Back to Journals » Clinical Ophthalmology » Volume 15
Survival Outcome and Prognostic Factors of Corneal Transplantation: A 15-Year Retrospective Cohort Study at King Chulalongkorn Memorial Hospital
Authors Reinprayoon U, Srihatrai P , Satitpitakul V , Puangsricharern V , Wungcharoen T , Kasetsuwan N
Received 31 August 2021
Accepted for publication 30 September 2021
Published 18 October 2021 Volume 2021:15 Pages 4189—4199
DOI https://doi.org/10.2147/OPTH.S336986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Usanee Reinprayoon,1,2 Parinya Srihatrai,2 Vannarut Satitpitakul,1,2 Vilavun Puangsricharern,1,2 Thitima Wungcharoen,2 Ngamjit Kasetsuwan1,2
1Excellence Center of Cornea and Limbal Stem Cell Transplantation, Department of Ophthalmology, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 2Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Correspondence: Usanee Reinprayoon
Excellence Center of Cornea and Limbal Stem Cell Transplantation, Department of Ophthalmology, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, 1873 Rama 4 Road, Pathumwan, Bangkok, 10330, Thailand
Tel +66 816186025
Email [email protected]
Purpose: To evaluate long-term survival outcomes and determine the prognostic factors of corneal transplantation performed at a tertiary referral hospital in Thailand.
Design: A 15-year retrospective cohort study.
Materials and Methods: One corneal graft per patient was selected; graft failure was defined as graft opacity due to recurrent disease or endothelial cell dysfunction. Kaplan–Meier survival analysis was performed. Median time to failure was compared using the Log rank test. Prognostic factors were identified using the Cox proportional hazards model.
Results: We enrolled 704 transplanted grafts. Surgical indications were optical (88.5%), therapeutic (10.2%), and tectonic (1.3%). The most common diagnoses were corneal opacity (25.3%), bullous keratopathy (15.8%), and regraft (14.8%). The overall survival rates at 1, 3, 5, and 10 years were 87.5%, 72.0%, 59.2%, and 41.7%, respectively. Univariate analysis identified age, primary diagnosis, graft size, pre-existing glaucoma, prior lens status, prior intraocular surgery, indication for surgery, donor endothelial cell density, and previous graft rejection as prognostic factors for graft failure. Multivariate analysis revealed three prognostic factors: primary diagnosis of perforation/peripheral ulceration/Mooren’s ulcer (hazard ratio [HR]=28.57; 95% confidence interval [CI], 6.32– 129.16; P< 0.001), active keratitis (HR=24.30; 95% CI, 5.88– 100.43; P< 0.001), regraft (HR=9.37; 95% CI, 2.27– 38.66; P=0.002), and pseudophakic/aphakic bullous keratopathy (HR=7.97; 95% CI, 1.93– 32.87; P=0.004); pre-existing glaucoma (HR=1.52; 95% CI, 1.13– 2.04; P=0.006); and previous graft rejection (HR=1.95; 95% CI, 1.54– 2.48; P< 0.001).
Conclusion: Overall corneal graft survival rate was high in the first postoperative year and decreased after that. Primary diagnosis, pre-existing glaucoma, and previous graft rejection negatively influenced graft survival.
Keywords: keratoplasty, Thailand, survival rate, risk factor, prognostic factor
Introduction
Corneal blindness is one of the leading causes of blindness worldwide.1 Recent global data from the Vision Loss Expert Group of the Global Burden of Disease Study2 has found that corneal opacity is currently ranked as the fifth leading cause of blindness among both the global population and within Southeast Asia, with the burden of corneal blindness being underestimated in many developing countries. According to the Thai Red Cross Eye Bank (TRCEB),3 around 15,463 people with corneal blindness are currently awaiting a transplant.
The etiologies of corneal blindness are based on social, geographic, and economic factors, with the majority of patients in developing countries having anterior corneal pathologies contrary to the high prevalence of corneal endothelial diseases reported in the US literature.4 The most common corneal diagnoses leading to corneal transplantation in Singapore are pseudophakic bullous keratopathy (PBK)/aphakic bullous keratopathy (ABK), postinfectious scarring, and regraft.5 By contrast, corneal blindness in Thailand6 is mostly due to corneal scarring from previous trauma or infection, corneal ulcer, surgical bullous keratopathy, and genetic diseases such as corneal dystrophy; these causes may have a considerable effect on regional or racial differences in prevalence.
The main treatment option for patients with corneal blindness is corneal transplantation. The first successful corneal transplantation was performed in 1905. This procedure is the most frequently performed solid organ transplantation and results in better survival outcomes than other transplantations.7 Various studies, mainly from graft registries in developed countries, have reported on the survival outcomes of corneal transplantation, including penetrating and lamellar keratoplasty. Although excellent survival and visual results have been reported for both penetrating and lamellar keratoplasty in the short-term,8,9 there are a limited number of published multicenter studies on long-term outcomes. A previously published study from the Western world, entitled The Australian Corneal Registry Study (ACGR), reported that the survival of penetrating corneal grafts was 62% after ten years.10 Similarly, Borderie et al’s study of the French population reported 5- and 10-year graft survival estimates of 74% and 64%, respectively.11 There have been few studies on long-term corneal graft survival in Asian populations, especially within Southeast Asia.5,12 The Singapore Corneal Transplant Study (SCTS) demonstrated that the survival rate of an optical purposed graft was up to 86.6% after the first postoperative year, which decreased to 52% after 10 years.5 This result is comparable to the aforementioned Western studies.10,11 Nevertheless, Dandona et al’s large-scale study conducted in India, a developing country, revealed significantly lower graft survival for an optical indication, with a rate of 46.5% after 5 years.12 Many factors can impact these survival rates, such as the time from harvesting to tissue preservation, eye bank procurement procedure, donor endothelial cell density, type of surgery, the experience of the surgeon, and postoperative management. Furthermore, most of the cornea donor tissues in the Singapore study were imported, which resulted in a short waiting time.
The shortage of donor corneal tissue is the main obstacle to treatment in developing countries.13 All donor grafts used in this study were harvested locally by the TRCEB. Even though the Buddhist population fundamentally believes in “making merit,” and the TRCEB conducts an active program for eye donation, the waiting list is still relatively long.14 Most patients have to wait for a donor for years; as a result, the progression of the disease during this period may affect visual prognosis and the outcome.
Therefore, the current study was conducted to investigate long-term survival outcomes and to identify significant prognostic factors of corneal transplantation in Thailand using local donor tissue. This may reflect the developing world’s circumstances within Southeast Asia in recent years. Furthermore, findings of this study may also reflect the general trend of the public healthcare system regarding corneal blindness prevention, corneal disease treatment, and management of postoperative transplant care in modern times.
Materials and Methods
This was a 15-year retrospective cohort study conducted from January 2000 to December 2013 at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University. The study was carried out in accordance with the tenets of the Declaration of Helsinki and has been registered in the Thai Clinical Trials Registry system (TCTR20141024001).
This study included data on a total of 1373 consecutive surgeries for all types of corneal transplantation procedures obtained from the TRCEB database. Four hundred and eighty-four cases (35.2%) were excluded due to incomplete data. The remaining 889 surgeries were reviewed. In multiple grafts, only one graft per patient was randomly selected for accurate statistical analysis. Randomization was performed using a STATA random number generator (version 10.1; Stata Corp., College Station, Texas, USA). As a result, 704 transplanted eyes were included in statistical analysis. Information on corneal donor tissue acquisition was obtained from the TRCEB, the main local corneal donor in Thailand.
We divided data into the following three variable sets to analyze them separately: preoperative, intraoperative, and postoperative data. A total of 26 indices were collected. Regarding primary diagnosis, the three main indications for corneal transplantation were optical, tectonic, and therapeutic purposes. Corneal grafting in keratoconus patients, defined as the reference group, had a graft failure incidence rate of 1 per 1000 patients per month.
Graft failure was defined as an irreversible loss of corneal clarity due to any cause. The possible risk factors for graft failure were categorized into preoperative, intraoperative, and postoperative factors. Preoperative factors included patient characteristics, such as sex, age, systemic conditions, primary diagnosis, waiting time for transplantation (the period between registration for transplantation and transplantation of the donor cornea), preoperative recipient status, preexisting glaucoma, or raised intraocular pressure (IOP), lens status, ocular surface disease, and history of previous intraocular surgeries. Corneal vascularization was defined as the invasion of blood vessels into the stromal cornea from 1–4 quadrants. Increased IOP was defined as IOP ≥ 21 mmHg when the glaucomatous optic disc’s structural and/or functional changes could not be assessed. The other preoperative factors were donor graft conditions, including age and sex of donors; endothelial cell density; death-to-enucleation time; and enucleation-to-surgery time. Intraoperative factors included the type of corneal transplantation (penetrating keratoplasty, triple operation, anterior lamellar keratoplasty, endothelial keratoplasty), other combined surgical procedures, intraoperative complications, and recipient graft size. Postoperative factors included postoperative complications such as raised IOP or glaucoma, corneal ulcer, and allograft rejection. Survival outcomes were reported as survival rates at 1, 3, 5, and 10 years.
Statistical Analysis
Data were analyzed using STATA version 10.1 (Stata Corp., College Station, Texas, USA). Descriptive statistics were used for the continuous and categorical variables. The corneal transplantation survival rates, which were analyzed overall and for different indications, were determined using the Kaplan-Meier method and were reported as survival curves. Eleven parameters were analyzed: recipient age, primary diagnosis, pre-existing glaucoma or raised IOP, graft rejection, donor endothelial cell density, prior intraocular surgery, lens status, recipient graft size, indication for surgery, waiting time to transplantation, and corneal vascularization. Furthermore, the possible risk factors were qualified and reported as hazard ratios (HRs). The results were also analyzed using the log-rank statistic to assess significant differences among strata. For variables with three or more categories, the reference category was chosen based on previous studies5 or the lowest hazard stratum. All variables were subjected to the Log rank test and Cox proportional hazards regression for univariate analysis to assess the significance of their influence on graft failure. All factors that achieved a P-value <0.2 on univariate analysis were entered into the multivariate analysis model and Cox regression was used to adjust for the effect of confounding factors. A P-value of <0.05 was considered to be statistical significance. For descriptive data, the mean with standard deviation was used.
Results
Seven hundred and four consecutive surgeries performed during the study period were included in the analysis. There were 393 (55.8%) males and 311 (44.2%) females (Table 1). Their mean age was 54.4 ±18.2 years. The median follow-up period was 31.7 months (range, 0.2–171.0 months). Eye laterality was observed in the right and left eyes of 358 (50.8%) and 346 (49.2%) patients, respectively. Keratoplasty was performed in 623 (88.5%), 72 (10.2%), and 9 (1.3%) eyes for optical, therapeutic, and tectonic purposes, respectively (Table 2). The three most common diagnoses were corneal opacity/scar (25.3%), PBK/ABK (15.8%), and regraft (14.8%) (Table 2). The waiting time to transplantation was analyzed in 690 eyes. Of these, optical indication had the longest median waiting time at 42.7 months (range, 0–194.3 months), whereas tectonic and therapeutic indications’ waiting times were 3.5 months (range, 0–161.4 months) and 0.6 months (range, 0–96.0 months), respectively.
 |
Table 1 Demographic Characteristics of the Study Population |
 |
Table 2 Indications and Primary Diagnosis of Corneal Transplantation |
The two most common procedures performed were penetrating keratoplasty and triple operations (Table 3). Data on intraoperative complications, such as vitreous loss, hyphema, and Descemet’s membrane detachment, were collected. Postoperative complications included graft rejection, delayed epithelialization, ocular surface problems, raised IOP or glaucoma, and ulcer on the graft, resulting in differences in management and outcomes.
 |
Table 3 Types of Corneal Transplantation |
The overall Kaplan-Meier median graft survival period was 81.7 months (range, 65.8–97.6 months). The overall survival rates at 1, 3, 5, and 10 years were 87.5%, 72.0%, 59.2%, and 41.7%, respectively (Figure 1). The incidence rate of graft failure was 9 per 1000 patients per month.
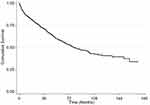 |
Figure 1 The Kaplan-Meier survival curve analysis of overall corneal grafts. |
Among various indications, grafts for optical indications had the highest survival, with rates of 91.7%, 75.8%, 62.5%, and 44.9% at 1, 3, 5, and 10 years, respectively, with a median follow-up period of 99.9 months (range, 76.4–123.3 months) (Figure 2). In therapeutic corneal transplants, the median follow-up period was 13.3 months (range, 5.4–57.7 months). The survival rates in this group were 56.9%, 44.3%, 36.1%, and 15.5% at 1, 3, 5, and 10 years, respectively. The overall proportion of patients with graft failure was the highest in patients aged 41–60 years (43.75%) and the lowest in patients under 21 years of age (26.67%). Survival rates categorized by diagnosis are shown in Table 4.
 |
Table 4 Survival Rates of Primary Diagnosis |
 |
Figure 2 The Kaplan–Meier graft survival rates for optical, therapeutic, and tectonic indications. |
Nine of the 11 parameters were found to be potential prognostic factors for graft failure by univariate analysis: recipient age, indication for surgery, primary diagnosis, prior intraocular surgery, lens status, pre-existing glaucoma or raised IOP, recipient graft size, history of graft rejection, and donor endothelial cell density.
For the recipient age, the patients aged 21–40 years had the highest graft survival rate, whereas those aged 41–60 years had the highest risk for graft failure (HR=1.93; 95% confidence interval [CI], 1.36–2.75; P<0.001). Tectonic indications (HR=6.22; 95% CI, 3.06–12.66; P<0.001) and therapeutic indications (HR=4.26; 95% CI, 3.13–5.79; P<0.001) were associated with higher risks of graft failure compared with optical indications. Patients with a history of intraocular surgery (HR=1.68; 95% CI, 1.32–2.12; P<0.001), pseudophakic eyes (HR=1.53; 95% CI, 1.18–1.98; P=0.001), and donors with low endothelial cell density (HR=0.9991; 95% CI, 0.9988–0.9994; P<0.001) had a significantly higher risk of graft failure. Recipient grafts with diameters of 7.5–7.9 mm were found to have the lowest risk of graft failure compared with the other groups. Large recipient grafts with diameters of 8.5–8.9 mm (HR=5.44; 95% CI, 2.75–10.75; P<0.001) and ≥9.0 mm (HR=4.91; 95% CI, 2.06–11.69; P<0.001) were associated with significantly higher risks of graft failure (Table 5). However, the patients with longer waiting times were not at a significantly increased risk of graft failure (HR=1.08; 95% CI, 0.80–1.45; P=0.623). The remaining three prognostic factors identified in univariate analysis were also included in the multivariate analysis, the details of which are described below.
 |
Table 5 Univariate Analysis of Graft Failure Association |
Multivariate analysis revealed three prognostic factors: primary diagnosis, pre-existing glaucoma, and history of graft rejection (Table 6). In the case of primary diagnosis, keratoconus diagnosis was identified as the reference group (HR=1), whereas major diseases related to graft failure included perforation/peripheral ulceration/Mooren’s ulcer (HR=28.57; 95% CI, 6.32–129.16; P<0.001), active keratitis (HR=24.30; 95% CI, 5.88–100.43; P<0.001), regraft (HR=9.37; 95% CI, 2.27–38.66; P=0.002), and PBK/ABK (HR=7.97; 95% CI, 1.93–32.87; P=0.004).
 |
Table 6 Multivariate Analysis of Graft Failure Association |
The second factor, pre-existing glaucoma or raised IOP, was the only ocular condition that negatively influenced graft survival (HR=1.52; 95% CI, 1.13 to 2.04; P=0.006).
The last prognostic factor was a history of graft rejection, a common postoperative complication of corneal transplantation. Graft rejection was a significant risk factor for graft failure (HR=1.95; 95% CI, 1.54–2.48; P<0.001).
Discussion
At present, corneal transplantation is one of the most favorable options for visual rehabilitation for those currently faced with corneal blindness.7 Several studies, which were mainly conducted in the developed world or Caucasian populations, have reported short-term graft survival outcomes and potential risk factors of graft failure. However, there are a limited number of studies assessing long-term corneal graft success in developing countries and non-Caucasian populations using local donor tissue.12,15
Previous large-scale studies of Asian eyes were conducted over a decade ago in India (1997) and Singapore (2008).5,12 In the study by Dandona et al12 in India, locally acquired donor tissue revealed a poor long-term graft survival rate and visual results. On the contrary, the graft survival success of the SCTS,5 which used internationally imported donor corneas, is similar to those of other major long-term studies from Western countries.8,10,16 These results may be related to differences in demographic profile, preoperative diagnosis, indications for surgery, or socioeconomic factors, as India is relatively less developed than Singapore. However, reliable data regarding corneal transplant survival using local donor tissue from the developing world are barely available at present, particularly in Southeast Asia.
The present study reported higher optical graft survival rates (91.0%, 76.1%, 61.3%, and 43.9% at 1, 3, 5, and 10 years, respectively) than did the study by Dandona et al (79.6%, 68.7%, and 46.5% at 1, 2, and 5 years, respectively).12 A study in Japan conducted by Ono et al15 showed a relatively higher long-term graft survival rate of 60.4% at 12 years; this is comparable to the rates reported in Western studies such as Williams et al’s ACGR study and Borderie et al’s study in France.10,11
The graft survival outcome for optical purposes at one and three years (91.7% and 75.8%, respectively) in the current study was higher than that in the SCTS5 (86.6% and 72.0%, respectively). Our results were comparable to those of the SCTS at 5 years but were slightly lower than the SCTS results at 10 years (44.9% in our study vs 52.0% in SCTS). However, our study’s overall Kaplan-Meier median graft survival period was negligibly lower than the median graft survival period in the SCTS (99.9 months vs 104.9 months).
The first-year survival rate of our study was comparable to that of the ACGR (91%) and the Cornea Transplant Follow-up Study (CTFS) (88%). Notably, our findings showed a gradual decrease in graft survival rate over time, which contributed to a lower long-term graft survival rate compared with data from the ACGR10 (62%), France11 (64%), and Sweden17 (71%) studies, which showed a rate of more than 60% at 10 years.
Despite the good overall survival outcome of corneal transplantation across various studies, the individual graft survival rate varies depending on the donor, recipient, surgeon, and environmental factors. In the current study, we confirmed that primary diagnosis, pre-existing glaucoma, and history of graft rejection were significant prognostic factors demonstrating a negative influence on graft survival by multivariate analysis. As in our study, primary diagnosis and glaucoma were also found to be significant prognostic factors in the SCTS. Other factors such as recipient age, recipient sex, inflammation, vascularization, perforation, recipient graft size, and donor endothelial cell count influenced graft survival in the SCTS.5 Similarly, Dandona et al found that primary diagnosis, recipient age, vascularization, quality of the donor cornea, and socioeconomic status affected transplant survival significantly.12
The difference in primary diagnosis distribution in individual studies, such as in the ACGR,10 the Canadian Corneal Graft Outcome Study (CGOS),16 and studies conducted in France,11 the USA,18 and China,19 were reported to be associated with graft success rate. Active keratitis, corneal perforation, PBK/ABK, and regrafts have been identified as significant poor prognostic factors for corneal transplantation.12,20
Although we found that the three most common primary diagnoses in our study (corneal opacity/scar, PBK/ABK, and regraft) were similar to those of Asian studies conducted in India,12 Japan,15 and Singapore,5 the overall graft survival rate varied. This might be related to other contributing factors such as donor quality, waiting time for transplantation, and socioeconomic issues. Nonetheless, our findings were inconsistent with those reported from the Western world, such as by the ACGR in Australia,10 the National Health Service Blood and Transplant Ocular Tissue Advisory Group (OTAG) in the UK,21 the Corneal Transplant Epidemiologic Study (CORTES) in Italy,22 a study in France,11 the CGOS in Canada,16 and the USA,18 in which keratoconus and PBK/ABK were the leading primary diagnoses (range, 21–47% in keratoconus; 25–43% in PBK/ABK).
As reported by many previous studies, keratoconus has typically been considered the disease with the highest graft survival after corneal transplantation.17,22 In our study, the survival rate was the highest in keratoconus treatment (95.6% at 5 years), although it accounted for only 4.4% of all diagnoses. In other studies, keratoconus was more prevalent than in our study, with a primary diagnosis of 9.7% in the SCTS,5 6.8% in India, and 6.7% in Japan. As mentioned previously, several studies conducted in Caucasian populations of the Western world indicated notably greater percentages of keratoconus cases, namely in the ACGR in Australia (32%),10 the CORTES in Italy (47%),22 a study in France (27.8%),11 the OTAG in the UK (45.8%),21 and the CGOS in Canada (20.7%),16 owing to the distribution of cases. Consequently, many keratoconus cases in Western countries might have contributed to a higher overall graft survival outcome.
We also found that the survival outcomes were worse in active keratitis, corneal perforation, and peripheral ulcer, accounting for 10.9% of all cases. The relatively high prevalence of these cases, which is aligned with a unique Asian corneal disease spectrum,23 might have contributed to a somewhat reduced long-term graft survival rate due to the active disease itself, the high probability of complications, and the quality of the donor cornea used for therapeutic and tectonic indications.
Our study’s other potential prognostic factors that significantly reduced graft survival outcomes were pre-existing glaucoma and a history of graft rejection. Consistent with prior studies from the West (ACGR, CCTS, France, and the USA),10,11,18,24 glaucoma was a significant risk factor affecting graft survival outcomes. A history of graft rejection was noted as a prognostic factor, similar to studies in Japan, China, the ACGR, France, and the CGOS.10,11,15,16,19
Six other factors, including recipient age, recipient graft size, lens status, prior intraocular surgery, endothelial cell density, and indication for surgery, were prognostic factors for graft failure by univariate analysis. Corneal neovascularization was shown not to significantly influence corneal graft survival, which is different from other studies; this might have been caused by incomplete and inaccurate retrospective data collection.
We have an efficient registration system for patients through the TRCEB. However, we have been experiencing a shortage of donors, in which we cannot achieve a demand-supply balance. Our patients must stay on waiting lists for a long period. Thus, the TRCEB must manage and optimize waiting times according to the urgency of the individual’s eye condition. In the TRCEB, eye conditions are classified as emergency or urgent cases, such as corneal perforation, uncontrolled corneal ulcers, and elective cases. In turn, elective cases are divided into bilateral corneal disease requiring bilateral corneal transplantation and unilateral corneal disease. Most patients with visual impairment needed to wait around 3–5 years for donor tissue in the present study. As a result, this long waiting time was likely to impact the outcome of corneal transplantation and was included as a possible risk factor in our study. However, there was no statistically significant difference in the survival rate among patients waiting for three or fewer years and those waiting three years or more. Although the influence of waiting time on survival outcome was not apparent, longer waiting time certainly impacted patients’ quality of life and economic burden in society.26
Dandona et al found that patients with lower socioeconomic status had a higher risk of graft failure, with a relative risk of 1.28 (95% CI, 1.16–1.42).12 On behalf of the OTAG study, Chua et al reported that hard-pressed patients had an increased risk of graft failure within 5 years than the least deprived patients after adjusting for confounding factors and indications (HR=1.3; 95% CI, 1.1–1.5; P=0.003).25 In our study, 14.3% of our patients were unemployed, suggesting a low socioeconomic status. This might have contributed to the association by increasing the risk of graft failure due to poorer compliance with postoperative care. Thus, we should educate high-risk patients to avoid all preventable potential risk factors of graft failure.
Limitations of our study include its retrospective design, missing data, recall bias, and incomplete medical record data, which might have affected the analysis. Excluded incomplete data by 35% may interfere and possibly overestimate the survival outcomes. We strongly believe that pre-registration for corneal transplantation in conjunction with complete follow-up data and patient education will solve this problem and improve the clinical results in the future.
Conclusion
In conclusion, corneal grafts’ overall long-term survival rate at King Chulalongkorn Memorial Hospital is comparable to that of major studies from Asian and Western countries. Most grafts are used for visual rehabilitation, which shows the best prognosis. Primary diagnosis, glaucoma, and history of graft rejection are significant prognostic factors that influence graft survival outcomes. Lastly, the shortage of donor corneas using local donor tissue in developing countries is problematic and should be qualified as an urgent public health concern.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University and carried out in accordance with the tenets of the Declaration of Helsinki. Owing to its retrospective design, patient’s written informed consent to study enrollment was waived by the Ethic Committee of the hospital. In addition, all patient data was deidentified.
Acknowledgments
We thank Wasan Panyasang, MSc, the Research Affairs of the Faculty of Medicine, Chulalongkorn University, Bangkok, for biomedical statistical consultation and Thai Red Cross Eye Bank, Thai Red Cross Society, Bangkok, Thailand, for data information.
Disclosure
The abstract was presented orally at the 36th Royal College of Ophthalmologists of Thailand Annual Meeting, November 25, 2015, Khon Kaen, Thailand and presented as a research poster at the 31st Asia-Pacific Academy of Ophthalmology Congress, March 24–27, 2016, Taipei, Taiwan. The authors report no conflicts of interest in this work.
References
1. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851.
2. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. doi:10.1016/S2214-109X(17)30393-5
3. Thai Red Cross Eye Bank. Statistics of February 2021. Available from: https://eyebankthai.redcross.or.th.
4. Eye Bank Association of America. 2016 Eye Banking Statistical Report. Washington DC: Eye Bank Association of America; 2017.
5. Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008;115(6):975–982 e1. doi:10.1016/j.ophtha.2007.08.049
6. Pariyakanok L, Erjongmanee S, Saonanon P. Indications for corneal transplantation in Thailand between 1996 and 2008. Asian Biomed. 2011;5(6):843–848.
7. Crawford AZ, Patel DV, McGhee CNJ. A brief history of corneal transplantation: from ancient to modern. Oman J Ophthalmol. 2013;6(Suppl 1):S12–S17. doi:10.4103/0974-620X.122289
8. Vail A, Gore SM, Bradley BA, et al. Conclusions of the corneal transplant follow up study. Br J Ophthalmol. 1997;81:631–636. doi:10.1136/bjo.81.8.631
9. Jones MN, Armitage WJ, Ayliffe W, et al. Penetrating and deep anterior lamellar keratoplasty for keratoconus: a comparison of graft outcomes in the United Kingdom. Invest Ophthalmol Vis Sci. 2009;50(12):5625–5629. doi:10.1167/iovs.09-3994
10. Williams KA, Muehlberg SM, Lewis RF, et al. Long-term outcome in corneal allotransplantation. The Australian Corneal Graft Registry. Transplant Proc. 1997;29(1–2):983. doi:10.1016/S0041-1345(96)00335-1
11. Borderie VM, Boëlle PY, Touzeau O, et al. Predicted long-term outcome of corneal transplantation. Ophthalmology. 2009;116(12):2354–2360. doi:10.1016/j.ophtha.2009.05.009
12. Dandona L, Naduvilath TJ, Janarthanan M, et al. Survival analysis and visual outcome in a large series of corneal transplants in India. Br J Ophthalmol. 1997;81(9):726–731. doi:10.1136/bjo.81.9.726
13. de By TM. Shortage in the face of plenty: improving the allocation of corneas for transplantation. Dev Ophthalmol. 2003;36:56–61.
14. Reinprayoon U, Maneerat N, Chansangpetch S, et al. Eye donation project: differences between donors versus refusers. Int J Eye Bank. 2015;3(1):17.
15. Ono T, Ishiyama S, Hayashidera T, et al. Twelve-year follow-up of penetrating keratoplasty. Jpn J Ophthalmol. 2017;61(2):131–136. doi:10.1007/s10384-016-0489-2
16. Sit M, Weisbrod DJ, Naor J, et al. Corneal graft outcome study. Cornea. 2001;20(2):129–133. doi:10.1097/00003226-200103000-00002
17. Claesson M, Armitage WJ. Ten-year follow-up of graft survival and visual outcome after penetrating keratoplasty in Sweden. Cornea. 2009;28(10):1124–1129. doi:10.1097/ICO.0b013e3181a2a7a6
18. Thompson RW
19. Pan Q, Li X, Gu Y. Indications and outcomes of penetrating keratoplasty in a tertiary hospital in the developing world. Clin Exp Ophthalmol. 2012;40(3):232–238. doi:10.1111/j.1442-9071.2011.02598.x
20. Fasolo A, Capuzzo C, Fornea M, et al. Risk factors for graft failure after penetrating keratoplasty: 5-year follow-up from the corneal transplant epidemiological study. Cornea. 2011;30(12):1328–1335. doi:10.1097/ICO.0b013e318206895a
21. Sibley D, Hopkinson CL, Tuft SJ, et al. Differential effects of primary disease and corneal vascularisation on corneal transplant rejection and survival. Br J Ophthalmol. 2020;104(5):729–734. doi:10.1136/bjophthalmol-2019-314200
22. Fasolo A, Frigo AC, Böhm E, et al. The CORTES study: corneal transplant indications and graft survival in an Italian cohort of patients. Cornea. 2006;25(5):507–515. doi:10.1097/01.ico.0000214211.60317.1f
23. Tran TM, Duong H, Bonnet C, et al. Corneal blindness in Asia: a systematic review and meta-analysis to identify challenges and opportunities. Cornea. 2020;39(9):1196–1205. doi:10.1097/ICO.0000000000002374
24. Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101(9):1536–1547. doi:10.1016/s0161-6420(94)31138-9
25. Chua PY, Azuara-Blanco A, Hulme W, et al. The effect of socioeconomic deprivation on corneal graft survival in the United Kingdom. Ophthalmology. 2013;120(12):2436–2441. doi:10.1016/j.ophtha.2013.07.050
26. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001;85(3):322–326. doi:10.1136/bjo.85.3.322
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
