Back to Journals » Clinical Ophthalmology » Volume 16
Suprachoroidal versus Intravitreal Triamcinolone Acetonide for the Treatment of Diabetic Macular Edema
Authors Zakaria YG, Salman AG, Said AMA, Abdelatif MK
Received 10 December 2021
Accepted for publication 9 February 2022
Published 11 March 2022 Volume 2022:16 Pages 733—746
DOI https://doi.org/10.2147/OPTH.S351853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yousra Gamal Zakaria, Abdelrahman Gaber Salman, Azza Mohamed Ahmed Said, Mona Kamal Abdelatif
Ophthalmology Department, Ain Shams University, Cairo, Egypt
Correspondence: Yousra Gamal Zakaria, Ophthalmology Department, Ain Shams University, Ramses Street, Abbassiya, Cairo, 11517, Egypt, Tel +21006799302, Email [email protected]
Purpose: This article aims to compare between intravitreal (IV) and suprachoroidal (SC) triamcinolone acetonide (TA) injection for the treatment of diabetic macular edema (DME) in terms of improvement in both best-corrected visual acuity (BCVA) and central macular thickness (CMT), and development of complications (intraocular pressure (IOP) rise and cataract progression), and to identify the efficient dose of TA using the SC route.
Patients and Methods: This prospective interventional randomized comparative study included 45 eyes of 32 patients, randomly divided into three groups, group I received 4 mg/0.1 mL intravitreal TA (IVTA), group II received 4 mg/0.1 mL suprachoroidal TA (SCTA), and group III received 2mg/0.1 mL SCTA. Patients were followed up for 6 months.
Results: At 1 month, a maximum reduction in CMT (147.33 ± 110.97 μm, 160.80 ± 98.25 μm and 65.64 ± 46.19 μm in groups I, II, and III, respectively) was observed, which was associated with the greatest improvement of BCVA (0.09 ± 0.09, 0.19 ± 0.10 and 0.10 ± 0.09 logMAR lines) in groups I, II, and III, respectively. At 3 months, CMT started to increase, and reduction was not statistically significant compared to baseline, except in group II (4 mg SCTA group) (149.80 ± 106.57 μm with P-value = 0.013). At 6 months, CMT and BCVA returned close to baseline except for group II which had a sustained reduction of 60.16 μm from baseline. Regarding steroid-related complications, IOP elevation of 10 mmHg or more was noted in 1 eye (6.7%), 2 eyes (13.3%), and 1 eye in groups I, II, and III, respectively. Three phakic eyes per group showed cataract progression.
Conclusion: SCTA is a safe and effective route for the treatment of DME, which has comparable effects to IVTA, and may even last longer.
Keywords: suprachoroidal space, diabetic macular edema, triamcinolone acetonide, intravitreal
Introduction
Diabetes mellitus (DM) is becoming a very important public health problem. The DM prevalence worldwide was estimated in 2019 to be 9.3% (463 million people) and is expected to rise to 10.2% (578 million) by 2030.1 Diabetic retinopathy (DR) is the most frequent and severe ocular complication of DM. It is the leading cause of blindness in the working-age population in developed as well as developing countries.2 Diabetic macular edema (DME) is one of the main causes of decreased vision in patients with DR.3
Treatment of DME has undergone a major shift over the years. The Early Treatment of Diabetic Retinopathy Study (ETDRS) was the first study to establish laser photocoagulation as a treatment line for DME. After 3 years of macular laser, a decrease of 50% or more in the incidence of moderate visual loss was noted in treated eyes with a reduction in central macular thickness (CMT), compared to untreated eyes.4 However, macular laser, both grid and focal, caused many complications, including progressive photoreceptor atrophy, visual field scotomas, choroidal neovascularization (CNV), and subretinal fibrosis.5
Intravitreal injection (IVI) of steroids has been used for the treatment of DME. This is due to the anti-inflammatory, angiostatic, and anti-permeability properties of corticosteroids. Also, it is due to the inhibition of the expression of vascular endothelial growth factor (VEGF) and key pro-inflammatory genes (eg tumor necrosis factor-alpha [TNF-α]).6 A slow-release bioerodible dexamethasone implant and an extended-release nonbioerodible fluocinolone acetonide insert are both approved for the treatment of DME and provide the advantage of reduced treatment burden.7
Anti-VEGF therapy has replaced laser photocoagulation as the mainstay of treatment. Excellent results of the initial anti-VEGF trial (the RISE/RIDE and VIVID/VISTA) were documented leading to the approval of ranibizumab and aflibercept for the treatment of DME by the US Food and Drug Administration (FDA) in 2012 and 2014, respectively. Moreover, bevacizumab has been used as an off-label treatment for DME.8 Currently, the role of triamcinolone acetonide (TA), either alone or combined with laser, could be considered mainly in refractory DME (Best-corrected visual acuity (BCVA) gain ≤5 letters or reduction in CMT ≤ 20% after the loading dose), especially in pseudophakic eyes.9
Suprachoroidal injection is a newly developed technique for drug delivery to the posterior segment. The suprachoroidal space (SCS) is a potential space that is located between the sclera and choroid. Injection of drugs into the SCS does not have the risk of intraocular penetration.10 Animal studies concluded that TA seemed the most promising drug formulation for SCS delivery to treat retinal diseases due to its low solubility and sustained-release property. Thus, it was thought it might be possible to obtain therapeutic levels of TA in the retina and choroid.11 Animal studies also showed that there were higher amounts of the drug in the retina and the SCS, and minimal amounts in the anterior segment compared with the intravitreal (IV) route. This may decrease complications commonly associated with TA, such as the development of glaucoma and cataract.12 It is even estimated to have a higher reach to the choroid-retina region than the IV route.13
Many clinical trials were done in humans to demonstrate the safety and efficacy of suprachoroidal injection of TA (SCTA). In macular edema complicating uveitis, Yeh et al DOGWOOD and Goldstein et al studies demonstrated the safety of SCTA.14 This led to another study (PEACHTREE study), which showed significant improvement in macular thickness compared to sham treatment.15
In macular edema due to vein occlusion, the TANZANITE study showed that the mean improvement from baseline BCVA, CMT, and the percentage of participants requiring no re-treatments were higher in the combination group (IV aflibercept and SCTA) than the monotherapy with IV aflibercept.16
Regarding DME, the HULK study showed a greater reduction in CMT in the group treated with SCTA, compared to the combination group with IV aflibercept. In another study, the TYBEE study demonstrated that the combination group (SCTA and IV aflibercept) showed greater improvement in CMT compared to the IV aflibercept group.17
Our prospective interventional study was done to compare between IV and SCTA in the treatment of DME in terms of efficacy and possible complications.
Patients and Methods
Our study was a prospective interventional randomized comparative study set up in the Ophthalmology Department, Ain Shams University hospitals. The study subjects were selected from patients attending the Ophthalmology outpatient clinics between August 2019 and July 2021. Ethics committee approval was obtained from the Institutional Review Board of the Faculty of Medicine, Ain Shams University, and all procedures conformed to the guidelines provided by the World Medical Association Declaration of Helsinki on ethical principles for medical research involving humans. Informed consent was taken from patients before participation. The sample size was calculated using the PASS program, setting alpha error at 5% and power at 80%.
We included patients aged 40–70 with type II DM, having clinically significant macular edema (CSME) with a CMT of more than 300 µm by spectral-domain optical coherence tomography (SD-OCT). We excluded: 1) Patients with retinal pathology other than DM, 2) Patients with proliferative diabetic retinopathy (PDR), 3) Patients who have intraocular pressure (IOP) ≥21 mmHg or known glaucoma patients, and 4) Patients who had any recent intraocular procedure within six months and 5) patients who have a poor resolution of OCT images due to media opacity.
Included patients were randomly divided into 3 groups: Group I received a single IV dose of 0.1 mL TA (Kenakort A by GlaxoSmithKline Brentford, Middlesex, TW8 9GS, United Kingdom) at a concentration of 4 mg per 0.1 mL. Group II received a single SC injection of 0.1 mL TA at a concentration of 4 mg per 0.1 mL. Group III received a single SC injection of 0.1 mL TA at a concentration of 2 mg per 0.1 mL.
At the time of initial assessment, all participants underwent thorough history taking, and full ophthalmological examination including BCVA, complete anterior and posterior segment examination using Zeiss slit lamp (Zeiss SL 120, Germany), with cataract grading using the LOCS III classification and IOP measurement using Goldmann applanation tonometry. Patients were dilated with Mydriacyl® (tropicamide ophthalmic solution, USP). Fundus photos of patients were taken using VX-20 Kowa fundus camera, Japan. OCT images were assessed using Retinascan RS 3000 advance, Nidek co. ltd, Gamagori, Japan. Images were taken including macula map and macula radial scans. The macula map was divided according to the ETDRS classification, and the CMT included the central 1 mm zone, which was used for statistical analysis. The Scleral thickness of patients who were randomized to groups II and III was assessed using an ultrasound biomicroscopy (UBM) machine (VuMax, Sonomed Escalon, the United States of America).
The procedure was performed under sterile conditions in the operating room using TA drawn from a single-use bottle. In group I, IVI was done using a 30 gauge needle at a distance of 3.5 mm from the limbus in pseudophakic patients, and 4 mm in phakic patients in the superior temporal quadrant. SCS injection was done using a custom-made 30 gauge needle with a sterile plastic sleeve, cut in such a way that approximately 1 mm of the needle was exposed, to prevent further penetration of the needle into the vitreous cavity (Figure 1). The injection was done at approximately 4 mm from the limbus in the superior temporal quadrant (Figure 2). After injection, the needle was removed and pressure was applied at the injection site using a cotton-tipped applicator over the entry site.
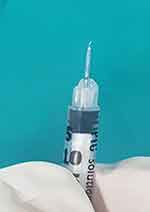 |
Figure 1 Syringe used for suprachoroidal injection. |
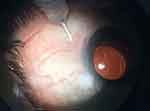 |
Figure 2 Injection of TA in the suprachoroidal space using a custom-made syringe. |
Assessment of IOP was done after injection, and topical antibiotic therapy (ofloxacin) was prescribed for 1 week. Follow-up visits were done the next day to assess serious adverse effects of injection as severe IOP rise or infection, and to ensure proper targeting of the SCS in groups II and III using UBM (Figure 3). One week after the injection, a full ophthalmological assessment was done including BCVA and IOP. At 1, 3, and 6 months, full ophthalmological examination and OCT were done. BCVA measurements were based on the Snellen chart and were converted to the logarithm of the minimum angle of resolution (logMAR) scale for statistical analysis. Clinical trial registration is on clinicaltrials.gov: NCT04069780.
 |
Figure 3 UBM after SC injection in case no.8 in the 4mg SC group. |
Statistical Analysis
Data were collected, revised, coded, and entered into the statistical package for social science (SPSS) version 23; qualitative data were presented as numbers and percentages and compared between groups using Chi-square test, while quantitative data were presented as mean, standard deviations, and ranges. Parametric data were compared between the three groups using One Way ANOVA, while non-parametric data were compared using the Kruskal–Wallis test. The follow-up for the studied parameters in each group was done by using Repeated Measures ANOVA. The confidence interval was set to 95%, and the margin of error accepted was set to 5%. As a result, the p-value was considered significant at the level of <0.05.
Results
We included 45 eyes (32 patients), with 15 eyes in each group. Mean ± SD age was 57.67 ± 6.62, 57.54 ± 6.33, and 60.25 ± 6.47 in groups I, II, and III, respectively (P-value=0.513). Females were more than males with no statistically significant difference between the 3 groups (P-value=0.486). The mean duration of diabetes was 14.00, 14.69, 14.00 years in the 3 groups, respectively (P-value=0.927) as shown in (Table 1).
 |
Table 1 Baseline Data |
BCVA did not show a statistically significant difference (P-value=0.310) between the 3 groups (Table 1) before injection, with mean BCVA 0.59, 0.73, and 0.67 logMAR in groups I, II, and III, respectively. Regarding the lens status, 10 eyes (66.7%) in group I, 9 eyes (60%) in group II, and 12 eyes (80%) in group III were phakic. IOP (P-value=0.204) and CMT (P-value=0.284) were not statistically different between the 3 groups pre-injection.
After injection, no serious adverse effects occurred in all 3 groups. IOP was preserved in the immediate post-operative period, with no patients requiring treatment for rising of IOP. On the first post-injection day, none of our patients showed any signs of infection or acute rise of IOP requiring treatment.
BCVA
BCVA showed no statistically significant differences between the 3 groups throughout the follow-up period (P-value= 0.347, 0.887, 0.864, and 0.679 at 1 week, 1 month, 3 months, and 6 months respectively) (Table 2). However, at 1 month, the improvement in BCVA compared to presentation was significant (Table 3) between the 3 groups (P-value=0.025). Eyes in group II showed the largest mean BCVA change 1 month after the treatment.
 |
Table 2 Comparison Between the 3 Groups Regarding BCVA Through the Follow-Up Period |
 |
Table 3 Comparison Between the 3 Groups Regarding the Difference in BCVA from Presentation to 1, 3, and 6 Months |
BCVA improved in the 3 groups at 1 and 3 months. However, it decreased at 6 months returning to near baseline values, except in group II, in which vision remained relatively stable (Table 4). At 1 month, BCVA significantly improved compared to baseline in all 3 groups (P-value= 0.004, 0, 0.009 in groups I, II, and III respectively), while at 3 months, it was still significant compared to baseline only in groups I and II (P-value= 0.012 and 0.002 respectively).
 |
Table 4 Follow-Up of BCVA in Each of the 3 Groups Throughout the Follow-Up Period |
At 6 months, BCVA returned to baseline values, except in group II, which showed the greatest and most sustainable improvement throughout the follow-up period (Table 3 and Figure 4).
 |
Figure 4 Chart showing the change of BCVA throughout the follow-up period in the 3 groups (logMAR). |
CMT
CMT showed no statistically significant differences between the 3 groups throughout the follow-up period (P-value= 0.276, 0.354, and 0.464 at 1 month, 3 months, and 6 months respectively) (Table 5).
 |
Table 5 Comparison Between the 3 Groups Regarding CMT Through the Follow-Up Period |
The change of CMT (Table 6) was highly significant in the 3 groups (P-value= 0, 0.001, and 0 in groups I, II, and III respectively) throughout the follow-up period (Figures 5,6 and 7). All the groups showed the most significant decrease in CMT after 1 month (P-value=0.005, 0.008, and 0.001 in groups I, II, and III, respectively) with the reduction of CMT (Table 7) showing a statistically significant difference between the 3 groups (P-value=0.027). Post-hoc analysis revealed that group II had the greatest reduction. CMT started to increase again after 3 months and returned back close to the presentation values after 6 months (P-value=1, 0.714, and 0.991 in groups I, II, and III, respectively).
 |
Table 6 Follow-Up of CMT Throughout the Follow-Up Period in the 3 Groups |
 |
Table 7 Comparison Between the 3 Groups Regarding the Difference in CMT from Presentation to 1, 3, and 6 Months |
 |
Figure 5 Radial OCT images of eye no.14 in group I ((A) At presentation, (B) 1 month after injection, (C) 3 months after injection, (D) 6 months after injection). |
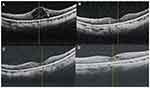 |
Figure 6 Radial OCT images of eye no.5 in group II ((A) At presentation, (B) 1 month after injection, (C) 3 months after injection, (D) 6 months after injection). |
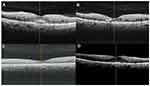 |
Figure 7 Radial OCT images of eye no.2 in group III ((A) At presentation, (B) 1 month after injection, (C) 3 months after injection, (D) 6 months after injection). |
Although group II had the highest CMT at baseline, by the end of the follow-up at 6 months, group II had the least CMT with a mean reduction of CMT of 60.18 µm (Table 7 and Figure 8).
 |
Figure 8 Chart showing the change of CMT throughout the follow-up period in the 3 groups (µm). |
Intraocular Pressure
IOP did not show statistically significant differences between the 3 groups throughout the follow-up period (P-value= 0.226, 0.543, 0.139, and 0.128 at 1 week, 1 month, 3 months, and 6 months respectively) (Table 8). Regarding the change in IOP throughout the follow-up period (Table 9), changes were highly significant only in group III (P-value=0.003) with a statistically significant rise in IOP at 1 month compared to baseline (P-value= 0.041), which decreased close to baseline at 6 months (P-value= 1) (Figure 9).
 |
Table 8 Comparison Between the 3 Groups Regarding IOP Through the Follow-Up Period |
 |
Table 9 Follow-Up of IOP in Each of the 3 Groups Throughout the Follow-Up Period |
 |
Figure 9 Chart showing the change of IOP throughout the follow-up period in the 3 groups (mmHg). |
The number of eyes with an IOP increase of 10 mmHg or more, was 1 (6.7%), 2 (13.3%), and 1 eye (6.7%) in groups I, II, and III, respectively. All cases were managed medically through topical antiglaucoma treatment and did not require further treatment.
Cataract
During the follow-up, 3 cases (30% of phakic cases) in group I, 3 cases (33.34% of phakic cases) in group II, and 3 cases (25% of phakic cases) in group III had progression in their cataract. Only one case in group II had a significant cataract causing reduction of BCVA, precluding proper examination and OCT assessment, requiring cataract surgery.
Discussion
Injection of corticosteroids into the SCS may achieve therapeutic drug levels in the retina while minimizing levels in the anterior chamber, thus, decreasing adverse effects, such as cataract and glaucoma.18
In this study, we compared intravitreal triamcinolone acetonide (IVTA) to 2 different doses of SCTA (4 and 2 mg). The aim of our research was to confirm the efficacy of suprachoroidal steroids compared to intravitreal injections, to find the appropriate effective dose of SCTA, and to detect the safety of this novel route of injection and its complications compared to IVTA. We could not find a similar study comparing IV and SC routes for the injection of steroids in the management of DME. Besides, no previous studies compared different doses of TA injected in the suprachoroidal space.
Our study showed that BCVA improved significantly in the 3 groups at 1 and 3 months. At 1 month, the difference from baseline was significant between the 3 groups, as the 4 mg SC group showed the greatest improvement in BCVA. However, BCVA returned to near baseline values at 6 months, except for the 4 mg SC group. Similarly, CMT significantly decreased in the 3 groups at 1 and 3 months. All groups showed the most significant reduction of CMT after 1 month with a statistically significant difference between the 3 groups, as the 4 mg SC group had the highest reduction. CMT started to increase again after 3 months and returned close to presentation values at 6 months except for the 4 mg SC group, which still had a mean reduction of 60.18 µm.
Regarding IOP elevation and progression of cataract, both routes have insignificantly different effects. The lower dose of SCTA did not show a lower risk of complications of TA.
One of the first studies to assess the safety and efficacy of SCTA in diabetic patients was (HULK) study,19 which aimed to study the safety and tolerability of SCTA injection in subjects with DME. It was a Phase 1/2 study that included 20 patients with DME. They injected CLS-TA, which is preservative-free triamcinolone, terminally sterilized aqueous suspension (XIPERE™ [Clearside Biomedical, Alpharetta, GA]) using a SCS microinjector (Clearside Biomedical), as monotherapy (in previously treated group) or combined with intravitreal aflibercept (in treatment naïve group). Throughout the follow-up, all participants were treated with CLS-TA administered suprachoroidally pro re nata (PRN). A greater reduction in CMT (128 um) was observed in the monotherapy group compared to the combination group (91 um). Despite this, more letter gain (8.5 letters) in the ETDRS letters was noted in the combination group compared to the monotherapy group (1.1 letters). Their results agree with ours, as we both had significant improvements in BCVA reflecting the reduction in CMT after SCTA injection. Although we did not inject intravitreal aflibercept as in the HULK study, we had a reduction in CMT that lasted for 6 months in the 4 mg SC group despite being injected only once at baseline.
This was followed by Phase 2 TYBEE study,17 which was performed in treatment-naïve participants to assess suprachoroidal CLS-TA in combination with IV injection of aflibercept at 0 and 3 months (active group) compared to aflibercept monotherapy monthly for 4 successive months (control group), and follow-up for 24 weeks. Patients could receive aflibercept at weeks 16, and 20 if needed (PRN). Although the active combination group showed a greater improvement in CMT compared to the control group (212.1 µm and 178.6 µm, respectively [P-value = 0.089]), the improvement of BCVA was less marked between the 2 groups (11.4 and 13.8 letters in the active and control groups, respectively). Although the combined treatment did not show better improvement in BCVA, it had an important advantage as it required fewer PRN treatments compared to the control group, which proves the longer effect of SC steroids over IV anti-VEGF.
This agreed with our study, as although we had a greater reduction in CMT in the 4 mg SC group compared to the IV group throughout the follow-up period, the improvement in BCVA was significantly greater in the 4 mg SC group only at 1 month. A major difference between our study and both HULK and TYBEE studies is that we did not inject anti-VEGF agents, and we injected our patients only once at presentation, which ensures the long-lasting effect of single-dose 4 mg SC injection of TA.
In our study, the improvement in CMT and BCVA was significant until the third month of post-injection in the IV and the 4 mg SC groups, which agreed with Tayyab et al,20 who injected 4 mg SCTA into treatment-resistant DME, and had significant CMT and BCVA improvements at 1 and 3 months, although they did not follow the patients till 6 months to detect the persistence of this improvement. While Yousef et al had persistent significant improvement in CMT and BCVA till 6 months post-injection of 4 mg SCTA contradicting our results, we had definite regression in CMT in the 4 mg SCTA group at 6 months, although BCVA was relatively stable compared to 3 months.21
One of the main complications of TA injection is the rise of IOP. An IOP rise of 10 mmHg or more was noted in 2 patients (10%) in the HULK study and in 5 patients (15%) in the active group in the TYBEE study following CLS-TA injection. This was comparable to our results, as we had a similar IOP rise in 1 patient (6.7%) of the IV group, 2 patients (13.3%) in the 4 mg SC group, and 1 patient (6.7%) in the 2 mg SC group, which was a lower percentage compared to Massin et al,22 who had IOP rise in 6 out of 12 patients after injection with 4 mg IVTA. Our results prove that TA injection would have a similar effect on IOP either injected intravitreally or in the SCS. The difference between IOP rises was insignificant between different doses of TA in our study, which agrees with that of Audren et al who compared 2 and 4 mg IVTA.23 On the other side, Tayyab et al found an insignificant rise in IOP following SCTA injection, although they had only 1 patient with a 5 mmHg rise that returned back to normal on antiglaucoma measures.
Regarding cataract progression in our study, there was a progression in the level of cataract in 3 cases (30% of phakic cases) in the IV group, 3 cases (33.34% of phakic cases) in the 4 mg SC group, and 3 cases (25% of phakic cases) in the 2 mg SC group, with no significant difference between the 3 groups, which was comparable to TYBEE study who had progression of cataract in 2 participants in the active group.
Conclusion
Our study suggests the efficacy of SCTA injection using a custom-made syringe compared to the IV route and would provide a safe alternative route of injection as both routes had relatively similar steroid-related complications. An advantage of the SC route over the IV one could be the longer effect on reduction in CMT and improvement of BCVA, which should be further assessed by larger studies for a longer follow-up period. We found the 4 mg dose of SCTA more effective and lasted longer than the 2 mg dose, without having a higher rate of steroid-related complications. However, as CMT had almost returned to baseline values in most of our patients, we believe that reinjection should be considered before 6 months.
One of the limitations of the study is the relatively small number of patients and, accordingly, using both eyes of some of our patients, so larger studies would provide stronger results.
Data Sharing Statement
All individual data collected during the trial after deidentification are available upon request from the corresponding author. No end date.
Acknowledgments
We are grateful to the staff and residents of the Ophthalmology Department, Ain Shams University, who helped in the recruitment of cases for our study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saeedi O, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation, Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Wu L, Fernandez-Loaiza P, Sauma J, Hernandez-Bogantes E, Masis M. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4(6):290–294. doi:10.4239/wjd.v4.i6.290
3. Bandello F, Parodi MB, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol. 2017;58:102–138.
4. Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761–764. doi:10.1016/s0161-6420(87)33527-4
5. Park YG, Kim EY, Roh YJ. Laser-based strategies to treat diabetic macular edema: history and new promising therapies. J Ophthalmol. 2014;2014:1–9. doi:10.1155/2014/769213
6. Lanzetta P, Veritti D. The role of steroids in the treatment of diabetic macular edema. Retina Today. 2009;4:41–44.
7. Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236. doi:10.1159/000499540
8. Sharma T. Evolving role of anti-VEGF for diabetic macular oedema: from clinical trials to real life. Eye. 2020;34(3):415–417. doi:10.1038/s41433-019-0590-0
9. Bandello F, Zucchiatti I, Lattanzio R, Preziosa C. Diabetic macular edema. In: Bandello F, Zarbin MA, Lattanzio R, Zucchiatti I, editors. Clinical Strategies in the Management of Diabetic Retinopathy. A Step-By-Step Guide for Ophthalmologists. Heidelberg, Germany: Springer; 2014:65–122.
10. Yamada N, Olsen TW. Routes for drug delivery to the retina: topical, transscleral, suprachoroidal and intravitreal gas phase delivery. Dev Ophthalmol. 2016;55:71–83.
11. Chen M, Li X, Liu J, Han Y, Cheng L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;203:109–117. doi:10.1016/j.jconrel.2015.02.021
12. Edelhauser HF, Verhoeven RS, Burke B, Struble CB, Patel SR. Intraocular distribution and targeting of triamcinolone acetonide suspension administered into the suprachoroidal space. Invest Ophthalmol Vis Sci. 2014;55:52–59.
13. Tyagi P, Kadam RS, Kompella UB. Comparison of suprachoroidal drug delivery with subconjunctival and intravitreal routes using noninvasive fluorophotometry. PLoS One. 2012;7(10):e48188. doi:10.1371/journal.pone.0048188
14. Yeh S, Kurup SK, Wang RC, et al.; for DOGWOOD Study Team. Suprachoroidal injection of Triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis: a randomized, Phase 2 study (DOGWOOD). Retina. 2019;39(10):1880–1888. doi:10.1097/IAE.0000000000002279
15. Yeh S, Khurana RN, Shah M, et al.; for the PEACHTREE Study Investigators. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: Phase 3 randomized trial. Ophthalmology. 2020;127(7):948–955. doi:10.1016/j.ophtha.2020.01.006
16. Campochiaro PA, Wykoff CC, Brown DM, et al.; for the Tanzanite Study Group. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the Tanzanite study. Ophthalmol Retina. 2017;2(4):320–328. doi:10.1016/j.oret.2017.07.013
17. Barakat MR, Wykoff CC, Gonzalez V, et al. Suprachoroidal CLS-TA plus intravitreal aflibercept for diabetic macular edema: a randomized, double-masked, parallel-design, controlled study. Ophthalmol Retina. 2021;5(1):60–70. doi:10.1016/j.oret.2020.08.007
18. Habot-Wilner Z, Noronha G, Wykoff CC. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: a targeted approach. Acta Ophthalmol. 2019;97:460–472. doi:10.1111/aos.14042
19. Wykoff CC, Khurana RN, Lampen SIR, et al. Suprachoroidal triamcinolone acetonide for diabetic macular edema: the HULK trial. Ophthalmol Retina. 2018;2:874–877. doi:10.1016/j.oret.2018.03.008
20. Tayyab H, Ahmed CN, Sadiq MAA. Efficacy and safety of suprachoroidal triamcinolone acetonide in cases of resistant diabetic macular edema. Pak J Med Sci. 2020;36(2):42–47. doi:10.12669/pjms.36.2.1194
21. Yousef MS, Abd Elhafez YA, Farag MH. Assessment of suprachoroidal injection of triamcinolone acetonide in cases of diabetic macular edema. Int J Med Arts. 2021;3(2):1384–1389. doi:10.21608/ijma.2021.55079.1230
22. Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111(2):218–224. doi:10.1016/j.ophtha.2003.05.037
23. Audren F, Lecleire-Collet A, Erginay A, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: phase 2 trial comparing 4 mg vs 2 mg. Am J Ophthalmol. 2006;142(5):794–799. doi:10.1016/j.ajo.2006.06.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
