Back to Journals » Clinical Ophthalmology » Volume 18
Surgical Outcomes of Ab Interno Trabeculotomy Without Phacoemulsification
Authors Mochizuki T, Hirooka K, Okada N , Onoe H, Tokumo K, Okumichi H , Kiuchi Y
Received 23 October 2023
Accepted for publication 13 December 2023
Published 3 January 2024 Volume 2024:18 Pages 9—16
DOI https://doi.org/10.2147/OPTH.S446168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tsukasa Mochizuki, Kazuyuki Hirooka, Naoki Okada, Hiromitsu Onoe, Kana Tokumo, Hideaki Okumichi, Yoshiaki Kiuchi
Department of Ophthalmology and Visual Science, Hiroshima University, Hiroshima, 734-8551, Japan
Correspondence: Kazuyuki Hirooka, Department of Ophthalmology and Visual Science, Hiroshima University, 1-2-3 Kasumi, Minami-Ku, Hiroshima, 734-8551, Japan, Tel +81-82-257-5247, Fax +81-82-257-5249, Email [email protected]
Purpose: The aim of this study was to evaluate ab interno trabeculotomy outcomes without phacoemulsification.
Methods: This retrospective study evaluated 118 eyes of patients aged 18 and above who underwent ab interno trabeculotomy between December 2017 and August 2022. When surgeries were performed in both eyes, only the eye undergoing the initial surgery was evaluated. Prior to and after surgery, the intraocular pressure (IOP) and mean number of IOP-lowering medications were compared. An IOP of ≤ 21 mmHg (A) and ≤ 18 mmHg (B) along with a ≥ 20% reduction in the preoperative IOP was defined as survival. Cases that required reoperation for glaucoma were defined as surgical failure. The Kaplan–Meier method was used to evaluate the survival rates. A Cox proportional hazards model was used to analyze the preoperative factors that influenced survival rates.
Results: At 36 months postoperatively, the 13.4 ± 2.8 mmHg average IOP was significantly decreased from the preoperative 23.5 ± 9.8 mmHg value (P < 0.0001). Moreover, a significant decrease in the mean number of the IOP-lowering medications to 2.3 ± 1.4 at 36 months was found versus the initial 3.9 ± 1.1 preoperative value (P < 0.0001). The survival rates for criteria A and B at 36 months postoperatively were 28% and 25%, respectively. Only the preoperative IOP was identified by multivariate analysis as a factor influencing survival rates (P < 0.0001). Hyphema in 36 eyes (30.5%) and an IOP spike in 20 eyes (16.9%) were the only observed complications. Additional glaucoma surgery was required in 27 eyes (22.9%) during the follow-up period.
Conclusion: Utilization of ab interno trabeculotomy effectively lowered the IOP and reduced the number of IOP-lowering medications. Patients with higher preoperative IOP exhibited better postoperative outcomes.
Keywords: microhook, Kahook Dual Blade, intraocular pressure, glaucoma
Introduction
Retinal ganglion cell damage that occurs during the disease referred to as glaucoma can lead to progressive optic neuropathy and is associated with specific morphological features of the optic nerve disc and progressive visual field defects.1 The only proven method that has definitively been shown to decrease the speed of the progression of the visual field damage is through the reduction of the intraocular pressure (IOP).2,3 However, surgery is typically performed when sufficient lowering or progression of disease cannot be prevented after utilization of the maximum tolerated medical and/or laser therapy.
For the treatment of glaucoma, trabeculectomy is the most widely performed surgical procedure.4,5 However, minimally invasive glaucoma surgery (MIGS) has recently become available and is now being utilized, as it can be performed at an earlier stage as compared to that for trabeculectomy.6,7 Trabecular meshwork incision surgery is the most crucial technique in MIGS that is being performed within the eye.8 The characteristics of this procedure include the use of specialized instruments for incising the trabecular meshwork. The Trabectome, Kahook Dual Blade (KDB), Tanito microhook, and suture trabeculotomy are among the various instruments that have been developed for MIGS. During ab interno trabeculotomy, the mechanism by which the IOP is reduced is via the creation of an incision in the inner wall of the Schlemm’s canal, which is known to cause resistance to the aqueous humor outflow. Thus, restoration of the physiological aqueous outflow pathways can be achieved through these incisions, which will facilitate the drainage of the aqueous humor into the collector channels.9 Since the physiological aqueous outflow pathway is utilized by the ab interno trabeculotomy, this leads to fewer complications, such as hypotony, wound leak, or shallow anterior chamber, as compared to procedures that do not utilize this pathway, such as filtration surgery.10 Furthermore, ab interno trabeculotomy keeps the conjunctiva and sclera tissue free from damage.
While the outcomes of ab interno trabeculotomy with phacoemulsification have been reported in many studies, there are limited reports on the specific details on the outcomes of ab interno trabeculotomy alone. However, not only are the outcomes of ab interno trabeculotomy alone important, but further details on cases where trabeculotomy intraocular surgery results in poor IOP reduction also need to be reported on and evaluated. Therefore, our current investigation evaluated the preoperative factors that may influence IOP reduction, in addition to examining postoperative outcomes and complications.
Materials and Methods
Between December 2017 and August 2022 at Hiroshima University Hospital, ab interno trabeculotomy as a standalone procedure was performed on patients who were 18 years and older, and who underwent at least 6 months of follow-up observation. If both eyes underwent the procedure within the specified period, the study only used the patient data from the first eye surgery. If the postoperative target IOP was around 18 mmHg, we chose ab interno trabeculotomy as the primary surgical method, with the exception of neovascular glaucoma. This analysis retrospectively extracted data from the medical records of the cases that met the inclusion criteria. This study was in accordance with the principles of the Declaration of Helsinki and was conducted following approval from the university’s ethics committee. Informed consent was obtained from the included cases prior to the initial surgery.
All patients initially underwent a basic surgical procedure that created an incision on the temporal side of the cornea, followed by filling of the anterior chamber with viscoelastic material. After using gonioscopy to identify the trabecular meshwork, the inferior trabecular meshwork was incised from the nasal side using a Tanito microhook (Inami & Co., Ltd., Tokyo, Japan) or KDB (New World Medical, Rancho Cucamonga, CA, USA) (Figure 1). Following irrigation of the intraocular space, wound closure was confirmed. Three times a day, patients were administered postoperative eye drops that included 1.5% levofloxacin and 0.1% fluorometholone, with 2% pilocarpine eye drops administered three to four times a day. Previously used glaucoma eye drops were discontinued before the surgery and then based on the postoperative IOP, were subsequently gradually reintroduced on an as-needed basis.
 |
Figure 1 Intraoperative findings determined by the Kahook Dual Blade. The Kahook Dual Blade was inserted into the anterior chamber through the corneal port at the temporal position. |
The surgical success or failure was defined based on two IOP criteria. The Kaplan–Meier method was used to evaluate the obtained results. An IOP of ≤21 mmHg (A) with a decrease rate of ≥20% or an IOP of ≤18 mmHg (B) with a decrease rate of ≥20% was defined as survival. Cases that required reoperation for glaucoma were defined as surgical failure. When two consecutive measurements indicated that the IOP criteria were not met, this was considered to be an event (death). The event (death) was considered to occur at the point where the glaucoma surgery or laser treatment was performed in these cases. The preoperative factors that influenced the above survival criteria were analyzed using Cox proportional hazards modeling. Preoperative IOP, central corneal thickness, axial length, condition of the lens, surgical instruments used, preoperative number of IOP-lowering medications, age, sex, preoperative mean deviation (MD) value, preoperative type of IOP-lowering medications, and glaucoma subtype were the factors included in the analysis. After initially performing a univariate analysis, a multivariate analysis was then performed on the factors that showed significant differences. For the mean number of IOP-lowering medications, 1 point was assigned for each glaucoma eye drop, while 2 points were assigned for combination of eye drops and oral carbonic anhydrase inhibitors. The Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA, USA) with the Swedish Interactive Thresholding Algorithm program either 30–2 or 24–2 was used to determine the preoperative MD value within 6 months prior to the surgery. Partial laser interferometry (IOL Master 700; Carl Zeiss Meditec) and central corneal thickness measurements obtained using a specular microscope (Topcon SP-3000; Topcon Corporation, Tokyo, Japan) were performed to determine the axial length measurements. IOP was measured using the Goldman applanation tonometer.
Statistical analysis was performed using JMP Pro 16 software (SAS Inc., Cary, NC, USA), with a significance level set at P < 0.05. All data are presented as the mean ± standard deviation. P-values were adjusted using the Bonferroni correction in cases where multiple measurements of the IOP and topical medication score were taken.
Results
There were 118 eyes (63 eyes of males and 55 eyes of females) evaluated in this study, with a patient mean age of 52.9 ± 18.1 years (Table 1). The mean follow-up period was 25.1 ± 16.5 months. When eyes were divided into glaucoma subtypes, there were 48 eyes with primary open-angle glaucoma, 20 eyes with exfoliation glaucoma, 9 eyes with steroid glaucoma, 2 eyes with chronic angle-closure glaucoma, 21 eyes with juvenile open-angle glaucoma, 1 eye with pigmentary glaucoma, and 17 eyes with secondary glaucoma.
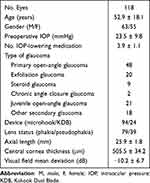 |
Table 1 Demographics and Preoperative Ocular Characteristics |
Significant IOP reductions were observed at every time point up to 36 months postoperatively, as compared to the preoperative IOP values (P < 0.0001 at every time point) (Table 2). There was a significant decrease in the mean number of postoperative IOP-lowering medications at every time point up to 36 months postoperatively (P < 0.0001 at every time point) (Table 3).
 |
Table 2 Preoperative and Postoperative Intraocular Pressure |
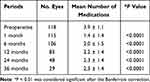 |
Table 3 Preoperative and Postoperative Medications |
Criteria A survival rates were 55% at 3 months postoperatively, 47% at 6 months, 40% at 12 months, 36% at 18 months, 36% at 24 months, and 28% at 36 months (Figure 2A). Criteria B survival rates were 53% at 3 months postoperatively, 46% at 6 months, 38% at 12 months, 33% at 18 months, 30% at 24 months, and 25% at 36 months (Figure 2B). Analysis of the preoperative factors that influenced the survival conditions was performed using the Cox proportional hazards models. For criteria A, a significant increase in the risk of failure was found for the preoperative IOP (Table 4). The univariate analysis for criteria B found significant differences for the following three factors: preoperative IOP with a risk ratio of 0.94, presence of carbonic anhydrase inhibitor eye drops with a risk ratio of 1.82, and primary open-angle glaucoma with a risk ratio of 1.56 (Table 5). The only significant factor found by the multivariate analysis for these factors was the preoperative IOP, which had a risk ratio of 0.94 (Table 5).
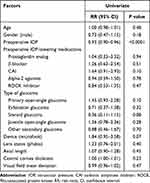 |
Table 4 Univariate Analyses for Possible Risk Factors for Failure After Trabeculotomy Ab Interno in Criteria a Using Cox Proportional Hazards Regression Models |
 |
Table 5 Univariate and Multivariate Analyses for Possible Risk Factors for Failure After Ab Interno Trabeculotomy in Criteria B Using Cox Proportional Hazards Regression Models |
The only observed postoperative complications were hyphema with niveau formation in 36 eyes (30.5%), transient IOP elevation in 20 eyes (16.9%), and ciliary body detachment in 1 eye (0.8%) (Table 6). There were 27 eyes (22.9%) that underwent glaucoma surgeries during the follow-up period. Of these surgeries, trabeculectomy was performed in 21 eyes, while 3 eyes underwent Ahmed glaucoma valve implantation, 2 eyes underwent ab externo trabeculotomy, with 1 eye receiving an Ex-PRESS glaucoma filtration device.
 |
Table 6 Postoperative Complications |
Discussion
Postoperative IOPs were significantly reduced at all of the time points in this surgical study when compared to the preoperative values. In addition, there was a corresponding decrease in the mean number of IOP-lowering medications used in these patients. A better IOP-lowering effect was observed following these surgeries in cases found to have a higher preoperative IOP.
Tanito et al11 previously evaluated microhook trabeculotomy alone and found that as compared to the preoperative IOP value, which was 22.4 ± 8.6 mmHg, the postoperative values at 12 and 24 months were 14.4 ± 3.0 mmHg (n = 64) and 13.9 ± 3.2 mmHg (n = 37), respectively. The number of preoperative IOP-lowering medications was 3.3 ± 1.1, and while this was significantly decreased for up to 30 months, this was not found to be significant at 36 months. Sieck et al12 evaluated the KDB-alone group (n = 32) and reported finding a significant reduction in the mean IOP and in the IOP-lowering medications from 20.4 ± 1.3 mmHg to 14.1 ± 0.9 mmHg and from 3.1 ± 0.2 to 2.3 ± 0.4 at 12 months, respectively. Wakil et al13 also evaluated a KDB-alone group (n = 23) that had a preoperative IOP of 24.3 ± 9.1 mmHg, which subsequently showed postoperative values of 16.9 ± 7.6 mmHg and 16.7 ± 7.6 mmHg at 12 and 18 months, respectively. However, there was a similar number of IOP-lowering medications observed between the before (2.9 ± 1.0) and after surgery (2.6 ± 1.2 at 12 months, 3.3 ± 1.1 at 18 months) values. Furthermore, at 24 months in a KDB-alone group (n = 33), Barkander et al14 reported finding a reduction in the mean IOP from 22.3 ± 5.8 mmHg to 13.9 ± 3.0 mmHg. Furthermore, there was also a reduction in the number of IOP-lowering medications from 3.5 ± 0.6 to 3.1 ± 0.9 (P = 0.047). In our current study, we found comparable IOP values to those reported for both the microhook and KDB surgeries. However, we evaluated a larger number of patients in addition to having a longer follow-up period as compared to that for the previous studies.
The Cox proportional hazards model analysis in our current study showed that a higher preoperative IOP was associated with better postoperative survival rates. Previous reports have also found that the higher preoperative IOP that was found during microhook-assisted trabecular meshwork surgery (including concurrent cataract surgery) was associated with a greater reduction in the postoperative IOP, although it was found that there were still relatively high postoperative IOP levels.15 However, both previous reports and our current results have found that greater postoperative IOP reductions were associated with higher preoperative IOPs. The presence of residual aqueous outflow resistance in the distal portion of the trabecular meshwork might be associated with this finding, as the surgical effect could potentially be limited in eyes having a lower preoperative IOP. Therefore, when the primary goal of performing surgery is to reduce the IOP, it is important to consider that patients with higher preoperative IOP could potentially be more likely to favorably respond to these types of procedures.
At 12 months postoperatively, it has also been reported that significantly better outcomes of microhook trabeculotomy are achieved after the use of preoperative ripasudil.16 However, our current study did not find any influence on the postoperative outcomes of ab interno trabeculotomy after the use of ripasudil. While the previous study evaluated patients who underwent microhook trabeculotomy with and without cataract surgery, our current study included only ab interno trabeculotomy alone. Furthermore, our present study also had a mean follow-up period of 25.1 ± 16.5 months. This is in contrast to the Okuda et al16 study in which the surgical outcomes were evaluated for a short period, up to 1 year postoperatively.
There were some limitations for our current study. First, this was a retrospective and single institution study. Second, due to the prior physician-prescribed use of IOP-lowering medications, the onset and duration of the IOP-lowering medications that were administered for each individual patient remains indeterminate. However, it has been previously reported that surgical failure of microhook trabeculotomy at 12 months postoperatively was significantly associated with a longer duration of >4.5 years of IOP-lowering medication use.17
In conclusion, the current study evaluated the postoperative outcomes of ab interno trabeculotomy and found that favorable surgical outcomes were achieved when there were significant reductions in the IOP and in the number of IOP-lowering medications. In addition, there was a greater effectiveness in IOP reduction through surgery in cases that had a higher preoperative IOP.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi:10.1001/jama.2014.3192
2. Chauhan BC, Mikelberg FS, Balaszi AG, LeBlanc RP, Lesk MR, Trope GE; Canadian Glaucoma Study Group. Canadian glaucoma study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–1036. doi:10.1001/archopht.126.8.1030
3. Heijl A; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
4. Kiuchi Y, Inoue T, Shoji N, Nakamura M, Tanito M. Glaucoma guideline preparation committee, Japan glaucoma society; the Japan glaucoma society guidelines for glaucoma 5th edition. Jpn J Ophthalmol. 2023;67(2):189–254. doi:10.1007/s10384-022-00970-9
5. Kirwan JF, Lockwood AJ, Shah P, et al.; Trabeculectomy Outcomes Group Audit Study Group. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology. 2013;120(12):2532–2539. doi:10.1016/j.ophtha.2013.07.049
6. Iwasaki K, Arimura S, Takamura Y, Inatani M. Clinical practice preferences for glaucoma surgery in Japan: a survey of Japan glaucoma society specialists. Jpn J Ophthalmol. 2020;64(4):385–391. doi:10.1007/s10384-020-00749-w
7. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
8. Kerr NM, Wang J, Barton K. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma. Clin Exp Ophthalmol. 2017;45(4):393–400. doi:10.1111/ceo.12888
9. Murakami-Kojima S, Takahashi E, Eguchi-Matsumoto M, Saruwatari J, Nakashima K, Inoue T. Risk factors for intraocular pressure elevation in a six-month period after ab interno trabeculotomy using a kahook dual blade. BMC Ophthalmol. 2022;22(1):327. doi:10.1186/s12886-022-02545-1
10. Jea SY, Francis BA, Vakili G, Filippopoulos T, Rhee DJ. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology. 2012;119(1):36–42. doi:10.1016/j.ophtha.2011.06.046
11. Tanito M, Sugihara K, Tsutsui A, Hara K, Manabe K, Matsuoka Y. Midterm results of microhook ab interno trabeculotomy in initial 560 eyes with glaucoma. J Clin Med. 2021;10(4):814. doi:10.3390/jcm10040814
12. Sieck EG, Epstein RS, Kennedy JB, et al. Outcomes of kahook dual blade goniotomy with and without phacoemulsification cataract extraction. Ophthalmol Glaucoma. 2018;1(1):75–81. doi:10.1016/j.ogla.2018.06.006
13. Wakil SM, Birnbaum F, Vu DM, McBurney-Lin S, ElMallah MK, Tseng H. Efficacy and safety of a single-use dual blade goniotomy: 18-month results. J Cataract Refract Surg. 2020;46(10):1408–1415. doi:10.1097/j.jcrs.0000000000000263
14. Barkander A, Economou MA, Jóhannesson G. Kahook dual-blade goniotomy with and without phacoemulsification in medically uncontrolled glaucoma. Clin Ophthalmol. 2023;17:1385–1394. doi:10.2147/OPTH.S409375
15. Tanito M, Sugihara K, Tsutsui A, Hara K, Manabe K, Matsuoka Y. Effects of preoperative intraocular pressure level on surgical results of microhook ab interno trabeculotomy. J Clin Med. 2021;10(15):3327. doi:10.3390/jcm10153327
16. Okuda M, Mori S, Ueda K, et al. Favorable effect of ripasudil use on surgical outcomes of microhook ab interno trabeculotomy. Graefes Arch Clin Exp Ophthalmol. 2023;2023:1–8.
17. Okuda M, Mori S, Takano F, et al. Association of the prolonged use of anti-glaucoma medications with the surgical failure of ab interno microhook trabeculotomy. Acta Ophthalmol. 2022;100(6):e1209–e1215. doi:10.1111/aos.15090
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

