Back to Journals » Infection and Drug Resistance » Volume 11
Synergy effect of meropenem-based combinations against Acinetobacter baumannii: a systematic review and meta-analysis
Received 24 April 2018
Accepted for publication 13 June 2018
Published 7 August 2018 Volume 2018:11 Pages 1083—1095
DOI https://doi.org/10.2147/IDR.S172137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Zhihui Jiang,1,2,* Xianxia He,3,* Jian Li2
1Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, China; 2Department of Pharmacy, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, China; 3Department of Drug Certification, Center for Certification and Evaluation, Guangzhou Food and Drug Administration, Guangzhou, China
*These authors contributed equally to this work
Purpose: The main objective of our meta-analysis was to examine the in vitro synergistic effect of meropenem-based combination therapies against Acinetobacter baumannii through a systematic review of the existing literature.
Methods: An extensive search was performed with no restrictions on date of publication, language, and publication type. Our study evaluated the main conclusions drawn from various studies describing the synergistic activity of combination therapies in vitro.
Results: In this review, 56 published studies were included. Our report included data on 20 types of antibiotics combined with meropenem in 1,228 Acinetobacter baumannii isolates. In time-kill studies, meropenem combined with polymyxin B and rifampicin showed synergy rates of 98.3% (95% CI, 83.7%–100.0%) and 89.4% (95% CI, 57.2%–100.0%), respectively, for Acinetobacter baumannii, modest synergy rates were found for meropenem combined with several antibiotics such as colistin and sulbactam, and no synergy effect was displayed in the combination of meropenem and ciprofloxacin, whereas in checkerboard method, the synergy rates of polymyxin B and rifampicin were 37.0% (95% CI, 0.00%–100.0%) and 56.3% (95% CI, 8.7%–97.8%), respectively.
Conclusion: We found that time-kill studies generally identified the greatest synergy, while checkerboard and Etest methods yielded relatively poor synergy rates. Further well-designed in vivo studies should be carried out to confirm these findings.
Keywords: Acinetobacter baumannii, meropenem, synergy, combination, in vitro
Introduction
The spread of Acinetobacter baumannii (A. baumannii), a major pathogen responsible for hospital infections, is difficult to control and its infections are difficult to treat owing to its ability to adapt to different environments and its intrinsic resistance to many antibiotics.1 Furthermore, an increasing number of multi-drug-resistant (MDR), even pan-drug-resistant A. baumannii strains have been isolated.2 Infections caused by MDR A. baumannii primarily occur in immunosuppressed patients, in patients with serious underlying diseases, and in those who underwent invasive procedures and treatment with broad-spectrum antibiotics.3 Meropenem, as a carbapenem antibiotic, has a low-toxicity profile and is highly resistant to serine β-lactamases produced by many MDR gram-negative bacteria, thus playing a key role in the treatment of various infections that are not readily treated by other antibiotics.4 However, reports regarding the yearly increase in meropenem-resistant A. baumannii strains have captured the attention of clinicians and microbiologists.5 A great focus has been placed on combination therapies to reduce A. baumannii resistance, and numerous experiments have been performed with meropenem-based therapies.6 However, systematic analysis of meropenem-based combination therapies is still lacking. To provide a basis and reference for clinical rationales behind combination therapies using meropenem, we systematically searched and analyzed three mainstream databases to evaluate the in vitro synergistic activity of meropenem with other antibiotics against A. baumannii.
Materials and methods
Search strategy and selection criteria
A broad literature search on the PubMed, Embase, and Web of Science databases was performed throughout March 2018 by two separate reviewers. Journal articles were selected without limitations on publication date, language, or publication type. Keywords and Boolean operators used for the searches were (meropenem) AND (baumannii) AND (synerg* OR combin*). We also used the related articles function to broaden our search. In addition, reference lists of the selected articles were also manually examined to find relevant studies that were not included in our initial searches.
All meropenem-based in vitro combinations tests (application of traditional testing methods including the checkerboard, Etest, and the time-kill method, which included both the static time-kill and the in vitro dynamic pharmacokinetic/pharmacodynamic model) were included in this study. Those testing meropenem in combination with compounds that are not available on the market worldwide, and the combination therapies with more than two drugs were excluded.
Data collection and statistical analysis
In this meta-analysis, data were independently extracted by two researchers using a premade data extraction form. From each study, the following information was extracted: 1) first author and the publication year; 2) susceptibility to meropenem; 3) types of antibiotics used; 4) total number of isolates tested; 5) in vitro combination testing methods. Antibiotic breakpoints were set based on the Clinical and Laboratory Standards Institute. Meropenem susceptibility and resistance were defined: susceptible, ≤2 mg/L; intermediate, 4 mg/L; resistant, ≥8 mg/L.
The outcome analyzed in the study was the in vitro synergy activity of combination therapy. For time-kill tests, synergy was defined as ≥2 log10 (at any time points in 24 hours) colony-forming units/mL decrease between the combination therapy and the most efficient agent, when used alone. With the checkerboard and Etest method, interactions were defined by the fractional inhibitory concentration index (FICI), which was calculated using the following formula: FICI = (MICAB/MICA) + (MICBA/MICB), where MICAB and MICBA were the minimum inhibitory concentrations (MIC) of drugs A and B in combination therapy, while MICA and MICB were the MIC of drugs A and B tested alone. The FICI value was interpreted as follows: ≤0.5, synergy; >0.5–4, indifference; >4, antagonism.7
Synergy rates with 95% CI and each isolate reported in a paper were calculated separately for each synergy method, where the number of isolates tested was defined as the sample size and the event was defined as synergy. As the event rates in most studies are 0 or 1, which are not normally distributed, we performed a logit transformation for the event rates. Results from each testing method were subgrouped by antibiotic type. Some time-kill studies utilized multiple drug concentrations on the same bacterial strains, and we chose a more common clinically achievable drug concentration. For studies applying multiple test methods, data of different methods were separately collected and analyzed. All statistical analyses were performed with the Stata 14.0 software (Stata Corporation 2015, College Station, TX, USA). We calculated various pooled synergy rates using both random- and fixed-effects models. Heterogeneity was assessed by I2. An I2 statistic of 0% indicated no observed heterogeneity. We used a fixed-effects model when I2 <50%; otherwise, the random-effects model was used.
Results
Studies included
Our search strategy initially yielded 1,161 potentially relevant citations from PubMed, Embase, and Web of Science (Figure 1). Excluding the studies that did not report any in vitro experiments evaluating the synergistic effect of meropenem-based combinations against A. baumannii, 56 published studies fulfilled the inclusion criteria and were included in this review.7–62 The characteristics of each included study are described in Table 1. In the analysis, 1,228 A. baumannii strains were subjected to 14 Etests, 42 checkerboard microdilution tests, and 40 time-kill assays to evaluate the synergistic activity of meropenem combination therapies using 20 types of antibiotics.
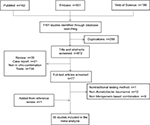  | Figure 1 Flow diagram for selection of studies. |
Time-kill data synthesis
Meropenem–polymyxin B combination therapies (Figure 2) were assessed in ten studies using 30 isolates, and yielded a synergy rate of 98.3% (95% CI, 83.7%–100.0%) with no heterogeneity (I2); the fixed-effect model was applied in the meta-analysis, and no isolates showed antagonism. As only one study was conducted on the meropenem-susceptible strains, subgroup analysis was not performed for this combination therapy.16,17,20,27,34,48,50,57,59,60
  | Figure 2 Forest plot and pooled synergy rates for meropenem–polymyxin B combinations in time-kill method. Abbreviation: ES, effect size. |
For meropenem–rifampicin combinations (Figure 3), pooling data from 20 isolates in four studies showed that the synergy rate was 89.4% (95% CI, 57.2%–100.0%), and no antagonism was observed.16,20,24,32 Heterogeneity (I2) for these studies was 51.9%; thus, the random-effect model was utilized in the analysis.
  | Figure 3 Forest plot and pooled synergy rates for meropenem–rifampicin combinations in time-kill method. Abbreviation: ES, effect size. |
For meropenem–colistin combinations (Figure 4), tests conducted on 132 isolates in 12 studies yielded a synergy rate of 60.4% (95% CI, 24.7%–91.8%), and no isolate was antagonistic.7,14,15,21,23,44,47,53,54,56,61,62 Heterogeneity (I2) was calculated to be 89.8%, and thus, the random-effect model was applied in the meta-analysis. There are five studies on meropenem-susceptible strains; however, no detailed information on the number of isolates was found in some of these studies, which resulted in the absence of subgroup analysis.
  | Figure 4 Forest plot and pooled synergy rates for meropenem–colistin combinations in time-kill method. Abbreviation: ES, effect size. |
For meropenem–sulbactam combination therapies (Figure 5), tests were performed on 69 isolates in five studies, and yielded a synergy rate of 54.8% (95% CI, 39.7%–69.6%) with no heterogeneity (I2), and the fixed-effect model was chosen.8,14,21,46,53 None of the isolates was antagonistic. Two studies evaluated the combination of meropenem and ampicillin/sulbactam, and synergy was observed in 0/1 and 1/2 strains, respectively.
  | Figure 5 Forest plot and pooled synergy rates for meropenem–sulbactam combinations in time-kill method. Abbreviation: ES, effect size. |
For meropenem–tigecycline combinations (Figure S1), six studies were performed on 36 isolates, which yielded a synergy rate of 24.5% (95% CI, 1.0%–58.4%), and no antagonism was found and heterogeneity (I2) for these studies was 46.5%.12,16,20,47,53,56
Two studies on nine strains tested meropenem–amikacin combinations, and the synergistic effect was found in 0/1 and 8/8 strains, respectively. One study evaluated meropenem–ciprofloxacin combination, and the synergistic activity was found in 18/40 strains.
Checkerboard microdilution data synthesis
For meropenem–polymyxin B combinations (Figure 6), tests were carried out on 99 strains in four studies, and showed a pooled synergy rate of 37.0% (95% CI, 0.0%–100.0%) and heterogeneity (I2) was 98.1%.13,26,39,51 Owing to its high heterogeneity, we used the random-effect model for statistical analysis. No isolates were found to be antagonistic.
  | Figure 6 Forest plot and pooled synergy rates for meropenem–polymyxin B combinations in checkerboard method. Abbreviation: ES, effect size. |
Thirteen studies with 189 isolates tested meropenem–colistin combination therapies and showed a pooled synergy rate of 58.8% (95% CI, 29.4%–85.6%) and a heterogeneity (I2) of 92.3% (Figure 7); thus, the random-effect model was applied in the meta-analysis.7,11,29,36,40,41,43,44,47,49,54,56,62 Two isolates were found to be antagonistic. There are six studies that include meropenem-susceptible strains; however, no detailed number of isolates was found in some of these studies, which resulted in the unavailability of subgroup analysis.
  | Figure 7 Forest plot and pooled synergy rates for meropenem–colistin combinations in checkerboard method. Abbreviation: ES, effect size. |
As shown in Figure 8, tests conducted on 405 isolates in eight studies showed that the synergy rate of meropenem–sulbactam combinations was 25.2% (95% CI, 16.1%–36.2%).9,25,28,30,35,37,46,52 A static heterogeneity (I2) of 79.4% was observed, and as a result, we chose the random-effects model. Three isolates were antagonistic. Considering that there was no detailed number of meropenem-susceptible isolates in three studies, the subgroup analysis was not applied. Six studies were performed on 309 strains in the meropenem–cefoprazone/sulbactam combinations (Figure S2), yielding a pooled synergy rate of 7.4% (95% CI, 1.4%–16.6%). Heterogeneity (I2) for these studies was 80.6%.26,30,32,33,35,52
  | Figure 8 Forest plot and pooled synergy rates for meropenem–sulbactam combinations in checkerboard method. Abbreviation: ES, effect size. |
Three studies testing meropenem–rifampicin combination therapies (Figure S3) yielded a synergy rate of 56.3% (95% CI, 8.7%–97.8%).24,26,32 Heterogeneity (I2) for these studies was 90.2%. Meropenem–ciprofloxacin combination therapies were tested in three studies (Figure S4), and yielded a pooled synergy rate of 0.3% (95% CI, 0.0%–3.5%).30,32,52 Heterogeneity (I2) was 33.0%. The combination of meropenem and tigecycline was tested in two studies, and seven of 35 strains showed synergy. Two studies tested the effect of meropenem in combination with amikacin, and synergistic activity was found in 67/128 strains.
Etest data synthesis
Four studies that evaluated the effect of meropenem–colistin combinations (Figure S5) on 122 isolates yielded a pooled synergy rate of 39.2% (95% CI, 0.0%–97.7%), and heterogeneity (I2) was found to be 95.9%.31,45,55,62 Three studies consisting of 102 strains investigated the effect of meropenem–sulbactam combinations (Figure S6), reporting a pooled synergy rate of 35.1% (95% CI, 21.4%–50.1%).37,38,55 Heterogeneity (I2) was 52.3%. One study with 40 strains tested the meropenem–rifampicin and meropenem–moxifloxacin combinations, and synergy was found in 1 and 0 strain, respectively. Two studies reported synergistic effect of 23/100 strains in meropenem–tigecycline combination therapies. Meropenem in combination with cefoprazone/sulbactam resulted in synergistic activity in 61/140 strains.
Discussion
The global emergence of MDR A. baumannii has spurred an interest in finding a more effective treatment strategy. The World Health Organization has identified antimicrobial resistance as one of the three most important problems affecting human health and carbapenem-resistant A. baumannii as a critical priority pathogen to help in prioritizing the research and development of new and effective antibiotic treatments.63 Combining antimicrobials is an effective treatment approach for MDR A. baumannii infections. Reports of meropenem-based combination against A. baumannii have been rising steadily during the past few years. Our results indicated that several antibiotics combined with meropenem could act synergistically in vitro against A. baumannii, especially for polymyxin B and rifampicin. Notably, colistin, also known as polymyxin E, showed a lower synergy rate than polymyxin B in the time-kill assays, while an opposite result was found in the checkerboard microdilution method. Several reasons could result in the inconsistent result. First, polymyxin B and colistin are mixture of cyclic polypeptides, which contain up to 39 and 36 distinct lipopeptides, respectively, and differences in the structures of these individual polypeptides and variations in the physicochemical property among different polymyxin products or even batches from the same company can contribute to variability in the antibacterial activity of polymyxin B and colistin.64 Second, the small sample size may skew the result though we performed a logit transformation for the event rate. Third, the time-kill method uses colony number as the judgment standard, while checkerboard microdilution and Etest methods use MIC in the formula. With no standardization of synergy test method, comparing results generated from different methods becomes a difficult task. As demonstrated in Table 2, it seems that higher rates of synergy are seen with time-kill assays than with checkerboard or Etest assays, which is consistent with the conclusion drawn by Ni et al.65 The synergy rate obtained from checkerboard microdilution and Etest methods is a static value and hard to reproduce.66 In terms of time-kill assay, it is a useful method to provide us kinetic information of antibiotic interaction with bacteria although time- and labor-intensive for routine use in a clinical diagnostic laboratory. Therefore, developing new kinetic test method such as luciferase-based reporter system or standardizing the interpretation of these existing test methods for synergy evaluation should be a feasible strategy to address these limitations.
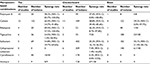  | Table 2 Summary of the pooled synergy rates Abbreviation: Tks, time-kill synergy. |
In time-kill assays, meropenem–polymyxin B combination showed the highest pooled synergy rate of 98.3%, which can be recognized as a very high degree of synergy to almost all isolates with different resistant profiles. Rifampicin in combination with meropenem displayed up to approximately 90% high synergy rate, and it is worth noting that amikacin may be a good partner of meropenem though only two studies can be found. Polymyxin B and amikacin are both molecules containing cations, which are capable of binding negatively charged lipopolysaccharides in the outer membrane of gram-negative bacteria, which thus leads to disruption of bacterial membrane permeability.67 As to rifampicin, more studies should be conducted to confirm our preliminary finding, and the underlying mechanism of the combination also needs to be addressed. Polymyxins are considered as the last-resort antibiotic for the treatment of gram-negative bacteria; however, they were also reported to be associated with nephrotoxicity and neurotoxicity,68 which often hindered their use in clinical settings. To reduce their toxicity, purification of polymyxins product and structure modification have been reported by several groups.64
Sulbactam, as a member of serine β-lactamase inhibitor, is unable to inhibit any carbapenemases but displays moderate activity against A. baumannii, so a pooled synergy rate of 54.8% generated by meropenem–sulbactam combination was expected. Carbapenemases, especially metallo β-lactamases (MBLs), have caused extensive concern on their rapid dissemination and ability to hydrolyze almost all β-lactam antibiotics except monobactams. Unfortunately, up till now, no MBL inhibitor has been clinically approved and all clinically used β-lactamase inhibitors are not active toward MBLs. It remains a substantial challenge to design MBL inhibitors, especially for broad-spectrum MBL inhibitors.
Conclusion
The pooled synergy data in this review suggested that combination therapies of meropenem with polymyxin B, rifampicin, and possibly amikacin as well could achieve high synergy rates against MDR A. baumannii isolates, and colistin and sulbactam could be secondary choice for the combination with meropenem. Tigecycline and ciprofloxacin combination had the least pooled synergy rates. Compared to static checkerboard and Etest method, time-kill assay is more like in vivo dynamic antibacterial process and generally exhibited the greatest of synergisms because of its kinetic synergy calculation method. Combination therapies are future alternatives to traditional monotherapies for infections caused by MDR bacteria. However, the studies included in this review were performed in vitro, which neglects the complicated interactions between antibiotics and hosts. There is still a long way from our results to their applications in clinic, and findings of this review should be verified by more well-designed in vitro and in vivo studies.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81403084); the Science and Technology Program of Guangzhou, China (201710010013 and 201509010012).
Disclosure
The authors report no conflicts of interest in this work.
References
Harding CM, Hennon SW, Feldman MF. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol. 2018;16(2):91–102. | ||
Doi Y, Bonomo RA, Hooper DC, et al. Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the Antibacterial Resistance Leadership Group. Clin Infect Dis. 2017;64(Suppl 1):S30–S35. | ||
Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5(12):939–951. | ||
Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55(11):4943–4960. | ||
El-Gamal MI, Brahim I, Hisham N, Aladdin R, Mohammed H, Bahaaeldin A. Recent updates of carbapenem antibiotics. Eur J Med Chem. 2017;131:185–195. | ||
Falagas ME, Mavroudis AD, Vardakas KZ. The antibiotic pipeline for multi-drug resistant gram negative bacteria: what can we expect? Expert Rev Anti Infect Ther. 2016;14(8):747–763. | ||
Tängdén T, Karvanen M, Friberg LE, Odenholt I, Cars O. Assessment of early combination effects of colistin and meropenem against Pseudomonas aeruginosa and Acinetobacter baumannii in dynamic time-kill experiments. Infect Dis. 2017;49(7):521–527. | ||
Ko WC, Lee HC, Chiang SR, et al. In vitro and in vivo activity of meropenem and sulbactam against a multidrug-resistant Acinetobacter baumannii strain. J Antimicrob Chemother. 2004;53(2):393–395. | ||
Kiffer CR, Sampaio JL, Sinto S, et al. In vitro synergy test of meropenem and sulbactam against clinical isolates of Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2005;52(4):317–322. | ||
Sader HS, Rhomberg PR, Jones RN. In vitro activity of beta-lactam antimicrobial agents in combination with aztreonam tested against metallo-beta-lactamase-producing Pseudomonas aeruginosa and Acinetobacter baumannii. J Chemother. 2005;17(6):622–627. | ||
Timurkaynak F, Can F, Azap OK, Demirbilek M, Arslan H, Karaman SO. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int J Antimicrob Agents. 2006;27(3):224–228. | ||
Scheetz MH, Qi C, Warren JR, et al. In vitro activities of various antimicrobials alone and in combination with tigecycline against carbapenem-intermediate or -resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51(5):1621–1626. | ||
Guelfi KC, Tognim MC, Cardoso CL, Gales AC, Carrara-Marrone FE, Garcia LB. In vitro evaluation of the antimicrobial activity of meropenem in combination with polymyxin B and gatifloxacin against Pseudomonas aeruginosa and Acinetobacter baumannii. J Chemother. 2008;20(2):180–185. | ||
Lee CH, Tang YF, Su LH, Chien CC, Liu JW. Antimicrobial effects of varied combinations of meropenem, sulbactam, and colistin on a multidrug-resistant Acinetobacter baumannii isolate that caused meningitis and bacteremia. Microb Drug Resist. 2008;14(3):233–237. | ||
Pankuch GA, Lin G, Seifert H, Appelbaum PC. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother. 2008;52(1):333–336. | ||
Lim TP, Tan TY, Lee W, et al. In vitro activity of various combinations of antimicrobials against carbapenem-resistant Acinetobacter species in Singapore. J Antibiot. 2009;62(12):675–679. | ||
Pankey GA, Ashcraft DS. The detection of synergy between meropenem and polymyxin B against meropenem-resistant Acinetobacter baumannii using Etest and time-kill assay. Diagn Microbiol Infect Dis. 2009;63(2):228–232. | ||
Kiratisin P, Apisarnthanarak A, Kaewdaeng S. Synergistic activities between carbapenems and other antimicrobial agents against Acinetobacter baumannii including multidrug-resistant and extensively drug-resistant isolates. Int J Antimicrob Agents. 2010;36(3):243–246. | ||
Körber-Irrgang B, Kresken M. In vitro activity of daptomycin combined with other antimicrobial agents against Gram-negative bacteria. Clin Microbiol Infec. 2010;16:S156–S157. | ||
Lim TP, Lee W, Sasikala S, et al. In vitro activity of antimicrobials in combination against clinical strains of extreme drug-resistant Acinetobacter bau-mannii to all antibiotics including polymyxin B in Singapore. Clin Microbiol Infec. 2010;16:S80. | ||
Pongpech P, Amornnopparattanakul S, Panapakdee S, et al. Antibacterial activity of carbapenem-based combinations against multidrug-resistant Acinetobacter baumannii. J Med Assoc Thai. 2010;93(2):161–171. | ||
Sarigüzel FM, Sümerkan B, Metan G. In-vitro activity of carbapenem/sulbactam combination against Acinetobacter baumannii strains. Erciyes Tip Dergisi. 2010;32(1):015–018. | ||
Srisupha-Olarn W, Burgess DS. Activity of meropenem and colistin alone and in combination against multidrug resistant Acinetobacter baumannii in a pharmacokinetic-pharmacodynamic model. Pharmacotherapy. 2010;30(10):401e. | ||
Chopra S, Matsuyama K, Tran T, Madrid P. Systematic discovery of synergistic novel antibiotic combinations targeting multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2012;40(4):377–379. | ||
Deveci A, Coban AY, Acicbe O, Tanyel E, Yaman G, Durupinar B. In vitro effects of sulbactam combinations with different antibiotic groups against clinical Acinetobacter baumannii isolates. J Chemother. 2012;24(5):247–252. | ||
Ozseven AG, Cetin ES, Aridogan BC, Ozseven L. In vitro synergistic activity of carbapenems in combination with other antimicrobial agents against multidrug-resistant Acinetobacter baumannii. African J Microbiol Res. 2012;6(12):2985–2992. | ||
Netto B, Vieira BJ, Hermes DM, Ribeiro VB, Zavascki AP. In vitro activity of non-bactericidal concentrations of polymyxin B in combination with other antimicrobials against OXA-23-producing carbapenem-resistant Acinetobacter baumannii. Braz J Infect Dis. 2013;17(4):502–504. | ||
Turk Dagi H, Kus H, Arslan U, Tuncer I. In vitro synergistic activity of sulbactam in combination with imipenem, meropenem and cefoperazone against carbapenem-resistant Acinetobacter baumannii isolates. Mikrobiyol Bul. 2014;48(2):311–315. | ||
Frantzeskaki F, Vourli S, Paramythiotou E, et al. In vitro and in vivo synergistic activity of high doses of ampicilline/sulbactame with colistin versus meropenem with colistin in ventilator associated pneumonia (VAP) caused by acinetobacter baumannii (AB). Intens Care Med. 2014;40(1):S195. | ||
Lu Y, Zhang Y, Zhou H, Yu F, Sun S, Rui Y. Combined drug sensitivity test of 50 strains of extensively drug-resistant Acinetobacter baumannii. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(11):1697–1701. | ||
Shah PG, Shah SR, Kamat SD, Kamat DV. Colistin-carbapenem combination therapy against carbapenem resistant gram negative bacilli infections: Clinical and an in vitro synergy study. Int J Pharm Pharmaceutical Sci. 2014;6(10):497–500. | ||
Sun Y, Wang L, Li J, et al. Synergistic efficacy of meropenem and rifampicin in a murine model of sepsis caused by multidrug-resistant Acinetobacter baumannii. Eur J Pharmacol. 2014;729:116–122. | ||
Xia J, Zhang D, Xu Y, Gong M, Zhou Y, Fang X. A retrospective analysis of carbapenem-resistant Acinetobacter baumannii-mediated nosocomial pneumonia and the in vitro therapeutic benefit of cefoperazone/sulbactam. Int J Infect Dis. 2014;23:90–93. | ||
Gall J, Lenhard J, Holden PN, Tsuji BT. Comparative pharmacodynamics of imipenem, doripenem, meropenem, and ertapenem in combination with polymyxin B against carbapenem-resistant Acinetobacter baumannii. Pharmacotherapy. 2015;35(11):e304–e305. | ||
Ke Q, Lü Y, Wang F. In vitro activity of meropenem in combination with sulbactam or cefoperazone-sulbactam against multidrug resistant acinetobacter baumannii. Chin J Infect Chemother. 2015;15(6):548–551. | ||
Le Minh V, Thi Khanh Nhu N, Vinh Phat V, et al. In vitro activity of colistin in antimicrobial combination against carbapenem-resistant Acinetobacter baumannii isolated from patients with ventilator-associated pneumonia in Vietnam. J Med Microbiol. 2015;64(10):1162–1169. | ||
Marie MA, Krishnappa LG, Alzahrani AJ, Mubaraki MA, Alyousef AA. A prospective evaluation of synergistic effect of sulbactam and tazobactam combination with meropenem or colistin against multidrug resistant Acinetobacter baumannii. Bosn J Basic Med Sci. 2015;15(4):24–29. | ||
Temocin F, Erdinc FS, Tulek N, et al. Synergistic effects of sulbactam in multi-drug-resistant Acinetobacter baumannii. Braz J Microbiol. 2015;46(4):1119–1124. | ||
Teo J, Lim TP, Hsu LY, et al. Extensively drug-resistant Acinetobacter baumannii in a Thai hospital: a molecular epidemiologic analysis and identification of bactericidal Polymyxin B-based combinations. Antimicrob Resist Infect Control. 2015;4(1):2. | ||
van Belkum A, Halimi D, Bonetti EJ, et al. Meropenem/colistin synergy testing for multidrug-resistant Acinetobacter baumannii strains by a two-dimensional gradient technique applicable in routine microbiology. J Antimicrob Chemother. 2015;70(1):167–172. | ||
Vourli S, Frantzeskaki F, Meletiadis J, et al. Synergistic interactions between colistin and meropenem against extensively drug-resistant and pandrug-resistant Acinetobacter baumannii isolated from ICU patients. Int J Antimicrob Agents. 2015;45(6):670–671. | ||
Yadav R, Landersdorfer CB, Nation RL, Boyce JD, Bulitta JB. Novel approach to optimize synergistic carbapenem-aminoglycoside combinations against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59(4):2286–2298. | ||
Bae S, Kim MC, Park SJ, et al. In vitro synergistic activity of antimicrobial agents in combination against clinical isolates of colistin-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60(11):6774–6779. | ||
Bedenić B, Beader N, Godič-Torkar K, et al. Postantibiotic effect of colistin alone and combined with vancomycin or meropenem against Acinetobacter spp. with well defined resistance mechanisms. J Chemother. 2016;28(5):375–382. | ||
Hong DJ, Kim JO, Lee H, et al. In vitro antimicrobial synergy of colistin with rifampicin and carbapenems against colistin-resistant Acinetobacter baumannii clinical isolates. Diagn Microbiol Infect Dis. 2016;86(2):184–189. | ||
Laishram S, Anandan S, Devi BY, et al. Determination of synergy between sulbactam, meropenem and colistin in carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii isolates and correlation with the molecular mechanism of resistance. J Chemother. 2016;28(4):297–303. | ||
Leite GC, Oliveira MS, Perdigão-Neto LV, et al. Antimicrobial combinations against pan-resistant Acinetobacter baumannii isolates with different resistance mechanisms. PLoS One. 2016;11(3):e0151270. | ||
Lenhard JR, Bulitta JB, Connell TD, et al. High-intensity meropenem combinations with polymyxin B: new strategies to overcome carbapenem resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2017;72(1):153–165. | ||
Liu X, Zhao M, Chen Y, et al. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents. 2016;48(5):559–563. | ||
Lenhard JR, Gall JS, Bulitta JB, et al. Comparative pharmacodynamics of four different carbapenems in combination with polymyxin B against carbapenem-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2016;48(6):719–724. | ||
Menegucci TC, Albiero J, Migliorini LB, et al. Strategies for the treatment of polymyxin B-resistant Acinetobacter baumannii infections. Int J Antimicrob Agents. 2016;47(5):380–385. | ||
Wang FJ, Lyu Y, Liu ZH, Li Y, Cui LQ. In vitro activity of different antibacterial agents in combination with each other against multidrug-resistant Acinetobacter baumannii. Chin Med J. 2016;129(19):2388–2389. | ||
Wang YC, Kuo SC, Yang YS, et al. Individual or combined effects of meropenem, imipenem, sulbactam, colistin, and tigecycline on biofilm-embedded Acinetobacter baumannii and biofilm architecture. Antimicrob Agents Chemother. 2016;60(8):4670–4676. | ||
Yang YS, Lee Y, Tseng KC, et al. In vivo and in vitro efficacy of minocycline-based combination therapy for minocycline-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60(7):4047–4054. | ||
Yavaş S, Yetkın MA, Kayaaslan B, et al. Investigating the in vitro synergistic activities of several antibiotic combinations against carbapenem-resistant Acinetobacter baumannii isolates. Turk J Med Sci. 2016;46(3):892–896. | ||
Büyük A, Yilmaz FF, Gül Yurtsever S, Hoşgör Limoncu M. Antibiotic resistance profiles and genotypes of Acinetobacter baumannii isolates and in vitro interactions of various antibiotics in combination with tigecycline and colistin. Tur J Pharm Sci. 2017;14(1):13–18. | ||
Gallo SW, Ferreira CAS, de Oliveira SD. Combination of polymyxin B and meropenem eradicates persister cells from Acinetobacter baumannii strains in exponential growth. J Med Microbiol. 2017;66(8):1257–1260. | ||
Ghazi IM, Grupper M, Nicolau DP. Antibacterial activity of human simulated epithelial lining fluid concentrations of amikacin inhale alone and in combination with meropenem against Acinetobacter baumannii. Infect Dis. 2017;49(11–12):831–839. | ||
Lenhard JR, Smith NM, Bulman ZP, et al. High-dose ampicillin-sulbactam combinations combat polymyxin-resistant Acinetobacter baumannii in a hollow-fiber infection model. Antimicrob Agents Chemother. 2017;61(3):e01268-16. | ||
Lenhard JR, Thamlikitkul V, Silveira FP, et al. Polymyxin-resistant, carbapenem-resistant Acinetobacter baumannii is eradicated by a triple combination of agents that lack individual activity. J Antimicrob Chemother. 2017;72(5):1415–1420. | ||
Manohar P, Shanthini T, Ayyanar R, et al. The distribution of carbapenem- and colistin-resistance in Gram-negative bacteria from the Tamil Nadu region in India. J Med Microbiol. 2017;66(7):874–883. | ||
Soudeiha MAH, Dahdouh EA, Azar E, Sarkis DK, Daoud Z. In vitro evaluation of the colistin-carbapenem combination in clinical isolates of A. baumannii using the checkerboard, Etest, and time-kill curve techniques. Front Cell Infect Microbiol. 2017;7:209. | ||
Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: World Health Organization; 2017. | ||
Roberts KD, Azad MA, Wang J, et al. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: last-line antibiotics against multidrug-resistant Gram-negative bacteria. ACS Infect Dis. 2015;1(11):568–575. | ||
Ni W, Shao X, di X, Cui J, Wang R, Liu Y. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents. 2015;45(1):8–18. | ||
Rand KH, Houck HJ, Brown P, Bennett D. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob Agents Chemother. 1993;37(3):613–615. | ||
Yadav R, Bulitta JB, Schneider EK, Shin BS, Velkov T, Nation RL, Landersdofer CB. Aminoglycoside concentrations required for synergy with carbapenems against Pseudomonas aeruginosa determined via mechanistic studies and modeling. Antimicrob Agents Chemother. 2017;61(12):e00722-17. | ||
Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Eur Soc Clin Microbiol Infect Dis. 2012;18(1):18–29. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

