Back to Journals » Clinical Interventions in Aging » Volume 18
Surgical Risks and Survival Outcomes in Robotic Pancreaticoduodenectomy for the Aged Over 80: A Retrospective Cohort Study
Authors Shyr BS, Yu JH, Chen SC, Wang SE, Shyr YM , Shyr BU
Received 6 March 2023
Accepted for publication 20 July 2023
Published 24 August 2023 Volume 2023:18 Pages 1405—1414
DOI https://doi.org/10.2147/CIA.S411391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Bor-Shiuan Shyr,* Jwo-Huey Yu,* Shih-Chin Chen, Shin-E Wang, Yi-Ming Shyr, Bor-Uei Shyr
General Surgery, Department of Surgery, Taipei Veterans General Hospital and National Yang Ming Chiao Tung University, Taipei, Taiwan, Republic of China
*These authors contributed equally to this work
Correspondence: Bor-Uei Shyr, General Surgery, Department of Surgery, Taipei Veterans General Hospital and National Yang Ming Chiao Tung University, 10 Floor 201 Section 2 Shipai Road, Taipei, 112, Taiwan, Republic of China, Tel +886-2-28757652, Fax +886-2-28757537, Email [email protected]
Aim: Whether to execute pancreaticoduodenectomy or not for older people could pose a dilemma. This study clarifies the safety and justification of robotic pancreaticoduodenectomy (RPD) for older individuals over 80.
Methods: A total of 500 patients undergoing RPD were divided into group O (≥ 80 y/o) and group Y (< 80 y/o) for comparison.
Results: There were 62 (12.4%) patients in group O. Surgical mortality was 1.6% for overall patients and higher in group O, 6.5% vs 0.9%; p = 0.001. The surgical complication was comparable between groups O and Y. Delayed gastric emptying and bile leakage were higher in group O, 9.7% vs 2.5%; p = 0.004, and 6.5% vs 0.9%; p = 0.001, respectively. Length of stay was also longer in group O, with a median of 26 vs 19 days; p = 0.001. Survival outcome after RPD was poorer in group O for overall periampullary adenocarcinomas, with a 5-year survival of 48.1% vs 51.2%; p = 0.025 and also for the subgroup of pancreatic head adenocarcinoma, with a 3-year survival of 27.4% vs 42.5%; p = 0.030.
Conclusion: RPD is safe and justified for the selected octogenarians and even nonagenarians, whoever is fit for a major operation. Nevertheless, pancreatic head cancer and higher mortality risk for the aged over 80 with advanced ASA score ≥ 3 should be informed as part of counselling in offering RPD.
Keywords: nonagenarian, octogenarian, pancreas, pancreaticoduodenectomy, robotic
Introduction
As life expectancy increases and health condition improves, so does the demand for better care and quality of life for older patients. The aging population is becoming a global phenomenon. The aged people over 80 will represent a consistent portion of this subset, reaching 12.7% of the population by 2080.1 Given the aging population and the associated increased incidence of malignancy with age, pancreatic adenocarcinoma, and periampullary malignancies are increasingly identified in the older brackets of the population.2–4 Pancreaticoduodenectomy, commonly known as the “Whipple procedure”, is a complex and challenging procedure and is considered the standard surgical treatment for the lesions in the periampullary region and pancreatic head. In the past, a major operation such as pancreaticoduodenectomy was not considered for older people after being diagnosed with a periampullary lesion. However, pancreaticoduodenectomy remains the only hope of a cure in patients with periampullary malignancy. As the percentage of patients in the older brackets increases, more patients seek surgical care as a chance to cure. Refusing patients for resection based on age alone seems unsustainable.2 Compared to the younger cohort, perioperative management of older people is more demanding mainly because of the major vulnerability of the aged, often concomitant with chronic comorbidities such as cardiovascular, metabolic, and pulmonary diseases. Therefore, older individuals are characterized by a reduced physiological reserve, which may lead to more severe and life-threatening complications and higher surgical mortality than their younger counterparts, especially those undergoing a major surgical procedure such as pancreaticoduodenectomy carrying high postoperative mortality and morbidity.1,5
Traditionally, open pancreaticoduodenectomy (OPD) is performed through a long saber slash, midline, or a rooftop abdominal incision which would cause excruciating pain and even probably result in detrimental effects on the patients.6 Recently, in addition to growing surgical expertise and improving postoperative management, minimally invasive pancreaticoduodenectomy (MIPD) with smaller wound and less pain might provide aged patients with a wide range of options and also enable more advanced age individuals to become eligible candidates for pancreaticoduodenectomy. There are some reports regarding MIPD by the laparoscopic approach being safely performed with favorable outcomes in aged patients.7–9 However, pancreaticoduodenectomy is characterized by an extensive visceral organ dissection, meticulous identification of critical vascular anatomy, and technically demanding reconstruction. Thus, the implementation of MIPD has lagged behind other abdominal procedures.10 With the introduction of da Vinci Robotic Surgical System ((Intuitive Surgical®, Sunnyvale, CA, USA) and approval by the US Food and Drug Administration in 2000,11 robotic surgery soon emerges to overcome the limitations of laparoscopic surgery because da Vinci Robotic Surgical System can provide Endowrist instruments increasing the range of motion mimicking open surgery, the high-definition 3-D vision of the surgical field, and tremor-free motion for both camera and instruments. These advances increase dexterity, improve ergonomics and reduce surgeon fatigue.10 However, spreading the RPD has also been sluggish, mainly because of the technical challenges, the relatively low surgical volume of this operation, and the cost.12 Despite the slow adoption of the robotic approach for pancreaticoduodenectomy, several studies have shown that RPD is a feasible and safe procedure compared to OPD.6,10,13,14
Only a few studies specifically evaluate the outcomes of pancreaticoduodenectomy in older people, and even a few authors focus on octogenarians.2–4,15,16 Therefore, the influence of increasing age on outcomes after pancreaticoduodenectomy is ambiguous. Whether the indication of surgery should be influenced by age remains a matter of debate, especially in those over 80 years.1,3,17–19 This study is to evaluate the perioperative characteristics and clarify the safety and justification of RPD for the aged over 80 years old. To our knowledge, no studies have reported the outcomes of RPD for advanced-age individuals such as octogenarians and nonagenarians.
Methods
Study Design and Study Population
Before the commencement, the study protocol was approved Data from patients with periampullary lesions undergoing RPD were identified for this retrospective study from a prospectively collected database from July 2014 to August 2022. According to the age at the time of RPD, the patients identified were categorized into two groups: group O (≥ 80 y/o), including octogenarian (80–89 y/o) and nonagenarian (90–99 y/o), and group Y (< 80 y/o). This study was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital (IRB-TPEVGH No.: 2023-02-006BC) and carried out in accordance with the IRB guidelines and regulations. The requirement for informed consent was waived in this retrospective cohort study with data anonymity. Patient selection for RPD was determined through the availability of a robotic machine and patient preferences after detailed counseling about the innovative nature of RPD and its advantages and disadvantages. Preoperative demographics and clinical variables were assessed, including sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, periampullary lesions, and tumor size. Surgical outcomes for evaluation included operation time, blood loss, conversion, vascular resection, surgical mortality, a variety of complications after RPD, length of stay (LOS), and lymph node yield (harvested number). Survival outcomes of periampullary adenocarcinomas were also determined.
Study Endpoints
The primary study endpoint was to clarify the safety and justification of RPD for the octogenarian and nonagenarian in group O by comparing the surgical outcomes in their younger counterparts, group Y. The secondary study endpoint was to assess the survival outcomes of periampullary adenocarcinomas in group O and Y.
Surgical Technique
RPDs were carried out with the assistance of Si or Xi da Vinci Robotic Surgical System® (Intuitive Surgical, Inc., Sunnyvale, CA, USA). All RPDs were performed using the same surgical technique by the same team led by Shyr YM. The robotic technique for the modified Blumgart pancreaticojejunostomy was described previously in detail.13 Briefly, after creating a pancreatic stump of approximately 0.5–1 cm, the divided distal jejunal limb was brought for an end-to-side pancreaticojejunostomy in a retrocolic fashion. A modified Blumgart pancreaticojejunostomy was performed using 3 or 2 transpancreatic U-sutures with 3-0 sutures made of polydioxanone (PDS™) as the outer-layer sutures which were not tied until all of the inner duct-to-mucosa sutures were placed and tied. A series of simple interrupted 4-0 sutures made of polydioxanone (MonoPlus®) was then carefully and accurately placed for the inner layer, duct-to-mucosa anastomosis; usually 6 sutures for a non-dilated pancreatic duct and 8–10 for a dilated pancreatic duct. After the inner layer duct-to-mucosa anastomosis was completed, the anterior outer-layer horizontal mattress sutures using previously held U-sutures were completed on the anterior surface of the jejunum and pancreas. Pancreatic duct stents were not routinely used except for a small pancreatic duct < 3 mm, for which a short, internal stent was placed. Thereafter, hepaticojejunostomy without stenting was completed on the same jejunal limb, with interrupted suturing for the non-dilated bile duct or continuous homemade double-needles suturing for the dilated bile duct. A hand-sewn gastrojejunostomy was performed using the extracorporeal approach involving a careful downward positioning of the stomach. The final position of gastrojejunostomy was antecolic, antiperistaltic, and inframesocolic near the umbilicus region. The right gastric artery was routinely divided in our practice. Pylorus-preserving pancreaticoduodenectomy was attempted initially whenever possible; otherwise, a limited antrectomy was done for those with ischemic pylorus after dividing the right gastric artery. Mesopancreas dissections were described in detail in our previous study.6 Oral intake with a clear liquid diet was started as early as possible, usually on postoperative days 1–3 if there is no nasogastric tube soon after RPD.
Definitions of Surgical Complications
Surgical mortality was defined as death within 90 days after surgery, including the period of admission for the operation or hospital readmission. Surgical complications were classified by the Clavien–Dindo classification.20 Postoperative pancreatic fistula (POPF) was referred to as a clinically relevant grade B or C pancreatic leakage based on the 2016 new grading system by the International Study Group for Pancreatic Fistula.21 Post-pancreatectomy hemorrhage (PPH), delayed gastric emptying (DGE), and chyle leak was classified according to the criteria proposed by the International Study Group of Pancreatic Surgery (ISGPS).22–24
Statistical Analysis
Statistical analysis was carried out using Statistical Product and Service Solutions version 26 software (SPSS Inc, IBM, Armonk, NY, USA). Categorical variables were presented as the number (percentage) and compared using Pearson’s χ2 test or Fisher’s exact test contingency tables. All continuous data were presented as median (range) and mean±standard deviation. The mean continuous variables distributed normally were compared between groups using the two-tailed Student’s t-test. The Wilcoxon rank-sum test was used for continuous variables without normal distribution. Survival rates were estimated using the Kaplan-Meier method. The Log rank test was used to compare differences between the survival curves. Statistical significance was set at P < 0.05.
Result
A total of 500 patients undergoing RPD were included in the study, with 438 (87.6%) in group Y (< 80 y/o) and 62 (12.4%) in group O (≥ 80 y/o), including 55 octogenarians and 7 nonagenarians (Table 1). The median age of the O group was 83 (range 80‑97), and of the Y group was 66 (range 19‑79) at the time of RPD. The age distribution of RPD patients is shown in Figure 1, with a median age of 67 years (range 19‑97). There was no significant difference in gender, BMI, and tumor size between group O and Y. Group O was associated with a higher rate of advanced ASA score > 3, 62.9% vs 29.5% in group Y; p < 0.001. The incidences of pancreatic head adenocarcinoma (45.2% vs 36.2%) and ampullary adenocarcinoma (32.3% vs 24.4%) were higher when compared to their younger counterparts; p = 0.033.
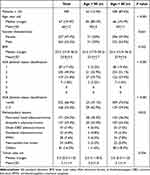 |
Table 1 Demographics for Patients with Periampullary Lesions Undergoing Robotic Pancreaticoduodenectomy |
 |
Figure 1 Distribution of patients undergoing robotic pancreaticoduodenectomy in each age decade. |
The operation time was longer in group O, with a median of 9.1 vs 8.0 hours in group Y; p = 0.039 (Table 2). Intraoperative blood loss, open conversion, and vascular resection were similar between these two groups. The surgical mortality was 1.6% for overall patients and higher in group O, 6.5% vs 0.9% in group Y; p = 0.010. The surgical mortality for each age decade is shown in Figure 2. There was no surgical mortality before the age of 60 years, whereas there were three (5.5%) in octogenarians and one (14.3%) in nonagenarians; p = 0.043. The causes of surgical mortality in group O included renal failure with multiple organ failure in one nonagenarian with an ASA score of 3, myocardial infarction in one octogenarian with an ASA score of 3 and one octogenarian with an ASA score of 4, and heart failure in one octogenarian with an ASA score of 3. All of the four patients in group O had pre-existing comorbidity and died of non-surgical complications which were not related to the RPD procedure itself. The overall surgical complication was comparable between these two groups, with 59.7% for group O and 57.3% for group Y. No statistical difference was noted regarding POPF, PPH, chyle leakage, wound infection, and lymph node yield, but DEG and bile leakage rates were higher in group O, 9.7% vs 2.5% in group Y; p = 0.004, and 6.5% vs 0.9% in group Y; p = 0.001 respectively. The LOS was also longer in group O, with a median of 26 vs 19 days in group Y; p = 0.001.
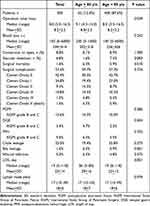 |
Table 2 Surgical Outcomes After Robotic Pancreaticoduodenectomy |
 |
Figure 2 Surgical mortality after robotic pancreaticoduodenectomy in each age decade. |
Survival outcome after RPD was poorer in group O for overall periampullary adenocarcinomas, with a 5-year survival of 48.1% vs 51.2% in group Y; p = 0.025 (Table 3) and also for the subgroup of pancreatic head adenocarcinoma, with a 3-year survival of 27.4% vs 42.5% in group Y; p = 0.030 (Figure 3). As to the subgroup of ampullary adenocarcinoma, the survival outcome was no statistical difference between groups O and Y.
 |
Table 3 Survival Outcomes for Periampullary Adenocarcinomas After Robotic Pancreaticoduodenectomy |
 |
Figure 3 Actuarial survival curves for group O (≥ 80 y/o) and group Y (< 80 y/o) with pancreatic head adenocarcinoma after robotic pancreaticoduodenectomy. |
Discussion
Since 70% of cancer is expected to occur in older people, this has imposed a new challenge for the healthcare system.25 This is particularly evident for pancreatic head cancer and other periampullary malignancies, as shown in this study with a higher incidence of pancreatic head adenocarcinoma (45.2% vs 36.2%) and ampullary adenocarcinoma (32.3% vs 24.4%) when compared to their younger counterparts, p = 0.033. Nevertheless, pancreaticoduodenectomy currently remains the only hope of a cure for these malignancies. In other words, a major resection with pancreaticoduodenectomy needs to be considered more frequently in older people.2–4,26 However, whether to execute pancreaticoduodenectomy or not for older people could pose a dilemma, especially for those aged over 80 years.1,3,17–19 Currently, the majority of the findings regarding the impact of age on the outcomes of pancreaticoduodenectomy are based on studies from OPD,1,3,17–19 a few from LPD,7–9 and none from RPD. Current evidence shows that robotic pancreatoduodenectomy (RPD) is feasible with a safety profile equivalent to either open pancreatoduodenectomy (OPD) or laparoscopic pancreatoduodenectomy (LPD).27
The major concern in considering a major operation would be the “biological age” instead of chronological age. Biological age uses biophysiological measures to more accurately determine an individual’s age-related risk of adverse outcomes.28 The aged individuals are often associated with multiple chronic comorbidities and are characterized by reduced functional and physiological reserves.1,5 As shown in this study, the advanced ASA score > 3 was high, up to 62.9% in Group O, compared to 29.5% in group Y, p < 0.001. The association of a more advanced ASA score > 3 could be a reflection of frailty and vulnerability in aged individuals who always need accurate preoperative evaluation and careful postoperative management.
The surgical risk would be the prime concern in considering RPD for the aged. Systematic review and meta-analysis with a combined total of 21,295 patients from 45 eligible studies of OPD by Pedziwiatr et al26 found that advanced age is a risk factor for increased non-surgical morbidity and, by extension, higher mortality. In this study, the overall surgical complication is comparable between these two age groups. There is no significant difference regarding intraoperative blood loss, POPF, PPH, chyle leakage, and wound infection in group O. Nevertheless; group O is associated with higher rates of delayed gastric emptying and bile leakage after RPD. As expected, the length of stay would also be longer in group O partly because of extra care work such as rehabilitation and a lengthy recovery program to be completed after a major operation.
The trade-off could be the risk of a higher surgical mortality rate in the older people group O as shown in this RPD study. The surgical mortality rate was 1.6% for the whole cohort in our series, and it was 6.5% higher in group O, compared with 0.9% in group Y, p = 0.010. There were three (5.5%) surgical mortality in octogenarians and one (14.3%) in nonagenarians; in contrast, no surgical mortality was noted before the age of 60. All of the four patients with surgical mortality in group O had pre-existing comorbidity, and the ASA score was at least 3 for all of them. They all died of non-surgical complications not related to the RPD procedure itself. Results from this study suggest that unfavorable physical status, such as advanced ASA score > 3 in aged patients, might lead to higher surgical mortality. A review of data from 812 patients undergoing pancreaticoduodenectomy in 10 referral centers in Italy by Quero et al1 found that chronological age was not recognized as a prognostic factor for major cumulative complications, while ASA ≥3 was the only confirmed independent risk factor for severe complications with an odds ratio of 2.98 [95% confidence interval 1.3–6.8]; p = 0.009, at multivariate analysis. The authors suggested that age should not be considered an exclusion criterion for pancreaticoduodenectomy, but a focused preoperative assessment is essential for adequate patient selection. These findings would remind us that the “biological age” with preserved functional and physiological reserves should be prime important in considering RPD for aged individuals instead of chronological age alone. Shiozawa et al29 suggested eligibility criteria specific to pancreaticoduodenectomy for octogenarians: (1) Cardiac function, ejection fraction: at least 40. (2) Pulmonary function, forced expiratory volume in 1 second (FEV1.0%): at least 50%. (3) Nutritional status, serum albumin level: at least 3.0 g/dl. (4) Daily activity status, Karnofsky performance status, at least 80%. (5) Psychological independence status, capable of self-determination with respect to surgery. In other words, RPD should not be precluded for the aged with good performance status, including octogenarians and even nonagenarians who are fit for a major operation, whenever accurate preoperative evaluation and careful postoperative management for the older people patients are ready. Therefore, RPD could be a safe and justified procedure for the selected individuals over 80 years old in terms of comparable overall surgical complications compared with their younger counterparts.
Survival outcomes after RPD in the older people group O are inferior for the overall periampullary adenocarcinoma and a subgroup of pancreatic head adenocarcinoma, but not for ampullary adenocarcinoma, as compared with those in the younger group Y. Unsurprisingly, the inferior survival outcome for the overall periampullary adenocarcinoma in the group O might be attributed to the subgroup of pancreatic head adenocarcinoma, which is also associated with poorer survival and comprises the majority of the overall periampullary adenocarcinoma in the group O in our series. A study of 586 consecutive surgically curable patients with diagnosed periampullary diseases by Okabayashi et al15 also showed significantly poorer 3-, 5-, and 10-year overall survivals in the octogenarian group than in the younger group, p =0 0.007. A study by Kim et al4 concluded that long-term survival was lower in the older patients treated for pancreatic cancer (16.6 vs 22.5 months, p = 0.048), which might reflect disease biology and inherent lower life expectancy with increasing age, rather than being directly related to surgical factors. Levi et al16 suggested that the biology of pancreatic head adenocarcinoma needs to be weighed against the inherent risk of the RPD itself and the quality of life in the aged. Given that survival outcomes could be poorer for pancreatic head adenocarcinoma but not for ampullary adenocarcinoma, RPD should be judiciously considered in terms of prognosis in different periampullary cancers for the aged.
Limitations
There are some limitations in the present study. While the data used for this study are collected prospectively, the retrospective nature of this study would still introduce selection bias. Moreover, this study only focused on the immediate postoperative period and did not study the extended postoperative period or quality of life, which could have significant impacts on outcomes, as mentioned by Kisch et al.3 In addition, the optimized outcomes presented are a collaborative effort among several surgeons who often work in tandem during the initial learning curve to ensure patient safety. As such, the results might not be generalizable to other smaller centers that are unable to allocate the required resources and infrastructure, as reminded by Zureikat and coworkers at the University of Pittsburgh.30
Conclusions
“Biological age” with preserved functional and physiological reserve is of prime importance in considering RPD for aged individuals. In other words, RPD should not be precluded for the aged with good performance status, including octogenarians and even nonagenarians who are fit for a major operation, whenever accurate preoperative evaluation and careful postoperative management for the aged patients are ready. The trade-off of RPD in those unfit for a major operation would result in higher surgical mortality, as seen in our older people group O. Nevertheless, given that survival outcome could be poorer for pancreatic head adenocarcinoma but not for ampullary adenocarcinoma, RPD should be judiciously considered in terms of prognosis in different periampullary cancers, especially for pancreatic head adenocarcinoma. Therefore, pancreatic head cancer and higher mortality risk for the aged over 80 with advanced ASA score > 3 should be informed as part of counselling in offering RPD. Otherwise, RPD could be a safe and justified procedure for the selected individuals over 80 years old in terms of overall surgical complications compared to the younger counterparts in our study.
Abbreviations
RPD, robotic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy; MIPD, minimally invasive pancreaticoduodenectomy; IRB, Institutional Review Board; BMI, body mass index; ASA, American Society of Anesthesiologists; LOS, length of stay; POPF, postoperative pancreatic fistula; PPH, Post-pancreatectomy hemorrhage; DGE, delayed gastric emptying; ISGPS, International Study Group of Pancreatic Surgery; LPD, laparoscopic pancreatoduodenectomy.
Data Sharing Statement
All the data used and/or analyzed during the current study are available from Bor-Uei Shyr upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital (IRB-TPEVGH No.: 2023-02-006BC) and carried out in accordance with the IRB guidelines and regulations. The requirement for informed consent was waived in this retrospective cohort study with data anonymity. The study complied with the Helsinki Declaration.
Consent for Publication
We have obtained consent for publication from all participants.
Acknowledgments
The authors would like to acknowledge the support of the Biobank of Taipei Veterans General Hospital and Common Well Foundation.
Author Contributions
All authors make a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation; take part in drafting, revising, or critically reviewing the article; give final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Taipei Veterans General Hospital (V112C-009, V112C-188, and V112B-001), the Ministry of Science and Technology (MOST 111-2314-B-075-073 -), and the Ministry of Health and Welfare (MOHW111-TDU-B-221-014015).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Quero G, Pecorelli N, Paiella S, et al. Pancreaticoduodenectomy in octogenarians: the importance of “biological age” on clinical outcomes. Surg Oncol. 2022;40:101688. doi:10.1016/j.suronc.2021.101688
2. Parasyris S, Hatzaras I, Ntella V, et al. Pancreaticoduodenectomy as a feasible choice for periampullary malignancy in octogenarians. Mol Clin Oncol. 2022;17(4):148. doi:10.3892/mco.2022.2581
3. Kisch SE, Nussbaum ER, Varsanik MA, et al. Octogenarians undergoing pancreaticoduodenectomy: assessing outcomes, disposition, and timing of chemotherapy. Surg Open Sci. 2022;7:58–61. doi:10.1016/j.sopen.2021.11.008
4. Kim SY, Fink MA, Perini M, et al. Age 80 years and over is not associated with increased morbidity and mortality following pancreaticoduodenectomy. ANZ J Surg. 2018;88(5):E445–E450. doi:10.1111/ans.14039
5. Kang CM, Lee JH, Choi JK, et al. Can we recommend surgical treatment to the octogenarian with periampullary cancer? National database analysis in South Korea. Eur J Cancer. 2021;144:81–90. doi:10.1016/j.ejca.2020.10.039
6. Shyr BU, Shyr BS, Chen SC, Shyr YM. Mesopancreas level 3 dissection in robotic pancreaticoduodenectomy. Surgery. 2021;169(2):362–368. doi:10.1016/j.surg.2020.07.042
7. Kim JS, Choi M, Kim SH, Choi SH, Kang CM. Safety and feasibility of laparoscopic pancreaticoduodenectomy in octogenarians. Asian J Surg. 2022;45(3):837–843. doi:10.1016/j.asjsur.2021.09.021
8. Chapman BC, Gajdos C, Hosokawa P, et al. Comparison of laparoscopic to open pancreaticoduodenectomy in elderly patients with pancreatic adenocarcinoma. Surg Endosc. 2018;2(5):2239–2248. doi:10.1007/s00464-017-5915-0
9. Jones LR, Zwart MJW, Molenaar IQ, et al. Robotic pancreatoduodenectomy: patient selection, volume criteria, and training programs. Scand J Surg. 2020;109(1):29–33. doi:10.1177/1457496920911815
10. Mantzavinou A, Uppara M, Chan J, Patel B. Robotic versus open pancreaticoduodenectomy, comparing therapeutic indexes; a systematic review. Int J Surg. 2022;101:106633. doi:10.1016/j.ijsu.2022.106633
11. Yates DR, Vaessen C, Roupret M. From Leonardo to da Vinci: the history of robot-assisted surgery in urology. BJU Int. 2011;108(11):
12. van Oosten AF, Ding D, Habib JR, et al. Perioperative outcomes of robotic pancreaticoduodenectomy: a propensity-matched analysis to open and laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. 2021;25(7):1795–1804. doi:10.1007/s11605-020-04869-z
13. Shyr BU, Chen SC, Shyr YM, Wang SE. Surgical, survival, and oncological outcomes after vascular resection in robotic and open pancreaticoduodenectomy. Surg Endosc. 2020;34(1):377–383. doi:10.1007/s00464-019-06779-x
14. Wang SE, Shyr BU, Chen SC, Shyr YM. Comparison between robotic and open pancreaticoduodenectomy with modified Blumgart pancreaticojejunostomy: a propensity score-matched study. Surgery. 2018;164(6):1162–1167. doi:10.1016/j.surg.2018.06.031
15. Okabayashi T, Sui K, Murokawa T, et al. Indications for pancreaticoduodenectomy affected postoperative outcomes in octogenarians. Ann Gastroenterol Surg. 2021;5(1):102–110. doi:10.1002/ags3.12395
16. Levi ST, Gough BL, Darcy CE, et al. Pancreatic resections: 30 and 90-day outcomes in octogenarians. Surg Oncol. 2021;37:101319. doi:10.1016/j.suronc.2020.01.002
17. Huang Y, Damodaran Prabha R, Chua TC, et al. Safety and efficacy of pancreaticoduodenectomy in octogenarians. Front Surg. 2021;8:617286. doi:10.3389/fsurg.2021.617286
18. Lee DY, Schwartz JA, Wexelman B, Kirchoff D, Yang KC, Attiyeh F. Outcomes of pancreaticoduodenectomy for pancreatic malignancy in octogenarians: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg. 2014;207(4):540–548. doi:10.1016/j.amjsurg.2013.07.042
19. Kim SY, Weinberg L, Christophi C, Nikfarjam M. The outcomes of pancreaticoduodenectomy in patients aged 80 or older: a systematic review and meta-analysis. HPB. 2017;19(6):475–482. doi:10.1016/j.hpb.2017.01.018
20. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
21. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–591. doi:10.1016/j.surg.2016.11.014
22. Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–768. doi:10.1016/j.surg.2007.05.005
23. Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi:10.1016/j.surg.2007.02.001
24. Besselink MG, van Rijssen LB, Bassi C, et al. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365–372. doi:10.1016/j.surg.2016.06.058
25. Langan RC, Huang CC, Mao WR, et al. Pancreaticoduodenectomy hospital resource utilization in octogenarians. Am J Surg. 2016;211(1):70–75. doi:10.1016/j.amjsurg.2015.04.014
26. Pedziwiatr M, Malczak P, Mizera M, et al. Pancreatoduodenectomy for pancreatic head tumors in the elderly - Systematic review and meta-analysis. Surg Oncol. 2018;7(3):346–364. doi:10.1016/j.suronc.2018.05.021
27. Napoli N, Kauffmann EF, Vistoli F, Amorese G, Boggi U. State of the art of robotic pancreatoduodenectomy. Updates Surg. 2021;73(3):873–880. doi:10.1007/s13304-021-01058-8
28. Diebel LWM, Rockwood K. Determination of biological age: geriatric assessment vs biological biomarkers. Curr Oncol Rep. 2021;23(9):104. doi:10.1007/s11912-021-01097-9
29. Shiozawa S, Usui T, Kuhara K, et al. Eligibility criteria specific to pancreaticoduodenectomy for octogenarians: single-center opinion. Anticancer Res. 2017;37(4):2037–2043. doi:10.21873/anticanres.11549
30. Zureikat AH, Beane JD, Zenati MS, et al. 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg. 2021;273(5):966–972. doi:10.1097/sla.0000000000003550
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
