaTPP) as a crosslinker. In addition, they render the desired attributes such as mucoadhesive and permeation enhancing properties. The ionic gelation utilizes polyelectrolytes which are able to crosslink and form hydrogel beads in the presence of counter ions. However, among the nanoparticle preparation methods using biomaterials, ionic gelation is one of the more affordable and simple methods to carry out research.5–8
Nanoencapsulation of drugs based on ethyl cellulose (EC) has been extensively studied for protecting drugs from degradation. The characteristics of the nanoencapsulation affect drug release behavior due to the interaction between the drugs and EC. In addition, the nanoencapsulation based on EC was designed to prolong the drug release period.3,4 Hence, the development of polymeric nanoparticles based on EC with better mucoadhesive properties is of high interest for various drug delivery systems. It was therefore the aim of this study to synthesize a cationic thiomer based on EC and to generate its nanoparticles. The nanoparticle was a thiomeric nanoparticle with distinctive properties.
Materials and Methods
Materials
2-(N-Morpholino)ethanesulfonic acid (MES hydrate), trinitrobenzensulfonic acid (TNBS), cysteamine, ethylene glycol, propylene glycol, t-butyl carbazate, sodium periodate, mucin from porcine stomach (type II: crude), sodium borohydride (NaBH4), sodium cyanoborohydride (NaCNBH3), sodium tripolyphosphate (NaTPP) and dialysis tubing cellulose membrane (molecular weight cut-off of 12 kDa), ethyl cellulose (∼145 mPaS 1% in H2O at 20 °C, molecular weight: ∼250,000), cellulase from T. viride (EC 3.2.1.4), glucose (hexokinase) assay kit, Caco-2 cells were all purchased from Sigma–Aldrich, Singapore. Dialysis cassette (molecular weight cut-off of 1 kDa) was obtained from Thermo Scientific USA.
Preparation of Aldehyde Polymer
Briefly, in 500 mL Erlenmeyer flask wrapped with aluminum foil, 20 mL of 8% (w/v) NaIO4 solution in distilled water was added to EC solution in ethanol 96% (v/v) at the final concentration of 1% (w/v) under continuous stirring for 2 h and 72 h at room temperature. Ethylene glycol (1 mL) was used to stop the oxidation process. The purification of the aldehyde product (EC-CHO) was conducted using dialysis tubing (molecular weight cut-off of 12 kDa; dialysis tubing cellulose membrane; Sigma, St Louis, MO) against distilled water for 3 days. The water was changed at least three times a day. The purified product was freeze dried (−78 °C, 0.01 mbar, VirTis, Gardiner, ME) and kept at 4 °C.6,8,9
Aldehyde Assay
The aldehyde assay was performed as described previously with slight modification. EC-CHO solution in 50% (v/v) ethanol (0.5 mL, 0.1%) and t-butyl carbazate (0.5 mL, 24.75 mM) in 1% (w/v) trichloro acetic acid in distilled water were mixed, and allowed to react for 24 h at room temperature. After the incubation, a volume of 200 µL was added to the aqueous TNBS solution (2 mL, 4 mM, 0.1 mM borate buffer, pH 8). The mixture was allowed to react for 30 min at room temperature, diluted with aqueous hydrochloric acid (0.5 M) and the absorbance of the solution was spectrophotometrically determined at 334 nm.9
Conjugation of Aldehyde Polymers to Cysteamine
First, 2% (w/v) EC-CHO in distilled water was prepared in three Erlenmeyer flasks, and MES hydrate was added to each flask at a final concentration of 0.1 M. Cysteamine was added in different amounts (0.125, 0.5 and 1 g). The pH value of each solution was adjusted to 5 and the solution was incubated for 3 h. Afterwards, 4 g NaCNBH3, serving as a reductor, was added, and the final mixture was stirred for 72 h at room temperature. In order to get rid of unreacted product and to isolate the polymer conjugates, the reaction mixtures were dialyzed six times in tubing (molecular weight cut-off of 12 kDa; dialysis tubing cellulose membrane; Sigma, St Louis, MO) at 10 °C in the dark. In detail, they were dialyzed once against distilled water, once against 0.2 mM HCl, then twice against the same medium but containing 1% (m/v) NaCl to quench ionic interactions between the cationic polymer and the ionic sulfhydryl compound. Then, the samples were dialyzed exhaustively twice against 0.2 mM HCl. Finally, the frozen polymer conjugates were lyophilized (−78 °C, 0.01 mbar, VirTis, Gardiner, ME) and stored at 4 °C until further use. To investigate the influence of NaCNBH3, controls were prepared in the same manner, but omitting NaCNBH3 for the reductive amination reaction.6,8,9
Determination of Free Thiol Group Content
The amount of covalently attached free thiol groups on EC-cysteamine conjugates was determined using Ellman’s reagent as described previously. The disulfide content was determined using NaBH4 for reduction reaction and Ellman’s reagent.6,8,9
Tablets Manufacture
Flat-faced tablets of unmodified EC and EC-cysteamine were prepared using a single punch eccentric press (Korsch EK, Germany) with a compaction pressure of 10 kN. The weight and diameter of tablets were 50 mg and 5.0 mm, respectively.6,9
Mucoadhesion Studies of Tablets
Unmodified EC, EC-CHO and EC-cysteamine tablets were put on a freshly excised intestinal porcine mucosa, which was attached to a stainless steel cylinder (diameter 4.4 cm; height 5.1 cm; apparatus 4-cylinder, USP). Afterwards, the cylinder was immersed in 500 mL of 100 mM phosphate buffer pH 6.8 in the dissolution apparatus according to the USP at a temperature of 37 °C and agitated at 125 rpm.6,9
Viscosity Studies
The viscosity study was performed by solubilizing 4 g of porcine mucin in 25 mL of demineralized water. The pH of the solution was diluted to a final volume of 50 mL with 200 mM phosphate buffer to obtain a pH value of 6.8. Unmodified EC, EC-CHO and EC-cysteamine, respectively, were suspended in demineralized water at a concentration of 6% (m/v). After optimizing dispersion, the obtained polymer suspensions were mixed with an equal volume of 8% (m/v) mucin solution. The suspension of 3% (m/v) polymer and 4% (m/v) mucin solution were used as references. Following sample preparation, each mixture was allowed to equilibrate for 20 min. The viscosity of each sample was measured at a temperature of 37 °C, 60 rpm, and a shear rate of 450 s−1 using a viscometer, Brookfield RV, with a small sample adapter.6,9,10–12
Differential Scanning Calorimetric (dsc) Analysis of Unmodified EC and EC-Cysteamine
The DSC profiles of unmodified EC, EC-CHO and EC-cysteamine were depicted using a PerkinElmer Thermal Analysis at a scanning rate of 10 °C/min from 20 to 300 °C under a nitrogen gas stream at a flow rate of 40 mL/min. Afterwards, 5 mg unmodified EC and EC-cysteamine were precisely weighed and put into flat-bottomed aluminum pans.6
Preparation of Nanoparticles
Briefly, a solution of 50 mg of EC-cysteamine in 50 mL of ethanol 96% (v/v) in two Erlenmeyer flasks wrapped with aluminum foil was prepared. Rhodamine 123 at a final concentration of 0.02 mg/mL was added to one of the Erlenmeyer flasks. Afterwards, 0.2% (w/v) NaTPP solution in the mixture of propylene glycol and distilled water in the volume ratio of 1:5 was added dropwise into each solution until a translucent appearance was obtained. The final mixture was mixed with the same volume of distilled water and stirred under permanent stirring for 10 min. The nanoparticles were recovered by centrifugation at 5000 g for 30 min. The nanoparticles loaded with rhodamine 123 were washed three times with demineralized water in order to remove free rhodamine 123. The washing solutions were separated by centrifugation as described previously. The purified nanoparticles were lyophilized and dissolved in ethanol 96% (v/v). The amount of rhodamine 123 in the ethanolic solution was determined by fluorescence spectrometry.8,9,13
Nanoparticle Characterizations
Particle size and zeta potential of the nanoparticles were measured by DLS (Particle and Zeta Sizer Malvern ZPS) at 25 °C. The morphology of the nanoparticles was observed using transmission electron microscope (TEM) JEOL 1010 operated at 80 kV of beam energy. Rhodamine 123 in the ethanolic solution was measured using fluorescence spectrometry at 550 nm excitation and 570 nm emission wavelength.8,9,13
Degradation Studies
Degradation of EC-cysteamine and EC-cysteamine nanoparticles were demonstrated in the presence of 0.1% (w/v) cellulase solution in 50 mM sodium acetate buffer pH 5. In brief, 0.1% (w/v) suspension of unmodified EC, EC-cysteamine and EC-cysteamine nanoparticles in the buffer solution were each incubated with the cellulase solution for 180 min at 37 °C. Generated glucose resulting from hydrolysis of all samples was measured using an enzymatic assay.8,11
Cytotoxicity Studies
Resazurin assay was applied in cytotoxicity studies to assess the viability of Caco-2 cells in the presence of 0.25% (w/v) unmodified EC, EC-cysteamine and EC-cysteamine nanoparticles. 1×105 Caco-2 cells were seeded per well in 24-well plates and grown in a humidified chamber at 37 °C, 5% CO2. MEM without phenol red and 5% (v/v) Triton X-100 plus medium served as low and high control, respectively. After the incubation of the cells with each tested polymer for 72 h, the medium was discharged from each well and 0.5 mL of 44 µM of resazurin solution was added to each well. The cells were then incubated at 37 °C, 5% CO2 for 3 h and fluorescence was recorded at 540 nm excitation and 590 nm emission wavelength using a Spectrophotometer DU® Series 600.8,9,12
In vitro Release Studies
The dialysis diffusion technique was used to evaluate the release of rhodamine 123 from EC-cysteamine nanoparticles. In brief, 75 mg of the EC-cysteamine nanoparticles loaded with rhodamine 123 were suspended in 10 mL phosphate buffer solution at pH 7.2 as the inner phase and transferred to a dialysis bag (molecular weight cut-off: 1 KDa). The dialysis bag was put into 250 mL outer phase with the same medium as the inner phase under continuous stirring at 37 °C. A 500 µL sample was withdrawn from the outer phase at a predetermined time and replaced with the same volume of the fresh medium. The amount of rhodamine 123 released from the nanoparticles was determined by fluorescence spectrometry at 550 nm excitation and 570 nm emission wavelength.13,14
Mucoadhesion Studies of EC-Cysteamine Nanoparticles
In brief, freshly porcine intestinal mucosa was mounted on a half pipe in the position of an angle of 45° in an incubator with 100% humidity and a temperature of 37 °C. After an equilibrium humidity, 75 mg of the lyophilized particles were administered to the mucosa and rinsed with phosphate buffer (100 mM, pH 6.5, 37 °C) at a constant rate of 1 mL/min. Afterwards, the mucosa was incubated in 25 mL ethanol 96% (v/v) for 20 min at 37 °C. The amount of the remaining rhodamine 123 was quantified using a spectrofluorometer as described in the release studies.8,9,13–15
Statistical Data Analysis
Statistical data analysis was performed using SPSS software and the statistical differences between the control group to the test groups were calculated by a one-way ANOVA test. A p-value <0.05 was considered statistically significant.
Results
Preparation of Aldehyde Polymer
The main methods for glucose ring opening of EC includes two steps, namely cleaving the C–C bonds of molecules with vicinal diols and the construction of aldehyde groups, leading to EC-CHO by the addition of NaIO4.16–18 Although the formation of thiomer using carbodiimide reaction has been well studied, novel strategies to construct the immobilization of free thiol groups are still of high interest for their better properties.
It was observed that once glucose ring opening occurs involving periodate oxidation, the solution turned cloudy. This observation directed to explore the concentration of NaIO4 for the oxidation. The amount of NaIO4 used in the present study was effective for glucose ring opening of HEC as reported in the previous study. The optimization process was attempted with increased amount of NaIO4 but it caused precipitation of NaIO4 in ethanolic solution of the polymer.
Aldehyde Assay
After the dialysis, EC-CHO served as a starting material for reductive amination. The results revealed that NaIO4 was suitable for the oxidation of EC and the resulting polymer was hydrated with very low solubility in distilled water. Therefore, EC-CHO could not be homogeneously mixed with the reagents for degree of oxidation (DO) assay. By lengthening the reaction time up to 72 h, the solubility was improved and led to 28% of DO.
Conjugation of Aldehyde Polymers to Cysteamine
The synthesis of EC-cysteamine conjugate was performed by reductive amination of EC-CHO using cysteamine with various concentrations (0.125, 0.5 and 1 g). The aldehyde groups underwent an amination substitution reaction, where the primary -NH group was derived from cysteamine. The thiolation was a simple step for the N-alkylation of cysteamine and allowed the reductive alkylation of cysteamine using NaCNBH3 as a reducing agent in MES buffer without adding a catalyst.19,20
Determination of Free Thiol Group Content
The determination of the number of free thiol groups on the conjugate (Figure 1) was attained by the Ellman test method using Ellman’s reagent, which is 5.5 ‘-dithio-bis (2-nitrobenzoic acid) (DNTB) in Ellman’s buffer medium.6,8,9,21 The addition of 0.125 g cysteamine resulted in the lowest amount of free thiol groups and disulfide bonds as shown in Table 1. In contrast, the use of 0.5 g cysteamine could effectively react with the aldehyde groups of EC-CHO, whereas further addition of cysteamine with amount of 1 g significantly could not further increase the thiolation. In addition, the controls displayed only an insignificant number of free thiol groups.
 |
Table 1 Amount of Free Thiol Groups and Disulfide Bonds Immobilized on EC–Cysteamine |
 |
Figure 1 Oxidation and reduction amination, EC (A), EC-CHO (B), EC-cysteamine (C). |
Mucoadhesion Studies of Tablets
The mucoadhesive study was conducted to prove the effect of free thiol groups on the mucoadhesion of unmodified EC, EC-CHO and EC-cysteamine tablets which were attached to the porcine intestine containing mucosa in phosphate buffer medium pH 6.8 at 37 °C. The adhesion time of each polymer tablet is depicted in Figure 2.
 |
Figure 2 Mucoadhesive properties of EC, EC-CHO and EC-cysteamine; indicated values are means of at least three experiments±SD. |
The results showed that EC-cysteamine tablets had an adhesion time of 5.4 h which was the longest amongst the three, whereas EC-CHO tablets had the capability of attaching to the intestine due to their wettability properties. The increase in mucoadhesive properties in EC-cysteamine tablets could be due to the formation of disulfide bonds between the cationic thiomers and mucus that contains cysteine. EC-cysteamine tablets were able to swell. The mucoadhesive properties are related to the swelling behavior. The ability to swell of EC-cysteamine tablets might influence the interdiffusion process between the polymer and the mucous layer, which gives rise to stronger adhesion properties.6,9,21–23
Viscosity Studies
The viscosity of EC-cysteamine became significantly higher after mixing with mucin as shown in Figure 3. An increase in the viscosity of EC-cysteamine conjugate was due to the formation of disulfide bonds between thiol groups found in the EC-cysteamine and cysteine within the mucous layer. The viscosity of unmodified EC and EC-CHO also increased but was insignificant. Since ionic interaction and hydrogen bonding with the mucin layer could occur, EC-CHO exerted a higher viscosity compared with unmodified EC, although the bond was not as strong as the disulfide bond. Since the viscosity study was carried out at pH 6.8, the carboxylic acid groups in mucin (from terminal sialic acid) are in the anion form. Therefore, ionic interactions between the cationic thiomer and mucin are also involved.6,9,21–23
 |
Figure 3 Viscosity values and rheological synergism for 3% (m/v) EC, EC-CHO and EC-cysteamine and their corresponding mixtures with mucin; indicated values are means of at least three experiments±SD. |
Differential Scanning Calorimetric (dsc) Analysis of Unmodified EC and EC-Cysteamine
The results of the thermal analysis using DSC were carried out starting at a temperature of 20 °C with a heating rate of 10 °C per minute, indicating a shift in the temperature of the endothermic peak and the enthalpy of unmodified EC and EC-cysteamine. Based on the results of the thermogram obtained, the glass transition temperature (Tg) of unmodified EC and EC-cysteamine was 276.82 and 250.48 °C, respectively (Figures 4 and 5). This could show a decrease in the melting point of unmodified EC influenced by changes in its structure. The process of glucose ring opening and cysteamine conjugation to the aldehyde groups on the backbone of EC-CHO might interfere with the Tg. The oxidation with 28% of DO might be unable to change the conformation and polymorphism of unmodified EC crystal. Thereby, cysteamine covalently bound to EC significantly contributed to a Tg shift. Accordingly, the DSC profile might be a valuable tool for characterization of the resulting thiomer.24,25
 |
Figure 4 Glass transition (Tg) curve of EC. |
 |
Figure 5 Glass transition (Tg) curve of EC-cysteamine. |
Preparation of Nanoparticles
NaTPP in aqueous solution can react with the amine groups of EC-cysteamine. Since both amine and free thiol groups of EC-cysteamine were in comparatively low amounts, the formation of nanoparticles based on EC-cysteamine might need the use of an additional method to generate nanoparticles. It was observed that the addition of distilled water after the crosslinking resulted in a reduced particle size with a narrowed size distribution. For the release studies, nanoparticles with the smallest particle size were selected. The amine functional groups in EC-cysteamine as a cationic thiomer interacted with free TPP ions to assemble the nanoparticles, which then might break into the smaller particle size after the addition of distilled water.
Nanoparticles Characterization
In the nanoparticle preparation, experimental parameters such as type of solvent used to solubilize NaTPP and the volume of distilled-water added to the polymer solution after the addition of NaTPP were critical (Figure 6). The addition of distilled water was crucial for the formation of EC-cysteamine nanoparticles. It could allow the precipitation in nanoscale thus both nanoparticles alone and nanoparticles loaded with rhodamine 123 were obtained after 10 min of incubation with a mean diameter of 123±25 nm and 94±14 nm, respectively. Propylene glycol was used as a wetting agent when an aqueous solution of NaTPP was introduced to ethanolic solution of EC-cysteamine. Hence, propylene glycol could prevent the aggregation, leading to non-nanoscale particles during the crosslinking process. The zeta potential of the nanoparticles alone and the nanoparticles loaded with rhodamine 123 were −14.7±4.6 and −7.97±3.89 mV, respectively. The free TPP ions resulted in the negatively charged nanoparticles. The zeta potential decreased as rhodamine 123 was entrapped within the nanoparticles. This can be explained by the fact that rhodamine 123 with amine groups would neutralize the charges in the nanoparticles.
 |
Figure 6 The morphology of EC-cysteamine nanoparticles alone (A) and EC-cysteamine nanoparticles loaded with rhodamine 123 (B). |
Degradation Studies
Cellulase was used to degrade the cellulose family, which has adjacent sugar molecules joined by β1→4 linkages. The degradation of EC-cysteamine and EC-cysteamine nanoparticles without rhodamine 123 was defined as the amount of glucose (mg) produced from the samples due to the activity of cellulase at 37 °C. As depicted in Figure 7, only EC-cysteamine and EC-cysteamine nanoparticles can be degraded. Since unmodified EC is practically insoluble in distilled water, the aqueous solution of cellulase could not contact with the EC particles, thus insignificant glucose was found after the incubation. The hydrolysis rate of EC-cysteamine was found to be significantly faster compared with EC-cysteamine nanoparticles. The degradation of EC-cysteamine nanoparticles was 1.2-fold reduced compared with EC-cysteamine. This finding might broaden the application of EC-cysteamine nanoparticles.
 |
Figure 7 Degradation of EC-cysteamine (▲) and EC-cysteamine nanoparticles (X); indicated values are means of at least three experiments±SD. |
Cytotoxicity Studies
The cytotoxicity profile of all the tested samples was conducted using the resazurin assay. The cells were viable after 72 h of exposure to different types of tested samples. The incubation of the cell with the tested samples could reduce resazurin efficiently, and the pink colored solution generated was analyzed spectrophotometrically. The same profile of cell survival was also obtained by HEC. These results were corroborated by previously published cytotoxicity of the cationic thiomer.8,9,12 Moreover, 0.25% (m/v) all the tested samples demonstrated more than 80% cell viability (Figure 8).
 |
Figure 8 Cytotoxicity studies of EC, EC-cysteamine and EC-cysteamine nanoparticles using resazurin; indicated values are means of at least three experiments±SD. |
Unmodified EC, EC-cysteamine and EC-cysteamine nanoparticles did not show any cytotoxicity as the results of the cell viability were 98.78, 93.25 and 84.43%, respectively. The cells treated with the nanoparticles showed the lowest viability. The exposure of the nanoparticles to the cells for 72 h could lead to their significant interaction thus interfering with the viability of cells. Hence, it could be assumed that the viability decreases when the sample undergoes modifications in its substructures (amine and thiol groups) and the formulation (nanoparticles) due to its comparatively effective surface area.
In vitro Release Studies
The release profile of rhodamine 123-loaded nanoparticles was measured at pH 7.2 and found to follow a biphasic pattern; a burst release followed by a slower release rate. As shown in Figure 9, the release profile was recorded within 8 h. This initial burst release might be attributed to the rhodamine 123 molecules adsorbed on the surface. Moreover, 12.57% (w/v) of rhodamine 123 was released within 1 h (Figure 9). The slower second phase with zero order could be related to the entrapped rhodamine 123 inside the nanoparticles due to the crosslinking process and disulfide bond formation within the nanoparticles.3–5,13,14
 |
Figure 9 Release studies of EC-cysteamine nanoparticles loaded with rhodamine 123; indicated values are means of at least three experiments±SD. |
Mucoadhesion Studies of EC-Cysteamine Nanoparticles
EC-cysteamine nanoparticles could be loaded with a fluorescent agent, rhodamine 123 due to the crosslinking and precipitation process in the nanoscale. Rhodamine 123 was mixed with EC-cysteamine solution in an organic solvent and thus 3.5% (w/w) of rhodamine 123 load was obtained. In the case of the tablets, the mucoadhesive properties were significantly improved by thiolation. Moreover, 36% (w/w) EC-cysteamine nanoparticles remained on the intestine after 3 h as shown in Figure 10. The residence time of nanoparticles based on EC-cysteamine might correspond to the number of free thiol groups on the surface of EC-cysteamine nanoparticles. While the amount of free thiol groups of EC-cysteamine was lower compared with HEC-cysteamine, the strength of the mucoadhesive properties of both cationic thiomers is quite similar.8,9
 |
Figure 10 Mucoadhesive properties of EC-cysteamine nanoparticles; indicated values are means of at least three experiments±SD. |
Discussion
The addition of NaIO4 caused the destruction of unmodified EC to build up EC-CHO and would grant an exact DO. The synthesis of thiomers with the use of carboxylate groups to form amide bonds does not work for the nonionic cellulose derivatives. EC-cysteamine as a cationic thiomer was obtained from the glucose ring opening of unmodified EC and the reductive amination. The available aldehydes on EC-CHO give rise to an effective C-N bond formation and allow immobilization of free thiol groups at room temperature in the presence of NaCNBH3. The amination reaction occurs due to the primary amine group derived from cysteamine. The mechanism for the reaction of thiomer formation can be described in the following way: in the first step, one of the I-O bonds from NaIO4 attacks one of the two hydroxyl groups of the vicinal diol; the second step is the formation of planar cyclic esters as part of an octahedral intermediate. The reaction of the polymer aldehyde (EC-CHO) with primary amine (cysteamine) produces an amine carbinol which can dehydrate to form imines. Protonated imines form quaternary imminium under weak acidic to neutral conditions.9,16–20
DSC is a technique that measures heat flow into or out of a material as a function of time or temperature. Polymer crystallinity can be determined with DSC by quantifying the heat associated with melting (fusion) of the polymer. The DSC profile of EC was characterized by Tg, which decreased after thiolation. It has been studied that after the polymers are melted, the changes in the structure of polymers decide the transformation of liquid into a glassy or crystalline state.24 The conjugation of cysteamine might increase free volume in the molecule of EC-cysteamine, leading to the decreased Tg. Intramolecular disulfide bonds in EC-cysteamine might decrease bond rotation, resulting in an increase in free volume, thus the decrease in Tg.24,25
Secondary amine groups on the backbone of EC-cysteamine demonstrated a stronger basicity compared with primary amine groups on polymer backbones. It has been reported that the protons prefer interaction with the stronger base.8 Therefore, the resulting ammonium ions of EC-cysteamine are stronger cations. These positive charges could significantly interact with negatively charged molecules to form the nanoparticles.8,9 Therefore, to synthesize nanoparticles based on EC-cysteamine, NaTPP solution in a mixture of propylene glycol and distilled water in a volume ratio of 1:5 was added to the polymer solution at room temperature. The nanoparticles were generated via inter- and/or intramolecular linkages between the amine groups protonated and the phosphate groups of NaTPP ions. The organic to aqueous phase ratio in NaTPP solution played an important role in the nanoparticle formation process. The size of the nanoparticles increased when the ratio decreased. NaTPP cannot completely dissolve when the concentration of the organic phase is increased. The presence of propylene glycol could prevent agglomeration of the generated EC-cysteamine nanoparticles. Moreover, the addition of distilled water could result in the precipitation in nanoscale and optimizes the crosslinking process within the newly formed surface of the resulting nanoparticles, thus strengthening their integrity.
The formation of nanoparticles had a significant effect on the degradability. The crosslinking process and disulfide bonds formation could not be ruled out in reducing degradation. The nanoparticle without rhodamine 123 was used in the degradation study to avoid the influence of rhodamine 123 in the glucose assay. In addition, the change in conformation and three-dimensional structure of unmodified EC that could be present after the thiolation and the crosslinking process significantly reduced cellulolytic enzyme activity. It was shown that the cellulase could selectively degrade EC-cysteamine into glucose.
The thiolation of unmodified EC produced the cationic thiomer with wettability properties. Thereby, the cells exposed to EC-cysteamine for 72 h underwent a decrease in viability due to better contact with the cells compared with unmodified EC dispersion. Amine functional groups on EC-cysteamine and the surface of EC-cysteamine nanoparticles can be protonated to confer positive charges, which could lead to cytotoxicity. 1,2,3 resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) is an indicator of cell cytotoxicity widely used to investigate the biocompatibility of the modified polymers. Mitochondrial enzymes are involved in the transference of electrons from NADPH + H+ to resazurin that causes the formation of resorufin, which is a strongly fluorescent dye. After the cells were exposed for 72 h to the different types of samples, the cells were capable of reducing resazurin efficiently and the pink colored solution appeared.26,27 The thiolation of unmodified EC and the formulation of nanoparticles did not show any significant effect on the cytotoxicity. A previous study with rats demonstrated that the toxicity of EC dispersion with doses of 0, 903, 2709 and 4515 mg/kg/day orally administered within 15 days showed no maternal deaths occurred.28
One of the most common developments in drug release is preparing polymeric nanocarriers that contain an active compound. Due to the increase in bioavailability and stability of drugs, nanoparticles have offered significant advantages for various drug formulations. To form its nanoparticles, EC-cysteamine as a matrix polymer utilized the ionic gelation and the precipitation method. The nanoparticles could provide prolonged drug release and simultaneously prolong their residence time along the gastrointestinal tract due to their mucoadhesive properties. Interestingly, previous studies demonstrated that EC offered a number of advantages such as stabilizing drugs in the extreme acidic environment, as EC is a pH-resistant polymer, and the decrease in the adverse effect of drugs along the gastrointestinal tract.3,4
EC-cysteamine-based polymeric nanoparticles could control the drug release with mucoadhesive properties. The release profile used rhodamine 123 as a drug model to reflect the critical information about the behavior of EC modification that might improve the efficacy of the entrapped drugs and might enrich the type of modified release. In addition, since EC can act as an emulsifying agent, EC-cysteamine could be formulated into various nanoemulsion formulations with controlled release and mucoadhesive properties.28
Conclusions
An attempt to change the properties of EC has been made in order to enrich the type of modified release and nanoparticle preparation method. EC-cysteamine synthesized by the glucose ring opening and the reductive amination is a cationic thiomer with the number of free thiol groups in the range of 210–261 µmol per gram of polymer. The nanoparticles based on EC-cysteamine, with comparatively strong lipophilic properties, exerted more stability in the cellulase solution than EC-cysteamine. Therefore, the conjugate might have the capability of stabilizing nanoparticles and nanoemulsion along the gastrointestinal tract to support the opportunity of attaching to the mucosa. The conjugate demonstrated the mucoadhesive properties for 5.4 h. In addition, the controlled release system could be provided by the nanoparticles, which were found to be non-toxic to the Caco-2 cell line. Accordingly, the conjugate could be explored to improve the bioavailability of drugs using various nanoparticulate drug delivery systems, which might open many gates to the most optimal therapeutic effect.
Acknowledgment
The research was funded by RISTEK DIKTI Indonesia, Skema WCR SK: 01/E1/KPT/2019.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi:10.1016/s0168-3659(00)003339-4
2. Kumari A, Yadav SK. Cellular interactions of therapeutically delivered nanoparticles. Exp Opinion Drug Deliv. 2011;8:141–151. doi:10.1517/17425247.2011.547934
3. Iman K, Jafari SM. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci Technol. 2016;53:34–48. doi:10.1016/j.tifs.2016.05.002
4. Naveen NR, Gopinath C, Kurakula M. Okra-thioglycolic acid conjugate-synthesis, characterization, and evaluation as a mucoadhesive polymer. Processes. 2020;8(316):1–19. doi:10.3390/pr8030316
5. Yang D, Chen W, Hu J. Design of controlled drug delivery system based on disulfide cleavage trigger. J Phys Chem B. 2014;118:12311–12317. doi:10.1021/jp507763a
6. Rahmat D, Rahman FAR, Nurhidayati L, Laksmitawati DR. Synthesis and characterization of hydroxypropyl cellulose-cysteamine conjugate as a novel cationic thiomer with lipophilic properties. Int J App Pharm. 2019;11:222–226. doi:10.22159/ijap.2019v11i1.30014
7. Santana SP, Salazar NF. Ionotropic gelation method in the synthesis of nano/microparticles for biomedical purposes. Polym Int. 2020;69:443–447. doi:10.1002/pi.5970
8. Rahmat D, Müller C, Barthelmes J, Shahnaz G, Martien R, Schnürch AB. Thiolated hydroxyethyl cellulose: design and in vitro evaluation of mucoadhesive and permeation enhancing nanoparticles. Eur J Pharm Biopharm. 2013;83:149–155. doi:10.1016/j.ejpb.2012.10.008
9. Rahmat D, Sakloetsakun D, Shahnaz G, Perera G, Kaindl R, Schnurch AB. Design and synthesis of a novel cationic thiolated polymer. Int J Pharm. 2011;411:10–17. doi:10.1016/j.ijpharm.2011.02.063
10. Hassan EE, Gallo JJ. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–495. doi:10.1023/A:1015812615635
11. Bondar RJL, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20:586–590. doi:10.1093/clinchem/20.5.586
12. Iqbal J, Shahnaz G, Dünnhaupt S, Müller C, Bernkop-Schnürch A. Preactivated thiomer as mucoadhesive polymers for drug delivery. Biomaterials. 2012;33:1528–1535. doi:10.1016/j.biomaterials.2011.10.021
13. Jonderian A, Maalouf R. Formulation and in vitro interaction of rhodamine-b loaded PLGA nanoparticles with cardiac myocyte. Front Pharmacol. 2016;7:1–7. doi:10.3389/fphar.2016.00458
14. Balzus B, Colombo M, Sahle FF, Zoubari G, Staufenbiel S, Bodmeier R. Comparison of different in vitro release methods used to investigate nanocarriers intended for dermal application. Int J Pharm. 2016;513:247–254. doi:10.1016/j.ijpharm.2016.09.033
15. Sarti F, Iqbal J, Müller C, Shahnaz G, Rahmat D, Schnürch AB. Poly(acrylic acid)–cysteine for oral vitamin b12 delivery. Anal Biochem. 2013;420:13–19. doi:10.1016/j.ab.2011.08.039
16. Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. 2007;28:975–983. doi:10.1016/j.biomaterials.2006.10.021
17. Crespin L, Biancalana L, Morack T, Blakemore DC, Ley SV. One-pot acid-catalyzed ring-opening/cyclization/oxidation of aziridines with N‑tosylhydrazones: access to 1,2,4-triazines. Org Lett. 2017;19:1084–1087. doi:10.1021/acs.orglett.7b00101
18. Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride: studies on direct and indirect reductive amination procedures (1). J Org Chem. 1996;61:3849–3862. doi:10.1021/jo960057x
19. Tadanier J, Hallas R, Martin JR, Stanaszek RS. Observation relevant to the mechanism of the reductive aminations of ketones with sodium cyanoborohydride and ammonium acetate. Tetrahedron. 1981;37:1309–1316. doi:10.1016/S0040-4020(01
20. Pletz J, Berg B, Breinbauer RA. General and direct reductive amination of aldehydes and ketones with electron-deficient anilines. Synthesis. 2016;48:1301–1317. doi:10.1055/s-0035-1561384
21. Hombach J, Palmberger TF, Schnürch AB. Development and in vitro evaluation of a mucoadhesive vaginal delivery system for nystatin. J Pharm Sci. 2009;98:555–564. doi:10.1002/jps.21457
22. Schnürch AB. Thiomers: a new generation of mucoadhesive polymers. Adv Drug Deliv Rev. 2005;57:1569–1582. doi:10.1016/j.addr.2005.07.002
23. Roldo R, Hornof M, Caliceti P, Schnürch AB. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur J Pharm Biopharm. 2004;57:115–121. doi:10.1016/s0939-6411(03)00157-7
24. Jadhav NR, Gaikwad VL, Nair KJ, Kadam HM. Glass transition temperature: basics and application in pharmaceutical sector. Asian J Pharm Sci. 2009;3:82–89. doi:10.4103/0973-8398.55043
25. Gombas A, Szabó-Révész P, Kata M, Regdon JG, Erõs I. Quantitative determination of crystallinity of a-lactose monohydrate by dsc. J Therm Anal Calorim. 2002;68:503–510. doi:10.1023/A:1016039819247
26. Erikstein BS, Hagland HR, Nikolaisen J, et al. Cellular stress induced by resazurin leads to autophagy and cell death via production of reactive oxygen species and mitochondrial impairment. J Cell Biochem. 2010;111:574–584. doi:10.1002/jcb.22741
27. Borra RC, Lotufo MA, Gagioti SM, Barros FDM, Andrade PM. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz Oral Res. 2009;23:255–562. doi:10.1002/jcb.22741
28. Melzera E, Kreuterb J, Danielsa R. Ethylcellulose: a new type of emulsion stabilizer. Eur J Pharm Biopharm. 2003;56:23–27. doi:10.1016/s0939-6411(03)00025-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
