Back to Journals » Journal of Asthma and Allergy » Volume 16
Symptom Burden, Health Status, and Productivity in Patients with Uncontrolled and Controlled Severe Asthma in NOVELTY
Authors Ding B , Chen S , Srivastava D, Quinton A, Cook W, Papi A , Reddel HK
Received 14 December 2022
Accepted for publication 13 April 2023
Published 11 June 2023 Volume 2023:16 Pages 611—624
DOI https://doi.org/10.2147/JAA.S401445
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Bo Ding,1 Stephanie Chen,2 Divyansh Srivastava,3 Anna Quinton,4 William Cook,2 Alberto Papi,5 Helen K Reddel,6 on behalf of the NOVELTY Scientific Community and the NOVELTY study investigators
1BioPharmaceuticals Medical, AstraZeneca, Gothenburg, Sweden; 2BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD, USA; 3ZS Associates, Pune, India; 4BioPharmaceuticals Business Unit, AstraZeneca, Cambridge, UK; 5Department of Translational Medicine, Università di Ferrara, Ferrara, Italy; 6The Woolcock Institute of Medical Research and the University of Sydney, Sydney, NSW, Australia
Correspondence: Bo Ding, AstraZeneca, Pepparedsleden 1, Mölndal, SE 431 83, Gothenburg, Sweden, Tel +46 31 776 2406, Email [email protected]
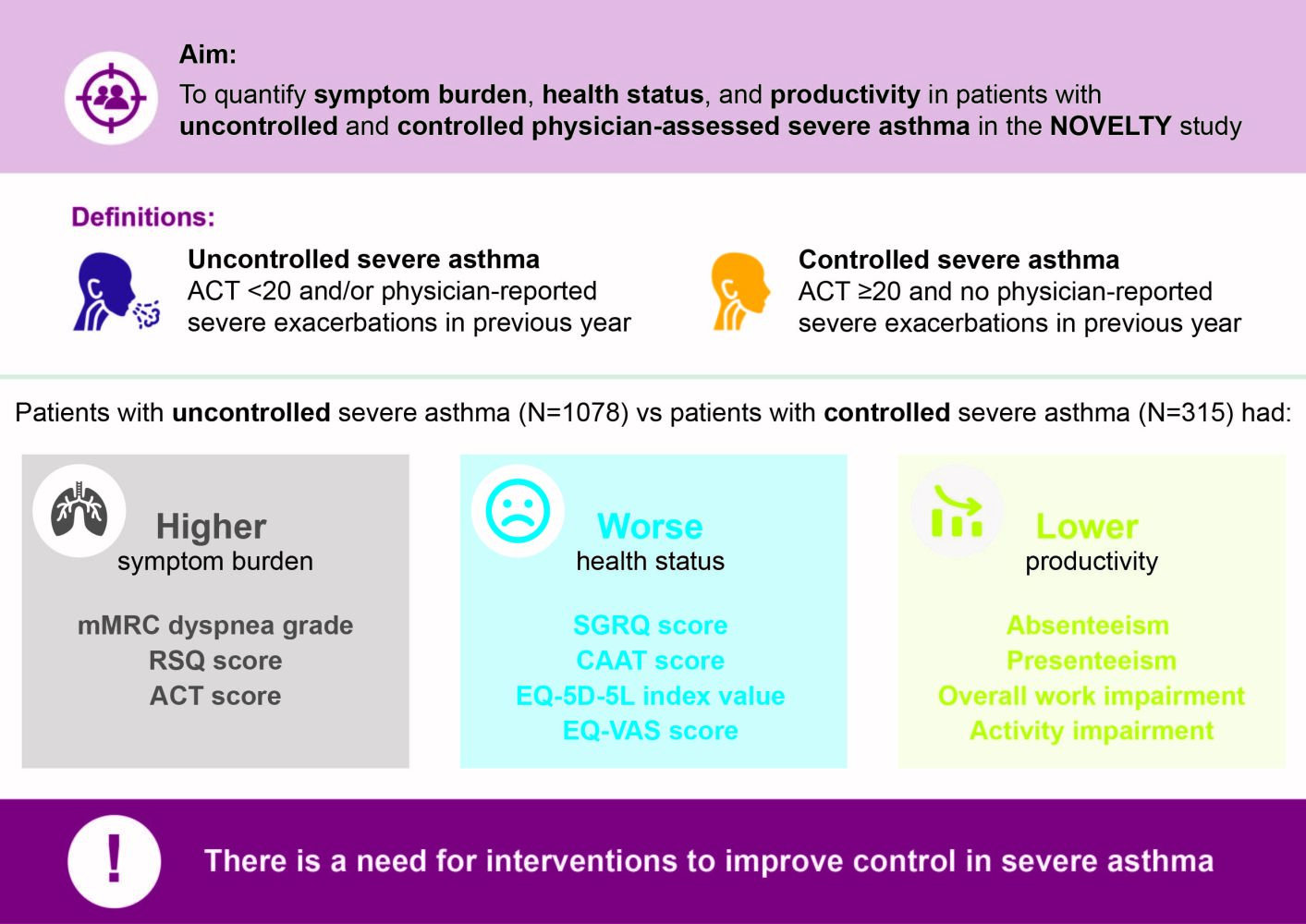
Background: Few studies have quantified symptom burden, health status, and productivity in patients with uncontrolled and controlled severe asthma. Up-to-date, real-world, global evidence is needed.
Objective: To quantify symptom burden, health status, and productivity in patients with uncontrolled and controlled severe asthma using baseline data from the NOVEL observational longiTudinal studY (NOVELTY; NCT02760329).
Methods: NOVELTY included patients aged ≥ 18 years (or ≥ 12 years in some countries) from primary care and specialist centres in 19 countries, with a physician-assigned diagnosis of asthma, asthma+chronic obstructive pulmonary disease (COPD), or COPD. Disease severity was physician-assessed. Uncontrolled severe asthma was defined by an Asthma Control Test (ACT) score < 20 and/or severe physician-reported exacerbations in the previous year; controlled severe asthma required an ACT score ≥ 20 and no severe exacerbations. Assessment of symptom burden included Respiratory Symptoms Questionnaire (RSQ) and ACT score. Assessment of health status included St George’s Respiratory Questionnaire (SGRQ), EuroQoL 5 Dimensions 5 Levels Health Questionnaire (EQ-5D-5L) index value, and EQ-5D-5L Visual Analog Score (EQ-VAS). Assessment of productivity loss included absenteeism, presenteeism, overall work impairment, and activity impairment.
Results: Of 1652 patients with severe asthma, asthma was uncontrolled in 1078 (65.3%; mean age 52.6 years, 65.8% female) and controlled in 315 (19.1%; mean age 55.2 years, 56.5% female). With uncontrolled versus controlled severe asthma, symptom burden was higher (mean RSQ score 7.7 vs 2.5), health status more impaired (mean SGRQ total score 47.5 vs 22.4; mean EQ-5D-5L index value 0.68 vs 0.90; mean EQ-VAS score 64.1 vs 78.1), and productivity lower (presenteeism 29.3% vs 10.5%).
Conclusion: Our findings highlight the symptom burden of uncontrolled severe asthma compared with controlled severe asthma and its impact on patient health status and productivity, and support the need for interventions to improve control of severe asthma.
Graphical Abstract:
Keywords: asthma control, health status, productivity, symptom burden, symptom control, uncontrolled severe asthma
Graphical Abstract:
Plain Language Summary
Asthma is a condition that affects the airways and has long-term effects on quality of life. Asthma affects about 1–18% of people in different countries. People with asthma can be grouped based on whether their doctor considers their asthma to be mild, moderate, or severe. They can also be grouped based on how well their asthma symptoms are under control (uncontrolled or controlled). This can be assessed by filling in a questionnaire about how their asthma has been in the past 4 weeks, and by whether they had any asthma flare-ups in the last year.
We used data from NOVELTY, a study that included over 1600 people with severe asthma. We found that asthma was uncontrolled in almost two-thirds of these people. They had more symptoms that affected their ability to work, and their overall health was not as good compared with the one-third of people with controlled severe asthma. Our findings are novel, as they reflect people with asthma in the real world, rather than people in clinical trials of new treatments.
Our findings show the burden of uncontrolled severe asthma and highlight that symptom control needs to be improved in people with uncontrolled severe asthma. Better treatment for people who have uncontrolled symptoms could reduce the impact of severe asthma on their quality of life.
Introduction
Asthma is a heterogeneous inflammatory disease of the airways that is estimated to affect 1–18% of people in different countries.1 Of patients with asthma, it is estimated that 3–10% have severe asthma.1 Good symptom control is an important goal in asthma management.1 However, even with optimized maximal therapy, not all patients with severe asthma achieve good symptom control.1 This represents a significant unmet need. Furthermore, asthma places a high burden on healthcare systems and on society due to loss of productivity.1 A survey conducted in the USA between 2008–2013 found that asthma was responsible for losses of $29 billion from asthma-related mortality, $50.3 billion in medical costs, and $3 billion from absenteeism (missed work and school days), highlighting the significant economic burden of asthma.2
One study of patients with severe asthma from specialist clinics reported that up to 81% of patients had uncontrolled disease (defined as patients who had clinically significant asthma exacerbations in the prior year, or an Asthma Control Questionnaire-5 score of ≥1.5).3 A Canadian study (N=158,516) reported that patients with uncontrolled severe asthma (defined based on an algorithm including data on medication and exacerbations in the last year) accounted for 60% of the overall direct healthcare costs of asthma from 1996 to 2000.4 Moreover, follow-up of 327 patients in the USA with severe or difficult-to-treat asthma enrolled in a cross-sectional study in 2001–2004 observed that for nearly half, asthma remained very poorly controlled a decade later (level of control defined by the National Heart, Lung, and Blood Institute asthma guidelines).5,6 These previous findings highlight the need for patients with uncontrolled severe asthma to receive improved or specialized care.
Most studies to date that have reported symptom burden, health status, and productivity loss in patients with uncontrolled severe asthma have involved patients only from specialist clinics.3,7 Few studies have included patients with severe asthma from both primary and specialist care. Furthermore, many of these studies have been from a single country and have defined severe asthma only by treatment step.8 One 2015–2016 cross-sectional, patient-completed internet-based survey (N=7820) of patients with any asthma severity compared poorly controlled, partly controlled, and well-controlled asthma, and found that well-controlled asthma was associated with lower healthcare resource utilization, costs, and work productivity loss, and less impaired health status. However, this study was limited to patients in the USA.9
In this analysis, we aimed to quantify symptom burden, health status, and productivity loss in patients with uncontrolled severe asthma from the NOVEL observational longiTudinal studY (NOVELTY), with patients with controlled severe asthma as a benchmark. NOVELTY includes patients from a real-world setting from both primary care and specialist centers in the Americas, Asia, Australia and Europe, providing a novel setting in which to evaluate the impact of uncontrolled severe asthma.
Methods
Study Design
This was a cross-sectional analysis using baseline data from the NOVELTY cohort (www.clinicaltrials.gov, NCT02760329). The study design of NOVELTY has been described previously.10 Briefly, NOVELTY included patients aged ≥18 years (or ≥12 years in some countries) with a physician-assigned diagnosis of asthma, chronic obstructive pulmonary disease (COPD), or both (asthma+COPD). Patients with a suspected (ie, not confirmed) diagnosis of asthma and/or COPD were also enrolled. Patients were enrolled at primary care and specialist centers in 19 countries in the Americas, Asia, Australia, and Europe (Supplementary Table 1).10 Data for patients from China were excluded from the present analyses due to a change in regulations about data transfer in May 2019.11
Study Population
Patients were included in these analyses if they had a physician-assigned diagnosis of asthma and physician-assessed severe asthma (regardless of their current asthma treatment) and completed the NOVELTY baseline study visit. Patients with a physician-assigned diagnosis of asthma+COPD were excluded, except for sensitivity analyses. Patients with severe asthma were grouped by their level of asthma control. Uncontrolled asthma was defined by an Asthma Control Test (ACT) score <2012 and/or ≥1 severe physician-reported exacerbation (defined as requiring systemic corticosteroids, emergency-room visit, or hospitalization during the 12-month period prior to the baseline visit). Controlled asthma was defined by an ACT score ≥2012 and no severe physician-reported exacerbations in the previous 12 months.
Study Assessments and Outcomes
Physicians recorded baseline and clinical characteristics, including physician-reported exacerbations in the previous year, as detailed previously.10 History of allergy was recorded by the physician, excluding food and medication allergies. Outcomes for this analysis were related to symptom burden, health status, and productivity loss. These outcomes were reported by patients online or by telephone at, or shortly after, the baseline visit.
Symptom burden was assessed using patient-reported outcome measures, including the modified Medical Research Council (mMRC) dyspnea scale,13 Respiratory Symptoms Questionnaire (RSQ),14 and ACT score.15 The mMRC dyspnea scale is a one-item measure, graded on a 0–4 scale, with a higher grade indicating worse disability due to dyspnea; mMRC dyspnea grade ≥2 is classified as clinically important dyspnea.16 The RSQ comprises four items related to respiratory symptoms over the previous 4 weeks and the impact they have on patient activity, with each item scored on a 0–4 scale. The total score (0–16) is the sum of all items, with higher scores indicating worse symptoms. The ACT measures asthma symptom control in the past 4 weeks and comprises five items scored on a 1–5 scale; the total score (5–25) is the sum of all items, with higher scores indicating better asthma control. ACT score 5–15 is classified as very poorly controlled symptoms, 16–19 as not well controlled, and ≥20 as well controlled.12
To assess health status, patient-reported outcome measures, including the St George’s Respiratory Questionnaire (SGRQ) and the question about overall health status that precedes it,17 Chronic Airways Assessment Test (CAAT),18 5-level EuroQoL 5-dimension (EQ-5D-5L) index value, and EQ-5D-5L Visual Analog Score (EQ-VAS),19 were used. The SGRQ is a 50-item questionnaire scored on a 0–100 scale, which was developed to measure overall health status in patients with obstructive airways disease. Higher scores indicate worse health status.17 The CAAT comprises eight items scored on a 0–5 scale, measuring the impact of symptoms on health status, and is modified with permission from the COPD assessment test20 to replace COPD-specific wording. The total score (0–40) is the sum of all items, with higher scores indicating worse health status.18 The EQ-5D-5L index value summarizes the EQ-5D-5L health states using a single summary number based on patient responses to five dimensions; the index value reflects how good or bad their health state is according to the preferences of the general population of the country/region. To obtain the EQ-5D-5L index value, a country-specific value set was used for each country (Supplementary Table 2). For countries where a standard EQ-5D-5L value set was not available, a “crosswalk” value set was used. A crosswalk value set is one that was created for the EQ-5D-3L and then adapted to fit the EQ-5D-5L descriptive system. For countries where standard EQ-5D-5L and EQ-5D-3L value sets were not available, a value set available from a country most closely resembling that country was used (Supplementary Table 2). The EQ-VAS indicates patients’ overall health status and is scored on a 0–100 scale. Higher scores indicate better health status on that day.19
Productivity loss experienced in the previous week was obtained from the patient-reported Work Productivity and Activity Impairment Questionnaire (WPAI).21 This included the percentage absenteeism (work time missed due to health), percentage presenteeism (impairment experienced while at work), percentage overall work impairment due to health, and percentage activity impairment due to health.
Statistical Analysis
Descriptive results are presented using mean, standard deviation (SD), median, and interquartile range for continuous variables, and frequency distributions for categorical variables. R statistical software version 4.1.2 was used in the analysis.22
Since physicians may assess asthma severity differently than recommended in guidelines, sensitivity analyses were performed by repeating the main analyses in patients in NOVELTY with asthma (regardless of physician-assessed severity) who were taking medium- or high-dose inhaled corticosteroid (ICS)+long-acting β2-agonist (LABA) with or without a long-acting muscarinic antagonist (LAMA), or any biologic therapy or maintenance oral corticosteroid (OCS); this corresponds to treatment steps 4 or 5 in the current (2022) Global Initiative for Asthma (GINA) report.1
Sensitivity analyses were also performed by repeating the main analyses in patients with physician-assessed severe asthma+COPD. This sensitivity analysis was performed to assess the generalizability of the findings in patients from a global, real-world setting.
Results
Patient Characteristics
A total of 1652 patients with severe asthma were included in this analysis. Of these, 315 (19.1%) had controlled severe asthma (mean age 55.2 years, 56.5% female), 1078 (65.3%) had uncontrolled severe asthma (mean age 52.6 years, 65.8% female), and 259 (15.7%) could not be classified due to missing ACT values at baseline (Table 1). Of the patients with severe asthma, 646 (39.1%) were recruited from primary care.
 |
Table 1 Baseline Demographic and Clinical Characteristics in Patients with Physician-Assessed Severe Asthma, including those with Uncontrolled Severe Asthma and Controlled Severe Asthma |
The uncontrolled severe asthma group had a lower proportion of North-East Asian patients (7.6% vs 22.5%), former smokers (28.5% vs 36.5%), and patients with a history of allergies (67.5% vs 74.6%), and a higher proportion of patients with a body mass index (BMI) ≥30 kg/m2 (39.5% vs 26.0%), than the controlled severe asthma group, respectively (Table 1).
Maintenance OCS were taken by 11.2% (mean [SD] daily dose 13.7 mg [13.0]) of patients with uncontrolled severe asthma and 6.7% (mean [SD] daily dose 12.8 mg [11.9]) of patients with controlled severe asthma. Almost one-third of patients with physician-assessed severe asthma were taking biologic therapy, the most common being omalizumab, taken by 19.6% and 24.4% of patients with uncontrolled and controlled severe asthma, respectively (Table 1).
Overall, nonrespiratory comorbidities were more common in the uncontrolled versus controlled severe asthma group (70.8% vs 60.6% had ≥1 nonrespiratory comorbidity, respectively), including gastro-esophageal reflux disease (20.9% vs 14.0%, respectively), depression (10.1% vs 2.5%, respectively) and obesity (10.3% vs 2.9%, respectively; Supplementary Table 3). The most common respiratory comorbidity in both groups was allergic rhinitis (28.8% and 27.6%, respectively); of respiratory comorbidities, obstructive sleep apnea (9.9% vs 6.3%, respectively) and bronchiectasis (9.2% vs 6.0%, respectively) were more common in the uncontrolled versus controlled severe asthma group.
Symptom Burden
Patients with uncontrolled severe asthma had a higher mMRC dyspnea grade than patients with controlled severe asthma (mean [SD] 1.5 [1.0] vs 0.8 [0.8], respectively). Additionally, there was a marked difference in distribution, with 45.1% of patients with uncontrolled severe asthma having clinically important dyspnea (mMRC dyspnea grade ≥2) compared with only 15.7% of those with controlled severe asthma (Figure 1).
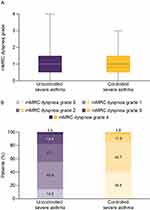 |
Figure 1 (A) Box plot of mMRC dyspnea grade and (B) proportion of patients by mMRC dyspnea grade in patients with uncontrolled severe asthma and controlled severe asthma. Abbreviation: mMRC, Modified Medical Research Council. Notes: The mMRC dyspnea scale is a one-item measure, graded on a 0–4 scale, with a higher grade indicating worse disability due to dyspnea.13,16 In panel A, the solid and dotted center lines denote the median and mean values, respectively. The boxes represent the interquartile range (25th to 75th percentiles), and the whiskers mark the minimum and maximum values. |
RSQ total scores were more than threefold higher in patients with uncontrolled severe asthma (mean [SD] 7.7 [4.0] vs 2.5 [2.2], respectively; Figure 2) than in patients with controlled severe asthma. In the uncontrolled severe asthma group compared with the controlled severe asthma group, a higher proportion of patients experienced shortness of breath more than once every day (30.3% vs 5.3%), needed rescue inhalers once or twice every day (22.7% vs 5.9%), or experienced moderate limitations in their activity (37.1% vs 6.3%) in the previous 4 weeks, respectively (Figure 3). A higher proportion of patients with uncontrolled severe asthma (73.4%) experienced nocturnal awakenings in the previous 4 weeks than those with controlled severe asthma (29.4%).
 |
Figure 2 Box plot of RSQ total score in patients with uncontrolled severe asthma and controlled severe asthma. Abbreviation: RSQ, Respiratory Symptoms Questionnaire. Notes: The RSQ comprises four items related to respiratory symptoms over the previous 4 weeks and the impact they have on patient activity, with each item scored on a 0–4 scale. The total score (0–16) is the sum of all items, with higher scores indicating worse symptoms.14 The solid and dotted center lines denote the median and mean values, respectively. The boxes represent the interquartile range (25th to 75th percentiles), and the whiskers mark the minimum and maximum values. |
 |
Figure 3 RSQ (A) shortness of breath, (B) limited activities, (C) rescue inhaler use, and (D) night awakening in patients with uncontrolled severe asthma and controlled severe asthma. Abbreviation: RSQ, Respiratory Symptoms Questionnaire. Notes: The RSQ comprises four items related to respiratory symptoms over the previous 4 weeks and the impact they have on patient activity.14 |
ACT score was lower in patients with uncontrolled severe asthma (mean [SD] 15.5 [4.6]) than with controlled severe asthma (22.4 [1.7]; Figure 4). Among patients with uncontrolled severe asthma with available data for ACT, 17.7% had an ACT score that indicated “well-controlled” asthma in the previous 4 weeks, but were classified as having uncontrolled severe asthma based on having experienced ≥1 severe physician-reported exacerbation in the previous 12 months. Almost half (49.4%) of patients with uncontrolled asthma were classified as having “very poorly controlled” asthma based on ACT score. All patients with controlled severe asthma were classified as having “well-controlled” asthma symptoms, since an ACT score ≥20 was required for patients to be classified as having controlled severe asthma.
 |
Figure 4 Box plot of ACT score in patients with uncontrolled severe asthma and controlled severe asthma. Abbreviation: ACT, Asthma Control Test. Notes: The ACT measures asthma symptom control and comprises five items scored on a 1–5 scale; the total score (5–25) is the sum of all items, with higher scores indicating better asthma control.12 The solid and dotted center lines denote the median and mean values, respectively. The boxes represent the interquartile range (25th to 75th percentiles), and the whiskers mark the minimum and maximum values. Uncontrolled severe asthma was defined by either an ACT score <20 or at least one severe physician-reported exacerbation in the previous 12 months. Controlled severe asthma was defined by ACT score ≥20 and no severe physician-reported exacerbations (defined as requiring systemic corticosteroids, emergency-room visit, or hospitalization during the 12-month period prior to the baseline visit). |
Health Status
A lower proportion of the uncontrolled severe asthma group rated their overall health as good (27.9%) or very good (3.6%) than those with controlled severe asthma (44.9% and 12.3%, respectively; Supplementary Table 4). SGRQ total score was approximately 25 points higher (mean [SD] 47.5 [20.5] vs 22.4 [13.8]) in patients with uncontrolled severe asthma than in patients with controlled severe asthma, respectively, indicating worse health status (Figure 5). The impact score (mean [SD] 37.8 [22.6] vs 14.6 [13.5]), symptom score (59.1 [20.3] vs 33.2 [19.1]), and activity score (58.1 [25.7] vs 30.4 [21.7]) were higher in the uncontrolled severe asthma group than in the controlled severe asthma group (Supplementary Table 4).
 |
Figure 5 Box plots for (A) SGRQ total score, (B) CAAT score, (C) EQ-5D-5L index value, and (D) EQ-VAS score in patients with uncontrolled severe asthma and controlled severe asthma. Abbreviations: CAAT, Chronic Airways Assessment Test; EQ-5D-5L, EuroQoL 5 Dimensions 5 Levels Health Questionnaire; SGRQ, St George’s Respiratory Questionnaire; VAS, visual analog score. Notes: The SGRQ is a 50-item questionnaire scored on a 0–100 scale, with higher scores indicating worse health status.17 The CAAT comprises eight items scored on a 0–5 scale, measuring the impact of symptoms on health status, and is modified with permission from the COPD assessment test,20 with higher scores indicating worse health status.18 The EQ-5D-5L index value summarizes the EQ-5D-5L health states using a single summary number based on responses to five dimensions, which reflects how good or bad a health state is. The EQ-VAS indicates patients’ overall health status and is scored on a 0–100 scale, with higher scores indicating better health status on that day.19 The solid and dotted center lines denote the median and mean values, respectively. The boxes represent the interquartile range (25th to 75th percentiles), and the whiskers mark the minimum and maximum values. |
CAAT score was higher in the uncontrolled severe asthma group (mean [SD] 20.3 [8.4]) than in the controlled severe asthma group (11.4 [7.0]; Figure 5). Among patients with uncontrolled and controlled severe asthma, 50.1% and 10.2% had a CAAT score of >20, respectively (Supplementary Figure S1).
Patients with uncontrolled severe asthma had a lower EQ-5D-5L index value (mean [SD] 0.68 [0.3] vs 0.90 [0.1], respectively; Figure 5), and EQ-VAS score was 14 points lower (mean [SD] 64.1 [19.2] vs 78.1 [15.1], respectively), than patients with controlled severe asthma (Figure 5).
Productivity Loss
Uncontrolled severe asthma was associated with high levels of productivity loss and activity impairment. Patients with uncontrolled severe asthma had a two- to threefold greater percentage of absenteeism (10.6% vs 4.0%), presenteeism (29.3% vs 10.5%), overall work impairment (32.4% vs 12.1%), and activity impairment (40.9% vs 17.3%) than patients with controlled severe asthma, respectively (Figure 6).
 |
Figure 6 Productivity loss in patients with uncontrolled severe asthma and controlled severe asthma. Abbreviation: WPAI, Work Productivity and Activity Impairment. Notes: Productivity loss measures were obtained from the WPAI Questionnaire which assessed employment status and absence from work during the past 7 days.21 This included absenteeism (percentage of work time missed due to health), presenteeism (percentage of impairment experienced while at work due to health), overall work impairment (absenteeism and presenteeism combined), and activity impairment (percentage of impairment in daily activities due to health). Error bars represent standard deviation. |
Sensitivity Analysis
Results for patients with asthma treated with a medium- or high-dose ICS+LABA, or a medium-dose ICS+LABA with a LAMA or any biologic therapy or maintenance OCS, reflecting patients with medium- or high-intensity treatment (N=1637), were similar in direction and magnitude to the main analysis (Supplementary Tables 5–7 and Supplementary Figures S2–S8). Data for patients with severe asthma+COPD (N=523) are shown in Supplementary Tables 8–10 and Supplementary Figures S9–S15, with findings also similar in direction and magnitude to the main analysis. A similar proportion of patients with asthma with medium- or high-intensity treatment were taking biologic therapy (30.1%), compared with the main analysis (30.4%); however, only a small proportion of patients with physician-assessed severe asthma+COPD were taking biologic therapy (9.4%).
Discussion
This global, real-world analysis highlights the symptom burden and productivity loss associated with uncontrolled severe asthma, including the comparison with controlled severe asthma. This study provides a novel setting, with patients recruited from clinical practice in primary and specialist care, and with physician-assigned diagnosis and physician-assessed severity, reflecting real-world physician perspectives. NOVELTY provides a holistic view, including input from both physicians and patients. A broad definition was used for patient selection, in contrast with the highly restrictive inclusion criteria used in clinical trials, making the findings more generalizable to real life. Additionally, data on symptom burden, health status, and productivity loss were available in one dataset, allowing for a more complete view of the burden of severe asthma.
Despite the availability of biologic therapies in many of the countries included in NOVELTY, the majority of patients with physician-assessed severe asthma had uncontrolled asthma (65.3%), defined as an ACT score <20 at baseline and/or at least one severe exacerbation during the 12 months prior to the baseline visit. The uncontrolled severe asthma group had a lower proportion of patients from North-East Asia than the controlled severe asthma group, which may reflect use of different criteria by physicians in this region to assess severity. A similar trend was observed in the sensitivity analysis in patients with asthma who were receiving medium- or high-intensity treatment. The slightly higher proportion of patients on biologics that was observed in the controlled severe asthma group than in the uncontrolled severe asthma group may reflect improvement in symptoms and/or exacerbations after starting medication. The uncontrolled severe asthma group had a higher proportion of patients with a BMI ≥30 kg/m2 than the controlled severe asthma group, which is consistent with a previous cross-sectional study, which found that patients classified as obese (BMI ≥30 kg/m2) were more likely to have uncontrolled asthma symptoms than patients with a normal BMI (BMI <25 kg/m2).23
Our findings in patients from primary and specialist care that patients with uncontrolled severe asthma had higher symptom burden, lower health status measures, and higher productivity loss than patients with controlled severe asthma are consistent with previous studies in specialist clinics. An Italian observational study of elderly patients with asthma reported that those with poorly controlled, partially controlled, and well-controlled asthma had significantly different mMRC dyspnea grades (mean [SD] 2.03 [0.90], 1.38 [0.80], 0.72 [0.71], respectively; P<0.001).24 These are similar to the mMRC dyspnea grades of patients with uncontrolled and controlled severe asthma in the present analyses (mean [SD] 1.5 [1.0] and 0.8 [0.8], respectively). In the present study, the difference in the mean SGRQ total score between patients with uncontrolled and controlled severe asthma was 25.1 (47.5 vs 22.4), which is much greater than the minimal clinically important difference (MCID) (≥4 points).25 This is consistent with a previous study in specialist clinics that reported a mean difference of 24.4 (47.0 vs 22.6) in SGRQ total scores of patients with uncontrolled and controlled severe asthma, respectively.3
For health status, the difference in mean EQ-5D-5L index value for uncontrolled versus controlled severe asthma was 0.22 (0.68 vs 0.90), which is much greater than the estimated MCID (0.037–0.069),26 demonstrating the magnitude of effect of uncontrolled severe asthma. This difference is much greater than in a large US internet-based survey that observed a difference of 0.1 in EQ-5D-5L index score when comparing patients with poorly controlled asthma and well-controlled asthma, albeit across the range of asthma severity.9 However, an observational study conducted in France, with patients recruited by specialist chest physicians, observed a difference of 0.24 in EQ-5D-5L scores between patients with uncontrolled severe asthma and well-controlled or partly controlled severe asthma (mean [SD] 0.59 [0.25] vs 0.83 [0.22]; P<0.01), a similar difference to the present analysis.7
In the present analyses, absenteeism, presenteeism, overall work impairment, and activity impairment were reported more frequently by patients with uncontrolled severe asthma than in patients with controlled severe asthma, which is consistent with previous studies in specialist clinics comparing patients (N=7820) with poorly controlled, partly controlled, or well-controlled asthma,9 patients (N=2529) with mild-to-moderate asthma or severe asthma,27 and patients (N=670) with uncontrolled or controlled severe asthma.3
The strengths of this study include the generalizability of the population to clinical practice. NOVELTY allowed broad patient recruitment, and provides an opportunity to assess symptom burden, health status, and productivity loss in patients with severe asthma in a real-world setting.10,11 While clinical trials are important to determine whether a treatment or intervention is efficacious, they are typically conducted only in restricted patient populations. Most patients from clinical practice are excluded because they do not meet the required eligibility criteria; in severe asthma, these exclusions are particularly on the basis of airflow obstruction, bronchodilator reversibility, and smoking status.28 Real-world therapeutic studies complement results from clinical trials by providing greater external validity once the efficacy and safety of a treatment have been confirmed under the strictly controlled conditions of clinical trials.29 Additionally, patient-reported outcomes are often unavailable in electronic health records and are not available in claims databases. The present findings indicate the burden of severe uncontrolled asthma in a broad population recruited from both primary and specialist care.
NOVELTY had some limitations.11 The recruitment of patients from clinical practice may have potentially led to biased selection of those patients making frequent healthcare visits, but the same applies to other studies mentioned above. In order to avoid the selection bias observed in many regulatory studies,28 NOVELTY enrolled an unselected population of patients with physician-assigned diagnosis and physician-assessed severity, which may potentially result in variation in criteria used by physicians to diagnose asthma or COPD and to classify disease severity, including compared with severe asthma guidelines.30 Patients were not required to be taking high-dose ICS and LABA and may not have had their treatment optimized, as required by severe asthma guidelines.30 However, this is also rarely done before entry to clinical trials of biologic therapies and reflects the real-world setting. In this analysis, a higher proportion of patients with uncontrolled severe asthma had missing data for symptom burden, health status and productivity measures compared with patients with controlled severe asthma. However, the only clinically important difference observed between patients with uncontrolled severe asthma who did or did not complete at least one patient-reported outcome was age; patients who completed at least one patient-reported outcome were slightly older than those who did not complete any, suggesting that younger patients were less likely to complete patient-reported outcomes (data not shown).
Conclusion
In conclusion, our findings highlight the symptom burden of uncontrolled severe asthma and its impact on patient health status and productivity. These results support recommendations for interventions to achieve good asthma control in more patients with severe asthma, which would reduce its symptom burden and its substantial impact on patient health status and productivity.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-The-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/. The NOVELTY protocol is available at https://astrazenecagrouptrials.pharmacm.com.
Ethics Approval and Informed Consent
The NOVELTY study protocol was approved in each participating country by the relevant independent ethics committees and institutional review boards (listed in full in Supplementary Table 11) and all patients provided written informed consent.
Acknowledgments
The authors would like to thank the patients who participated in this study and the NOVELTY Scientific Community and the NOVELTY study investigators who are listed in full in Supplementary Tables 12 and 13. The authors also thank Jaime Solorzano (AstraZeneca) for his contribution to this analysis. Medical writing support, under the direction of the authors, was provided by Karen Cilliers, PhD, CMC Connect, a division of IPG Health Medical Communications, funded by AstraZeneca, Cambridge, UK, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175(9):1298-1304). Divyansh Srivastava is a current employee of Citicorp Services India Pvt Ltd, Pune, India.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The NOVELTY study is funded by AstraZeneca.
Disclosure
B Ding is an employee of AstraZeneca. S Chen is an employee and shareholder of AstraZeneca. D Srivastava is a former employee of ZS Associates. A Quinton is an employee and shareholder of AstraZeneca. W Cook is an employee of AstraZeneca. A Papi has received research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Pfizer, Sanofi, and Teva; consulting fees from AstraZeneca, Avillion, Chiesi, Elpen Pharmaceuticals, GlaxoSmithKline, IQVIA, Novartis, and Sanofi; and payment or honoraria from AstraZeneca, Avillion, Boehringer Ingelheim, Chiesi, Edmond Pharma, Elpen Pharmaceuticals, GlaxoSmithKline, IQVIA, Menarini, MSD, Mundipharma, Novartis, Sanofi, Teva, and Zambon; payments to his institution from Agenzia Italiana del farmaco (AIFA), AstraZeneca, Chiesi, GlaxoSmithKline and Sanofi. HK Reddel has participated in advisory boards for AstraZeneca, Chiesi, GlaxoSmithKline, Novartis, and Sanofi-Genzyme; has received honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, Getz, GlaxoSmithKline, Sanofi, and Teva Pharmaceuticals for independent medical educational presentations; received independent research funding from AstraZeneca, GlaxoSmithKline, and Novartis; and received consulting fees from AstraZeneca and Novartis. She is Chair of the Global Institute for Asthma Science Committee and a member of the Australian National Asthma Council Guidelines Committee.
References
1. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; 2022. Available from: https://ginasthma.org/reports/.
2. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi:10.1513/AnnalsATS.201703-259OC
3. Müllerová H, Cockle SM, Gunsoy NB, Nelsen LM, Albers FC. Clinical characteristics and burden of illness among adolescent and adult patients with severe asthma by asthma control: the IDEAL study. J Asthma. 2021;58(4):459–470. doi:10.1080/02770903.2019.1708095
4. Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17(2):74–80. doi:10.1155/2010/361071
5. Dolan CM, Fraher KE, Bleecker ER, et al. Design and baseline characteristics of the epidemiology and natural history of asthma: outcomes and Treatment Regimens (TENOR) study: a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2004;92(1):32–39. doi:10.1016/S1081-1206(10)61707-3
6. Haselkorn T, Szefler SJ, Chipps BE, et al. Disease Burden and Long-Term Risk of Persistent Very Poorly Controlled Asthma: TENOR II. J Allergy Clin Immunol Pract. 2020;8(7):2243–2253. doi:10.1016/j.jaip.2020.02.040
7. Lucas C, Aly S, Touboul C, Sellami R, Guillaume X, Garcia G. Patient-Reported Outcome in Two Chronic Diseases: a Comparison of Quality of Life and Response Profiles in Severe Migraine and Severe Asthma. Patient Relat Outcome Meas. 2020;11:27–37. doi:10.2147/PROM.S222597
8. Stewart J, Kee F, Hart N. Using routinely collected primary care records to identify and investigate severe asthma: a scoping review. NPJ Prim Care Respir Med. 2021;31(1):1. doi:10.1038/s41533-020-00213-9
9. Lee LK, Ramakrishnan K, Safioti G, Ariely R, Schatz M. Asthma control is associated with economic outcomes, work productivity and health-related quality of life in patients with asthma. BMJ Open Respir Res. 2020;7(1):e000534. doi:10.1136/bmjresp-2019-000534
10. Reddel HK, Gerhardsson de Verdier M, Agustí A, et al. Prospective observational study in patients with obstructive lung disease: NOVELTY design. ERJ Open Res. 2019;5(1):00036–02018. doi:10.1183/23120541.00036-2018
11. Reddel HK, Vestbo J, Agustí A, et al. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur Respir J. 2021;58(3):2003927. doi:10.1183/13993003.03927-2020
12. Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi:10.1016/j.jaci.2006.01.011
13. Fletcher CM, Clifton M, Fairbairn AS, et al. Standardized questionnaires on respiratory symptoms: a statement prepared for, and approved by, the Medical Research Council’s Committee on the Aetiology of Chronic Bronchitis. BMJ. 1960;2(5213):1665.
14. Karlsson N, Atkinson MJ, Müllerová H, et al. Validation of a diagnosis-agnostic symptom questionnaire for asthma and/or COPD. ERJ Open Res. 2021;7(1):00828–02020. doi:10.1183/23120541.00828-2020
15. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
16. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Prevention, Diagnosis and Management of COPD: 2023 Report. 2022. Available from: https://goldcopd.org/2023-gold-report-2/.
17. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
18. Tomaszewski E, Atkinson MJ, Janson C, et al. Chronic Airways Assessment Test: psychometric properties in patients with asthma and/or COPD. Respir Res. 2023;24(1):106. doi:10.1186/s12931-023-02394-6
19. EQ-5D. EQ-5D User Guides. Available from: https://euroqol.org/publications/user-guides.
20. Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
21. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi:10.2165/00019053-199304050-00006
22. R Core Team. R: a language and environment for statistical computing. Available from: https://www.r-project.org/.
23. Barros LL, Souza-Machado A, Correa LB, et al. Obesity and poor asthma control in patients with severe asthma. J Asthma. 2011;48(2):171–176. doi:10.3109/02770903.2011.554940
24. Milanese M, Di Marco F, Corsico AG, et al. Asthma control in elderly asthmatics. An Italian observational study. Respir Med. 2014;108(8):1091–1099. doi:10.1016/j.rmed.2014.05.016
25. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi:10.1081/COPD-200050513
26. McClure NS, Sayah FA, Xie F, Luo N, Johnson JA. Instrument-Defined Estimates of the Minimally Important Difference for EQ-5D-5L Index Scores. Value Health. 2017;20(4):644–650. doi:10.1016/j.jval.2016.11.015
27. Chen H, Blanc PD, Hayden ML, et al. Assessing productivity loss and activity impairment in severe or difficult-to-treat asthma. Value Health. 2008;11(2):231–239. doi:10.1111/j.1524-4733.2007.00229.x
28. Brown T, Jones T, Gove K, et al. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52:1801444. doi:10.1183/13993003.01444-2018
29. Price D, Brusselle G, Roche N, Freeman D, Chisholm A. Real-world research and its importance in respiratory medicine. Breathe. 2015;11(1):26–38. doi:10.1183/20734735.015414
30. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
