Back to Journals » Clinical Ophthalmology » Volume 17
Surgical Time and Postoperative Symptoms Study in Pterygium Excision and Amniotic Membrane Graft Using Celularity Triple Layer Dehydrated Amniotic Membrane
Authors Rivera-Morales P, Barnard L, Linderman W, Gill M, Diaz V
Received 6 April 2023
Accepted for publication 20 June 2023
Published 11 July 2023 Volume 2023:17 Pages 1967—1974
DOI https://doi.org/10.2147/OPTH.S410452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Paola Rivera-Morales, Luke Barnard, Wendy Linderman, Mohsain Gill, Vicente Diaz
Department of Ophthalmology and Visual Sciences, Yale School of Medicine, New Haven, CT, USA
Correspondence: Vicente Diaz, Department of Ophthalmology, Yale School of Medicine, 40 Temple St, New Haven, CT, 06510, USA, Email [email protected]
Purpose: To evaluate a novel sutureless glueless technique using a triple-layer dehydrated amniotic membrane (TLDAM) for pterygia excisions in surgical time, postoperative pain, epiphora, irritation, and FBS.
Methods: Twenty eyes with pterygia underwent excision with mitomycin C. The conjunctival defect was closed with TLDAM placed on the dried scleral bed with the edges of the amniotic membrane tucked under the edges of the conjunctival defect. Surgical times were measured from injection of lidocaine to final placement of bandage contact lens. After a bandage contact lens was placed, the eye was patched until POD1. Patients graded self-administered questionnaires to rate pain, FBS, irritation, and epiphora on a scale of 1– 5 (1-none; 5-severe) at POD1 and POW1.
Results: Surgical times ranged from 6:55 to 12:00, with mean of 8:29. Compared with a previous study of sutureless glueless methodology, the difference in mean surgical time was 11.9 (p < 0.0001). Mean questionnaire scores were as follows: POD1 pain 1.8, FBS 2.3, irritation 1.0, and epiphora 2.6; POW1 pain 1.5, FBS 1.6, irritation 1.6, and epiphora 1.6. Compared to previous studies, this technique showed significantly improved pain at POD1 (p=0.0086, p< 0.0001, p< 0.0001, p< 0.0001) and POW1 (p=0.0002, p=0.0016, p< 0.0001). Significant improvement in irritation and FBS was noted at POD1 and POW1. See Table 1 for full analysis.
Conclusion: The sutureless glueless technique using TLDAM is a safe and effective technique compared to current standard methods. There appears to be a significant benefit regarding surgical time and postoperative pain, irritation, epiphora, and FBS compared to previous studies.
Keywords: pterygium, amniotic membrane, triple layer dehydrated, sutureless, glueless
Introduction
Pterygium is a benign fibrovascular proliferative condition of the ocular surface that extends from the bulbar conjunctiva onto the surface of the cornea. It can cause vision loss due to invasion of the visual axis, progressive scarring, and irregular astigmatism. Among its most recognized risk factor is long-term exposure to ultraviolet (UV) radiation.1 It has also been associated with factors such as smoking, alcohol consumption, geography, heat, dust, and a dry climate.2,3 Currently, the only available treatment is the surgical removal of the lesion, for which many surgical procedures can be used. Different surgical techniques have been described in the literature for removing pterygia, including bare sclera, conjunctival autograft, simple closure, and amniotic membrane (AM).
Nonetheless, the incidence of recurrence is still of great concern, with estimates between 5% and 15% worldwide, according to current literature.4 Conjunctival autograph and amniotic membrane have been reported among the closure techniques with the lowest recurrence rates.5 It has been shown that chronic inflammation and young age play a significant role in pterygium recurrence.6,7 Recurrence rates are influenced by surgical approach, as well as the patient’s race and ethnicity. Pterygium recurrence is more common in Hispanic and black patients after AM transplantation and conjunctival autograft.8
Various graft fixation methods are currently used and studied to find the best in terms of time, comfort, and cost. Sutures are the most prevalent form of autograft fixation, although they have disadvantages such as longer operating time, postoperative discomfort, inflammation, and scarring.9 Fibrin glue is extensively used for various reasons, including simple graft attachment, faster surgical times, and less postoperative discomfort.10,11 However, it has several drawbacks, including high cost, the risk of infection transfer, and inactivation by iodine preparations.12 Autologous blood is a natural method with no additional costs or hazards and can significantly reduce postoperative irritations.13 However, it has been reported that autologous blood may not be the best method for stabilizing the autograph.14
Amniotic membranes contain a variety of growth factors that aid in wound healing, act as a scaffold for re-epithelialization, have anti-inflammatory properties and have shown to be effective in the treatment of epithelial defects.15–17 The use of AM does not require sutures, which helps in reducing surgical time and provides greater postoperative comfort. When an AM is applied, epithelium migrates on the membrane and the amnion becomes incorporated in the cornea, a termed “graft”.18 The amnion allows keratocytes to migrate through the stroma and deposit collagen/scar tissue, which aids in the formation of tissue at the site. Available amnions including fresh, cryopreserved (Amniograft), freeze dried (Ambio dry), and vacuum dried (Omnigen), have demonstrated efficacy to varying degrees; the latter two, however, offer advantages such as ease of storage and shipping at room temperature.18 Some studies have found that a single layer of AM may not be enough to treat corneal ulcers, since the membrane usually disappears faster than the ulcer healed, opening the discussion that multiple-layer membranes were of greater benefit.19,20 A previous study compared Biovance 3L Ocular, a decellularized, dehydrated human AM (DDHAM), AMBIO2, a dehydrated human AM (DHAM), and AmnioGraft, a cryopreserved human AM (CHAM). It was found that DDHAM promoted a higher initial inflammatory response with a declining trend across time and better results overall, suggesting greater ocular cell compatibility in vivo than the other AM. Their results demonstrated that DDHAM is a fully decellularized AM, whereas DHAM and CHAM contain residual cells and DNA. The study also found that DDHAM best supported the cellular activities of human corneal epithelial cells (HCECs), enhanced an initial inflammatory response, and prevented a prolonged inflammatory response in HCECs under an in vitro inflammatory condition.21
Biovance 3L Ocular (Celularity), is a triple-layer dehydrated AM (TLDAM) comprised solely of amniotic tissue and the basement membrane is intact. In addition, the decellularization process removes any residual cells, cellular debris, DNA, growth factors, and cytokines. These characteristics promote rapid cellular attachment within hours, and the attachment stimulates the release of growth factors and cytokines. In this study, we aim to evaluate a novel, sutureless and glueless surgical technique using a TLDAM for pterygia excisions in terms of surgical time and postoperative pain, epiphora, irritation, and foreign body sensation.
Methodology
This prospective study was performed at Yale Hospitals. This study was deemed exempt from review by the Yale Institutional Review Board (HIC# 2000031314), and the study conformed to the provisions of the Declaration of Helsinki. All participants provided informed consent. The sample size in this study was twenty patients who underwent pterygia excision between March 2021 and January 2022. Exclusion criteria included a history of recurrent pterygium, immune-related disease, glaucoma in the studied eye, other concurrent ocular surface pathology, ocular surface or eyelid disease, poor general health, and concurrent or anticipated enrollment in an interventional clinical trial involving either an investigational medicinal product or medical device.
Surgical Technique
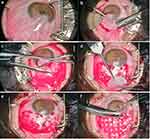
All surgeries were performed by a single surgeon, Vicente Diaz. Each affected eye was subjected to standard sterile preparation and draping. The procedure was performed under peribulbar lidocaine anesthesia. Dissection was performed by creating a peristome 3mm from the limbus, then dissecting underneath the pterygium, and removing it en bloc with a hemostat and creating 1×1 cm bare scleral bed. Both nasal and temporal pterygia were present at our study. A neurosurgical sponge soaked in mitomycin-C (MMC) was placed on the subconjunctival opening. The amniotic membrane sheet was placed directly on the dried scleral bed with the edges shaped to size and then tucked under the edges of the conjunctival defect (Figure 1). Immediately postoperatively, a bandage contact lens was placed, and the eye was patched until postoperative day 1. Surgical times were measured from injection of lidocaine to final placement of bandage contact lens. The bandage contact lens was removed at the post-operative week 1 visit. The patients were followed up monthly for the first 6 months and at 3-month intervals thereafter by the same surgeon for up to a year.
Main Outcome Measures
The initial examination was done on the first postoperative day, and a subsequent follow-up visit at week one. A 5-point scale questionnaire adapted from Lim-Bon-Siong et al22 was used to measure patients graded self-administered rate of pain, foreign body sensation (FBS), irritation, and epiphora on a scale of 1–5 (1 – none, 2 – very mild, 3 – mild, 4 – moderate, 5 – severe) at postoperative day 1 (POD1) and week 1 (POW1).
Statistical Analysis
Surgical times and questionnaire data were stored for analysis. Statistical analyses were carried out with GraphPad software Prism 9.4.1 (458). A T-test was used to evaluate the statistical significance between our study’s mean surgical time and Xu23 et al. This test was also used to compare our data on pain, irritation, FBS, and epiphora with Donepudi et al24 and Kucukerdonmez et al25 reported values. A P value less than 0.05 was considered statistically significant.
Results
A total of 20 patients (15 male patients, 5 female patients; age range 35–73 years) were included in whom excision of pterygium was carried out. All patients completed the 12-month follow-up and there was no recurrence of the pterygium in our patients. Our surgical time values for the 20 patients ranged from 6:55 to 12:00 minutes, with a mean of 8:30 ± 0.06 minutes (Figure 2).
 |
Figure 2 Pterygium excision surgical times from injection of lidocaine to the final placement of bandage contact lens. The blue line represents the mean surgical time of 8:30 minutes. |
Table 1 summarizes our mean results compared to previous studies. When analyzing our mean surgical time with those of Xu et al23 (20:4 ± 2.1 minutes), a study where conjunctival graft tissue junction was welded directly using an electrocautery pen (ECP), the difference in mean surgical time was 11.9 minutes (p < 0.0001). Patients’ self-reported degree of pain ranged from none to mild, with a mean of 1.8 ± 0.8 on POD1 and 1.5 ± 0.5 on POW1. Compared to both studies of Donepudi et al24 and Kucukerdonmez et al,25 our technique showed significantly less pain on both POD1 and POW1. Our mean irritation value was significantly less than both studies for POD1 (p = 0.0116, p < 0.0001 respectively) and significantly less than Kucukerdonmez et al25 sutures technique on POW1 (p < 0.0001). Mean foreign body sensation and epiphora values were also significantly less than some of the POD1 and POW1 values of both previous studies. See Table 1 for full statistical analysis.
 |
Table 1 Surgical Time and Postoperative Day 1 and Week 1 Parameters of Pain, Irritation, Foreign Body Sensation, and Epiphora Compared to Previous Studies |
Figure 3 shows our mean postoperative results for irritation, foreign body sensation, epiphora, and pain compared to Kucukerdonmez et al study.25 Our sutureless and glueless technique using TLDAM resulted in lower postoperative scores for all symptoms compared to the sutures group and most of the fibrin glue group values at POD1 and POW1.
Figure 4 depicts the postoperative appearances at day 1 and week 1. Complete epithelialization of the amniotic membrane graft was achieved 7 days after surgery in all patients.
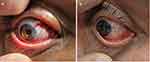 |
Figure 4 Pictures of (a) postoperative day 1 and (b) week 1 of a patient. |
Discussion
Pterygium has been treated surgically over the years using a variety of methods; therefore, surgeons continue to search for the most effective procedure. The primary goals are to improve patients’ vision, comfort, and cosmetic appearance with the lowest recurrence rate possible. A previous study by Syam et al26 found that conjunctival autograph caused scarring at the site of the donor conjunctiva in 36.6% of patients; therefore, it is not an ideal method for large pterygium cases. A prospective, interventional pilot study compared outcome parameters of conjunctival autograph fixation with sutures versus a similar sutureless and glueless technique.27 Our technique had better outcomes in parameters of pain, FBS, and epiphora. Due to suture discomfort, high cost of fibrin glue, risk of infection, and instability of fixation with autologous blood, we examined the efficacy of a sutureless and glueless technique for fixation of a triple-layer dehydrated amniotic membrane.28,29 To our knowledge, this is the first study to assess the clinical outcomes of this novel technique. It aims to lessen surgical time, patient discomfort, and surgery costs. Compared to previous studies, ours showed significantly best results in surgical time, pain, FBS, and epiphora.23–25 Our technique achieved the lowest mean surgical time so far published in the literature for pterygium surgery.
Using a sutureless and glueless technique has several advantages. Creating a safer and more comfortable postoperative period for patients allows for rapid normalization of their lifestyle and productivity. Removing the use of sutures allows for a shortened surgical time which saves surgeons and hospitals valuable operating room time, as well as provides a consistent technique that does not depend on suturing expertise. In addition, removing fibrin glue reduces the risk of infection and significantly less surgical cost, allowing the procedure to be accessible to a broader range of patients. It has been found that using fibrin glue may result in a gap between the graft and sclera, which may prevent rapid reepithelization.30 Hence, tucking the amniotic membrane under the edges of the conjunctival defect allows for direct contact and reepithelization, reducing recurrence rates.31
In ophthalmology, amniotic membranes are frequently used as scaffolds to treat epithelial damage and promote wound healing.32,33 It is currently one of the preferred techniques due to low recurrence rates and the ability to cover large defects.34 Celularity Biovance-3L is a three-layer, DDHAM. Its three-layer construction improves handling and ease of use for treating ocular surface disease. Unlike other placental-based allografts, the TLDAM is completely devoid of cells, hormones, cytokines, and growth factors. This leaves a clean scaffold that can be populated with autologous cells and growth factors after application to a surgical site.
It has been demonstrated that decellularizing human amniotic membranes have no impact on graft function and reduces the likelihood of an inflammatory response or graft rejection.35 A previous in vitro study on the efficacy of decellularized amniotic membranes demonstrated that within 24 hours, fibroblasts and keratinocytes were attached to a greater extent to the decellularized surface due to the lack of an overwhelming variety of extracellular matrix proteins and cytokines.36 Choosing amniotic membranes as scaffolds for reepithelization has advantageous properties like low immunogenicity, anti-inflammatory properties, and reduction of fibrosis.37 Human amniotic membrane allografts have proven to be effective at improving wound healing. A previous study demonstrated that multilayer amnion/chorion allografts can be more effective at wound healing than single-amnion layer membranes.38
Although advanced wound care products can be highly priced, benefits such as shortened surgical time and reduced rates of complications may compensate for the high cost.39 Ease of product storage and handling characteristic should also be considered when assessing wound care. Since all the cells and associated growth factors have been removed in the TLDAM due to decellularization, it has a 10-year shelf life that can be shipped and stored at room temperature. This study showed the best results so far in the literature regarding surgical time while also having a significant impact on patients’ postoperative comfort. Therefore, it is a safe technique that allows both surgeons and patients to benefit from it. We highly recommend this technique, and it should continue to be studied in the future on a broader cohort.
Acknowledgment
The abstract of this paper was presented at ARVO 2022 as a poster presentation with interim findings. The poster’s abstract was published in ARVO Annual Meeting Abstract at IVOS Journal. In addition, an article by Vicente Diaz was published to Ophthalmology Times about this project.
Funding
There is no funding to report.
Disclosure
Vicente Diaz, consultant for Celularity. The authors report no conflicts of interest in this work.
References
1. Modenese A, Gobba F. Occupational exposure to solar radiation at different latitudes and pterygium: a systematic review of the last 10 years of scientific literature. Int J Environ Res Public Health. 2017;15(1):37. doi:10.3390/ijerph15010037
2. Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999;6(3):219–228. doi:10.1076/opep.6.3.219.1504
3. Rezvan F, Khabazkhoob M, Hooshmand E, Yekta A, Saatchi M, Hashemi H. Prevalence and risk factors of pterygium: a systematic review and meta-analysis. Surv Ophthalmol. 2018;63(5):719–735. doi:10.1016/j.survophthal.2018.03.001
4. Young AL, Cao D, Chu WK, et al. The Evolving Story of Pterygium. Cornea. 2018;37(Suppl 1):S55–S57. doi:10.1097/ICO.0000000000001744
5. Rock T, Bramkamp M, Bartz-Schmidt KU, Rock D. A retrospective study to compare the recurrence rate after treatment of pterygium by conjunctival autograft, primary closure, and amniotic membrane transplantation. Med Sci Monit. 2019;25:7976–7981. doi:10.12659/MSM.915629
6. Sheppard JD, Mansur A, Comstock TL, Hovanesian JA. An update on the surgical management of pterygium and the role of loteprednol etabonate ointment. Clin Ophthalmol. 2014;8:1105–1118. doi:10.2147/OPTH.S55259
7. Anguria P, Ntuli S, Carmichael T. Young patient’s age determines pterygium recurrence after surgery. Afr Health Sci. 2014;14(1):72–76. doi:10.4314/ahs.v14i1.11
8. Campagna G, Adams M, Wang L, Khandelwal S, Al-Mohtaseb Z. Comparison of pterygium recurrence rates among different races and ethnicities after primary pterygium excision by surgeons in training. Cornea. 2018;37(2):199–204. doi:10.1097/ICO.0000000000001453
9. Kodavoor SK, Ramamurthy D, Solomon R. Outcomes of pterygium surgery-glue versus autologous blood versus sutures for graft fixation-an analysis. Oman J Ophthalmol. 2018;11(3):227–231. doi:10.4103/ojo.OJO_4_2017
10. Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004;88(7):911–914. doi:10.1136/bjo.2003.032854
11. Panda A, Kumar S, Kumar A, Bansal R, Bhartiya S. Fibrin glue in ophthalmology. Indian J Ophthalmol. 2009;57(5):371–379. doi:10.4103/0301-4738.55079
12. Celik T. In situ blood coagulum versus sutures for autograft fixation after pterygium excision. Curr Eye Res. 2018;43(8):977–980. doi:10.1080/02713683.2018.1470247
13. Singh PK, Singh S, Vyas C, Singh M. Conjunctival autografting without fibrin glue or sutures for pterygium surgery. Cornea. 2013;32(1):104–107. doi:10.1097/ICO.0b013e31824bd1fb
14. Maiti R, Mukherjee S, Hota D. Recurrence rate and graft stability with fibrin glue compared with suture and autologous blood coagulum for conjunctival autograft adherence in pterygium surgery: a meta-analysis. Cornea. 2017;36(10):1285–1294. doi:10.1097/ICO.0000000000001270
15. Fernandes M, Sridhar MS, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction. Cornea. 2005;24(6):643–653. doi:10.1097/01.ico.0000151501.80952.c5
16. Dhillon HK, Bahadur H, Raj A. A comparative study of tarsorrhaphy and amniotic membrane transplantation in the healing of persistent corneal epithelial defects. Indian J Ophthalmol. 2020;68(1):29–33. doi:10.4103/ijo.IJO_617_19
17. Dekaris I, Mravicic I, Barisic A, Draca N, Pauk M. Amniotic membrane transplantation in the treatment of persistent epithelial defect on the corneal graft. Coll Antropol. 2010;34(Suppl 2):15–19.
18. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–131. doi:10.1016/j.preteyeres.2018.04.003
19. Dekaris I, Gabric N, Mravicic I, et al. Multilayer vs. monolayer amniotic membrane transplantation for deep corneal ulcer treatment. Coll Antropol. 2001;25:23–28.
20. Chen H-J, Pires RTF, Tseng SCG. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–833. doi:10.1016/s0002-9394(00)00918-1
21. Mao Y, Protzman NM, John N, et al. An in vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and a clinical case study. J Biomed Mater Res B Appl Biomater. 2023;111(3):684–700. doi:10.1002/jbm.b.35186
22. Lim-Bon-Siong R, Valluri S, Gordon ME, Pepose JS. Efficacy and safety of the ProTek (Vifilcon A) therapeutic soft contact lens after photorefractive keratectomy. Am J Ophthalmol. 1998;125(2):169–176. doi:10.1016/s0002-9394(99)80087-7
23. Xu F, Li M, Yan Y, Lu K, Cui L, Chen Q. A novel technique of sutureless and glueless conjunctival autografting in pterygium surgery by electrocautery pen. Cornea. 2013;32(3):290–295. doi:10.1097/ICO.0b013e31824f8c15
24. Donepudi GD, Ramesh S, Govindarajulu M, et al. Early postoperative outcomes of pterygium surgery: sutures versus autogenous serum in-situ fixation of limbal conjunctival autograft. Life Sci. 2019;221:93–98. doi:10.1016/j.lfs.2019.02.019
25. Kucukerdonmez C, Karalezli A, Akova YA, Borazan M. Amniotic membrane transplantation using fibrin glue in pterygium surgery: a comparative randomised clinical trial. Eye. 2010;24(4):558–566. doi:10.1038/eye.2009.136
26. Syam PP, Eleftheriadis H, Liu CS. Inferior conjunctival autograft for primary pterygia. Ophthalmology. 2003;110(4):806–810. doi:10.1016/S0161-6420(02)01970-X
27. Natung T, Keditsu A, Shullai W, Goswami PK. Sutureless, glue-less conjunctival autograft versus conjunctival autograft with sutures for primary, advanced pterygia: an interventional pilot study. J Clin Diagn Res. 2017;11(8):NC04–NC07. doi:10.7860/JCDR/2017/23839.10419
28. Uy HS, Reyes JM, Flores JD, Lim-Bon-Siong R. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology. 2005;112(4):667–671. doi:10.1016/j.ophtha.2004.08.028
29. Boucher S, Conlon R, Teja S, et al. Fibrin glue versus autologous blood for conjunctival autograft fixation in pterygium surgery. Can J Ophthalmol. 2015;50(4):269–272. doi:10.1016/j.jcjo.2015.04.011
30. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104(6):974–985. doi:10.1016/s0161-6420(97)30197-3
31. Jain AK, Bansal R, Sukhija J. Human amniotic membrane transplantation with fibrin glue in management of primary pterygia: a new tuck-in technique. Cornea. 2008;27(1):94–99. doi:10.1097/ICO.0b013e318158b47f
32. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204. doi:10.1007/s10561-017-9618-5
33. Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–121. doi:10.5500/wjt.v4.i2.111
34. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001;108(3):449–460. doi:10.1016/s0161-6420(00)00567-4
35. Gholipourmalekabadi M, Farhadihosseinabadi B, Faraji M, Nourani MR. How preparation and preservation procedures affect the properties of amniotic membrane? How safe are the procedures? Burns. 2020;46(6):1254–1271. doi:10.1016/j.burns.2019.07.005
36. Bhatia M, Pereira M, Rana H, et al. The mechanism of cell interaction and response on decellularized human amniotic membrane: implications in wound healing. Wounds. 2007;19(8):207–217.
37. Ramuta TZ, Kreft ME. Human amniotic membrane and amniotic membrane-derived cells: how far are we from their use in regenerative and reconstructive urology? Cell Transplant. 2018;27(1):77–92. doi:10.1177/0963689717725528
38. Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater. 2015;103(5):1133–1140. doi:10.1002/jbm.b.33265
39. Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9:115. doi:10.1186/1472-6963-9-115
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.