Back to Journals » Clinical Interventions in Aging » Volume 15
Supplement of Lipid Emulsion to Epinephrine Improves Resuscitation Outcomes of Asphyxia-Induced Cardiac Arrest in Aged Rats
Authors Huang L, Ren Q, Yu S, Shao Y, Chen Y, Huang X
Received 21 June 2020
Accepted for publication 12 August 2020
Published 22 September 2020 Volume 2020:15 Pages 1701—1716
DOI https://doi.org/10.2147/CIA.S268768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Lijun Huang,1 Qiusheng Ren,1 Shenghui Yu,1 Ya Shao,1 Yijun Chen,2 Xin Huang1
1Department of Anesthesiology, Ningbo Yinzhou People’s Hospital, The Affiliated People’s Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China; 2Department of Anesthesiology, Ningbo First Hospital, Ningbo Hospital of Zhejiang University, Ningbo, Zhejiang, People’s Republic of China
Correspondence: Lijun Huang
Department of Anesthesiology, Ningbo Yinzhou People’s Hospital, The Affiliated People’s Hospital of Ningbo University, No. 251, East Baizhang Road, Ningbo, Zhejiang Province 315040, People’s Republic of China
Email [email protected]
Objective: The goal of the study was to investigate the efficacy of lipid supplement to epinephrine-based therapy in resuscitation of asphyxia-induced cardiac arrest in aged rats.
Methods: The study included two parts: in experiment A, rats underwent asphyxial cardiac arrest and cardiopulmonary resuscitation, randomized to receive epinephrine and normal saline (control group, n=22), epinephrine and intralipid 20% (long-chain triglycerides (LCT) group, n=22) or epinephrine and lipovenoes 20% (LCT/medium-chain triglcerides (MCT) group, n=22). Return of spontaneous circulation, recurrence of asystole after resuscitation, hemodynamic metrics, arterial blood gas values, neurological assessment score and indexes of pulmonary transudation were recorded. In experiment B, rats using the same model and resuscitation protocol were randomly divided into 21 groups: Control 0, Control 20, Control 40, Control 60, Control 80, Control 100, Control 120, LCT 0, LCT 20, LCT 40, LCT 60, LCT 80, LCT 100, LCT 120, LCT/MCT 0, LCT/MCT 20, LCT/MCT 40, LCT/MCT 60, LCT/MCT 80, LCT/MCT 100 and LCT 120 (n=10, the subscripts represent respective endpoint of observation in minutes). Myocardial bioenergetics were determined.
Results: In experiment A, the LCT and LCT/MCT groups had a shorter time to return of spontaneous circulation (ROSC) (P=0.001and P< 0.001, respectively) and higher survival rate (P=0.033 and P=0.014, respectively) compared with the Control group. The LCT/MCT group had higher MAP (P< 0.001 and P=0.001, respectively), HR (P< 0.001 and P=0.004, respectively) and RPP (P< 0.001 and P< 0.001, respectively) compared with the Control and LCT groups, respectively. In experiment B, the LCT/MCT group had a higher energy charge compared with the control group at 20 (P< 0.001) and 40 (P< 0.001) minutes. The LCT group had higher energy charge compared with the Control group at 40 (P< 0.001) and 60 (P< 0.001) minutes.
Conclusion: The supplement of lipid emulsion to epinephrine improves resuscitation outcomes of asphyxia-induced cardiac arrest than epinephrine alone in our in vivo model of aged rat. LCT/MCT emulsion may be superior to LCT emulsion in epinephrine-based resuscitation.
Keywords: asphyxia, cardiac arrest, epinephrine, lipid emulsion, cardiopulmonary resuscitation
Introduction
Cardiac arrest (CA) is a main cause of death and disability, which affects approximately 300,000 people per year in the United States.1 Despite advances in resuscitation therapies and techniques, the survival rate of CA remained unchanged at 8% over the past decades.2 In addition, neurological disorders, cardiac insufficiency and other organ dysfunction after return of spontaneous circulation (ROSC) are still intractable.3,4 Several epidemiological studies have revealed that the risk of CA increases with age.5 Decreased organ function and underlying diseases make aged populations more prone to low success rate of cardiopulmonary resuscitation (CPR), and substantial mortality and morbidity after ROSC.6 So far, the majority of researches investigating CA have used juvenile animal models.7,8 The main discouraging factor for conducting a study on aged animals is the low success rate of CPR and the poor post-CA survival. However, performing CA in aged animals may be more clinically relevant, for CA of aged populations is intractable and in large numbers.
Epinephrine, which is recommend by the American Heart Association guidelines, is the first-line medication in resuscitation of CA.9 It exerts a vasoconstriction effect, augments coronary perfusion pressure (CCP), and facilitates restoration of cardiovascular circulation.10 However, its role in CPR is controversial. The activation of adrenergic receptors increases myocardial oxygen consumption and peripheral vascular tone, which may lead to post-resuscitation myocardial dysfunction and deterioration of pulmonary oxygen exchange.11,12 In addition, a large dose of epinephrine is not effective in improving neurological outcomes after resuscitation.13 These unwarranted side effects of sole epinephrine application during CPR suggest that further treatment is required.
Lipid emulsions, including long-chain triglycerides (LCTs) and long- and medium-chain triglycerides (LCTs/MCTs), are widely used in clinical scenarios. Intravenous administration of lipid emulsion has been shown to successfully reverse local anesthetic-induced cardiovascular collapse in animal models.14 Clinical case reports have also confirmed efficacy of lipid emulsion in rescuing local anesthetic overdose.15 The application of lipid emulsion in rescuing drug overdose associated cardiac arrest is not confined to local anesthetics, but also to other lipophilic drugs.16–18 These data may hint the “lipid sink” theory, in which lipophilic drug are extracted by lipid emulsion, reducing tissue concentration of the toxin.19 An alternative theory is the “lipid flux,” whereby lipid supplies the myocardial cell with sufficient fatty acid which can be directly utilized by mitochondria, thus countering the impeded energy supply induced by myocardial ischemia and anoxia.20 Furthermore, there are differences between LCT and LCT/MCT in efficiency of oxidation and hydrolysis, ketone bodies production and metabolic pathways of energy utilization.21 Nevertheless, few studies focus on the rescuing effect of lipid on CA aside from lipophilic drug poisoning, nor addressing the choice of lipid emulsions.
No present data have reported the effects of lipid supplement to epinephrine during resuscitation of asphyxia-induced cardiac arrest in aged rat. We hypothesized that the combination of lipid emulsion and epinephrine provided better resuscitation outcomes than epinephrine alone after asphyxia-induced cardiac arrest in aged rat. Furthermore, LCT/MCT provides benefits over LCT lipid emulsion. Accordingly, we established an aged rat model of asphyxia-induced cardiac arrest with the primary aim of recording hemodynamic parameters and energy charge during the initial observation time of 120 minutes after onset of resuscitation, and determining resuscitation outcomes and hemodynamic metrics at the endpoint of 120 minutes. Secondarily, neurological outcomes, hemodynamic parameters, arterial blood gas values, lung wet-to-dry ratio and permeability index were determined 72 hours after onset of resuscitation to account for the possible difference between them.
Materials and Methods
Animal Preparation
All the experimental procedures were conducted according to the guidelines of the National Institutes of Health for the care and use of laboratory animals (NIH publication No 80–23), with the approval of the Animal Care and Use Committee of the Ningbo University. 20-month-old healthy male Sprague-Dawley rats (Sippe-Bk Lab Animal Co., Ltd., Shanghai, People's Republic of China) were used. They were housed in cages (4 rats per cage) at the animal center of Ningbo University in a standard 12-hour reverse day/night cycle at a humidity of 55 to 60% and an ambient temperature of 20–26°C. The rats had an ad libitum diet of stock laboratory diet and tap water. They had a recovery period of at least 2 weeks following transportation to Ningbo University before experimental manipulations.
A behavioral test for neurologic assessment was performed the day before CA/CPR. The rats were fasted for 12 hours with free access to water before surgery. Anesthesia was induced with 4% sevoflurane. After reaching a deep level of anesthesia, tracheal intubation was performed orally and rats were mechanically ventilated (tidal volume, 6–7.5 mL/kg; FiO2, 0.3; respiratory rate, 70–80 breaths/min; inspiratory/expiratory ratio, 2:3, maintaining the end-tidal carbon dioxide tension at 40 mmHg). Sevoflurane was adjusted to 3–4% to maintain the depth of anesthesia during surgery. Body temperature was maintained at 37.0–37.5°C by a thermal blanket underneath the rat’s body and a heating lamp kept at a safe distance. Electrocardiogram was recorded using three subcutaneous needle electrodes. The skin of the invasive operating area was shaved, sterilized and locally anesthetized with 1% lidocaine before incision. The right femoral artery was cannulated for blood sample collection and continuous monitoring of arterial blood pressure. The right femoral vein was cannulated for intravenous drug and fluid administration. The left internal jugular vein was cannulated for central venous pressure monitoring. All the rats were allowed to stabilize for 20 minutes to recover from anesthesia induction and invasive procedures. Then an arterial blood gas analysis was performed for measuring baseline parameters.
Experiment A
Experimental Protocol
A total of 66 rats were divided by the random table method into three groups: Control, LCT, and LCT/MCT groups according to a random number table (n=22). After the post-surgical stabilization, the rats received 0.5 mg/kg pancuronium for prevention of respiratory movement. CA was induced by stopping mechanical ventilation. CA was defined as a decrease in mean arterial pressure (MAP) below 10 mmHg. Resuscitation was initiated 30 seconds after onset of CA (baseline time, designated T0). Mechanical ventilation was restarted (tidal volume, 6–7.5 mL/kg; FiO2, 1.0; respiratory rate, 70–80 breaths/min; inspiratory/expiratory ratio, 2:3), external chest compression over the lower-middle sternum was manually performed (300 compressions per minute, a depth of 1/3 anterior-posterior chest diameter) and intravenous medications (preheated to 37°C before administration) were given: a bolus of 10 μg/kg epinephrine was administered at 1-minute intervals until ROSC (limiting maximum cumulative dose to 100 μg/kg), meanwhile normal saline (Control group), Intralipid 20% (LCT group) or Lipovenoes 20% (LCT/MCT group) were administered as a 3mL/kg bolus over 30 seconds followed by a 3 mL/kg infusion at a rate of 1.0 mL/kg/min for 3 minutes. The criterion of ROSC was defined as a spontaneous heart rate-blood pressure product (RPP) more than 20% of baseline value for at least 1 minute. Chest compression was ceased once spontaneous circulation was achieved or an elapsed time of 40 minutes without return of ROSC. Hemodynamic parameters, central venous pressure were monitored for 120 minutes after onset of resuscitation (T0).
After the observation period of 120 minutes, arterial blood was collected for blood gas analysis, all the intravascular catheters were removed, the vessels were ligated, and the incisions were sutured. Anesthesia was discontinued and the tube was removed after reaching adequate spontaneous respiration. All the rats were placed in an incubator tempered at 35°C for 3 days. A behavioral test for neurologic assessment was performed for the survival rats once more 72 hours after resuscitation. Then the rats were generally anesthetized, orally intubated and mechanically ventilated in the same protocol as before, the arterial blood pressure and the central venous pressure were measured as before except that the catheters were inserted at a more proximal point than the past ligation. Arterial blood was collected for blood gas analysis and further study, and the animals were sacrificed by overdose anesthesia with intraperitoneal injection of ketamine and xylazine.
Lung Wet-to-Dry Ratio and Permeability Index
After thoracotomy, the inferior lobe of the right lung was taken and weighed immediately (wet weight) and again after drying at 65°C for 72 hours (dry weight). The lung wet-to-dry ratio was calculated as wet weight/dry weight. The left lung was intubated and lavaged three times with 10 mL of normal saline. The bronchoalveolar lavage fluid (BALF) was centrifuged at 1000 revolutions per minute (rpm) for 10 minutes in a thermostatic centrifuge at 4°C, and the supernatant was removed. The blood sample collected 72 hours after onset of resuscitation was centrifuged at 3000 rpm for 10 minutes, the separated plasma was recentrifuged at 3000 rpm for 10 minutes, and the supernatant was removed. Protein concentrations of BALF in the supernatant and serum were measured by the bicinchoninic acid (BCA) protein assay, using the BCA Protein Assay Kit (Beyotime Biotechnology Inc, Shanghai, People's Republic of China) according to the manufacturer’s instructions. Lung permeability index was calculated as the protein concentration in BALF/serum ratio.22
Neurological Assessment Score
A behavioral test for neurologic assessment was performed the day before CA/CPR and 72 hours after onset of resuscitation. Testing was performed in a quiet and dim room by an investigator who was blinded as to the grouping of the rats. Consciousness, breathing pattern, food and water intake, vibrissae movement, motoric function, and interaction with the environment were scored (0 = no deficit; 100 = most severe deficit).23–25
Experiment B
Experimental Protocol
A second group of 210 rats were assigned to 21 groups (10 rats per group) by the random table method based on differing observation time. The groups were defined as Control 0, Control 20, Control 40, Control 60, Control 80, Control 100, Control 120, LCT 0, LCT 20, LCT 40, LCT 60, LCT 80, LCT 100, LCT 120, LCT/MCT 0, LCT/MCT 20, LCT/MCT 40, LCT/MCT 60, LCT/MCT 80, LCT/MCT 100 and LCT 120 (the subscripts represent respective endpoints of observation in minutes). The rats in Control 0–120, LCT 0–120 and LCT/MCT 0–120 received the same protocol of cardiac arrest and resuscitation as those in Control, LCT and LCT/MCT groups in experiment A. At the end of respective observation period in each group (0, 20, 40, 60, 80,100, 120 minutes), all the surviving rats were killed with overdose anesthesia, cardiac tissues were rapidly taken, rinsed to remove any residual blood and stored at - 80°C for further study.
Energy Charge
Adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) of cardiac tissue were measured by high-performance liquid chromatography. Sample preparation was conducted by the Stehr26 and Li14 procedure. Separations were carried out at 30°C. The mobile phase was a mixture of eluent A and B (pH: 6.0, 99:1, v:v) at a flow rate of 1.0 mL/min. Eluent A was 12 mmol/L Na2HPO4 and 88 mmol/L NaH2PO4 (pH: 6.0) and eluent B was a mixture of 85% eluent A and 15% acetonitrile. An injection volume of 20 μL was used for both standard samples and tissue extract samples. The detection wavelength was 245 nm. Equally treated external standards of known concentrations were applied to check retention times and to permit sample quantification based on the analysis of peak area. Energy charge was calculated as: (ATP+0.5*ADP)/(ATP+ADP+AMP).
Statistical Analysis
The number of animals in each group was determined based on our preliminary study (n=10) in which the survival rates at 120 minutes were 40.0%, 80.0%, and 80.0%, respectively. Effect size of 0.40 was acquired by calculation according to the survival rate. A sample size of 21 per group was obtained by PASS 11.0, with α = 0.05 and β = 0.2. We enrolled 22 rats per group to account for attrition.
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) for Windows. Normal distribution measurement data were presented as mean ± standard deviation (SD), non-normally distributed data were presented as medians and interquartile range, and frequencies were used in the case of categorical variables. Differences in baseline parameters, time to CA, time to first heartbeat, time to ROSC, arterial blood gas values, hemodynamic metrics at time point, RPP recovery ratio, neurological assessment score, wet-to-dry ratio, lung permeability index and energy charge in the three groups were examined by one-way ANOVA, and Bonferroni post-tests for data that had homogeneity of variance, or Dunnett T3 post-tests for data that exhibited heterogeneity of variance when significance was achieved. Dichotomous outcomes were analyzed with Fisher exact test. The cumulative epinephrine dose was analyzed using Wilcoxon and Mann–Whitney U-test. Differences in continuously recorded heart rate (HR), mean arterial blood pressure (MAP), heart rate-blood pressure product (RPP) and coronary perfusion pressure (CPP) during the 120-minute observation period were analyzed by two-way repeated-measure ANOVA and Bonferroni post-tests when indicated by significance of difference. P < 0.05 was considered significant. Curve fitting and bar charts were performed by GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Baseline Values
No differences were observed in weight, baseline hemodynamic metrics or baseline arterial blood gas values among the Control, LCT and LCT/MCT groups in experiment A (Table 1), and the Control0-120, LCT0-120 and LCT/MCT0-120 groups in experiment B (Tables 2 and 3).
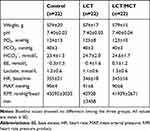 |
Table 1 Baseline Values of Weight, Blood Gas Values and Hemodynamic Metrics for Control, LCT and LCT/MCT Groups in Experiment A |
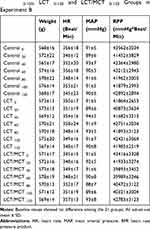 |
Table 2 Baseline Values of Weight, Hemodynamic Metrics for Control 0-120, LCT 0-120 and LCT/MCT 0-120 Groups in Experiment B |
 |
Table 3 Baseline Values of Blood Gas Values for Control 0-120, LCT 0-120 and LCT/MCT 0-120 Groups in Experiment B |
Resuscitation Outcomes
Resuscitation outcomes for the three groups are shown in Table 4. Animals in each group achieved CA for approximately 100 seconds, and no significant difference was demonstrated among the three groups in time to CA. Compared with the Control group, the LCT and LCT/MCT groups had shorter time to ROSC (Control group vs LCT group, 113±36 vs 78±20 seconds, P=0.001; Control group vs LCT/MCT group, 113±36 vs 73±16 seconds, P<0.001), and higher survival rate (Control group vs LCT group, 36.4% vs 72.7%, P=0.033; Control group vs LCT/MCT group, 36.4% vs 77.3%, P=0.014). All the rats that survived at 120 minutes had no further deaths during the 72 hours after onset of resuscitation. The comparison of time to CA, time to first heartbeat, rate of ROSC, mortality after ROSC or epinephrine cumulative dose among the three groups demonstrated no statistical differences.
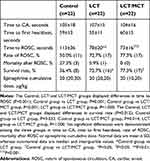 |
Table 4 Resuscitation Outcomes for Control, LCT and LCT/MCT Groups in Experiment A |
Hemodynamic Measures
Hemodynamic parameters are represented as MAP, HR, and RPP in the three groups, and are presented graphically in Figure 1. Within 120 minutes after the onset of resuscitation, the MAP of the LCT and LCT/MCT groups were higher than that of the Control group (Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), and the MAP of the LCT/MCT groups was higher than that of LCT group (LCT group vs LCT/MCT group, P=0.001). Significant differences were demonstrated among the three groups in MAP at 2 (P=0.037; Control group vs LCT group, P=0.038), 4 (P=0.018; LCT group vs LCT/MCT group, P=0.018), 10 (P<0.001; Control group vs LCT group, P=0.005; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 20 (P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 30 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 40 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 50 (P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 60 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 70 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 80 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 90 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 100 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 110 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001) and 120 minutes (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001). The HRs of the LCT and LCT/MCT groups were higher than that of the Control group (Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), and the HR of the LCT/MCT groups was higher than that of the LCT group (LCT group vs LCT/MCT group, P=0.004). Significant differences were demonstrated among the three groups in HR at 4 (P<0.001; Control group vs LCT group, P=0.001; Control group vs LCT/MCT group, P<0.001), 6 (P=0.024; LCT group vs LCT/MCT group, P=0.020), 20 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P=0.027), 30 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P=0.013), 40 (P<0.001; Control group vs LCT group, P=0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 50 (P<0.001; Control group vs LCT group, P=0.002; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 60 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 70 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 80 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P=0.030), 90 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 100 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 110 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001) and 120 minutes (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001). The RPP of the LCT and LCT/MCT groups were higher than that of the Control group (Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), and the RPP of the LCT/MCT groups was higher than that of the LCT group (LCT group vs LCT/MCT group, P<0.001). Significant differences were demonstrated among the three groups in RPP at 4 (P<0.001; Control group vs LCT group, P=0.002; Control group vs LCT/MCT group, P=0.019), 6 (P=0.024; Control group vs LCT/MCT group, P=0.016; LCT group vs LCT/MCT group, P=0.016), 10 (P<0.001; Control group vs LCT group, P=0.002; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P=0.001), 20 (P<0.001; Control group vs LCT group, P=0.023; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 30 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 40 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 50 (P<0.001; Control group vs LCT group, P=0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 60 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P=0.037), 70 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 80 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P=0.026), 90 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 100 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 110 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001) and 120 minutes (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001). The LCT/MCT group resulted in a more complete recovery of RPP at 20 (P<0.001), 40 (P<0.001), 60 (P<0.001), 80 (P<0.001), 100 (P<0.001) and 120 minutes (P<0.001) than did the Control group. The LCT group resulted in a more complete recovery of RPP at 40 (P=0.001), 60 (P<0.001), 80 (P<0.001), 100 (P<0.001) and 120 minutes (P<0.001) than the Control group. The LCT/MCT group resulted in a more complete recovery of RPP at 20 (P<0.001), 40 (P<0.001), 60 (P=0.023) and 80 minutes (P=0.021) than the LCT group.
 |
Figure 1 Continued. |
 |
Figure 1 Hemodynamic parameters for all rats that survived to 120 minutes in experiment A. |
Coronary Perfusion Pressure
CPP (coronary perfusion pressure, defined as simultaneously recorded diastolic arterial pressure minus central venous pressure12) in the three groups is graphically demonstrated in Figure 2. Within 120 minutes after the onset of resuscitation, the CPP of LCT and LCT/MCT groups wa higher than that of the Control group (Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001). There was no statistical significance when comparing CPP between the LCT and the LCT/MCT group during the 120-minute observation period. Significant differences were demonstrated among the three groups in CPP at 2 (P=0.001; Control group vs LCT group, P=0.001; Control group vs LCT/MCT group, P=0.039), 4 (P=0.041; LCT group vs LCT/MCT group, P=0.037), 10 (P=0.011; Control group vs LCT group, P=0.033; Control group vs LCT/MCT group, P=0.011; LCT group vs LCT/MCT group, P<0.001), 20 (P<0.001; Control group vs LCT/MCT group, P=0.001; LCT group vs LCT/MCT group, P=0.008), 30 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 40 (P<0.001; Control group vs LCT group, P=0.014; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 50 (P<0.001; Control group vs LCT/MCT group, P<0.001; LCT group vs LCT/MCT group, P<0.001), 60 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 70 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 80 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 90 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 100 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), 110 (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001) and 120 minutes (P<0.001; Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001).
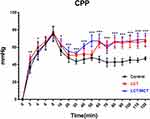 |
Figure 2 CPP vs time for rats that survived to 120 minutes in experiment A. |
Blood Gas Analysis and Hemodynamic Metrics 120 Minutes and 72 Hours After Onset of Resuscitation
Arterial blood gas values and hemodynamic metrics 120 minutes and 72 hours after onset of resuscitation in Experiment A are shown in Table 5. In regard to blood gas values 120 minutes after onset of resuscitation, the LCT group had higher PO2 (Control group vs LCT group, P<0.001), HCO3− (Control group vs LCT group, P=0.027) and BE (Control group vs LCT group, P=0.016), and lower lactate (Control group vs LCT group, P=0.043), when compared with the Control group; the LCT/MCT group had higher pH (Control group vs LCT/MCT group, P=0.001), PO2 (Control group vs LCT/MCT group, P<0.001), HCO3− (Control group vs LCT/MCT group, P<0.001) and BE (Control group vs LCT/MCT group, P<0.001), and lower lactate (Control group vs LCT/MCT group, P=0.002), when compared with the Control group; the LCT/MCT group had higher HCO3− (LCT group vs LCT/MCT group, P=0.020) and BE (LCT group vs LCT/MCT group, P=0.018) compared with the LCT group. In regard to blood gas values 72 hours after onset of resuscitation, the LCT and LCT/MCT groups had higher pH (Control group vs LCT group, P=0.017; Control group vs LCT/MCT group, P=0.032), PO2 (Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P=0.001), HCO3− (Control group vs LCT group, P=0.002; Control group vs LCT/MCT group, P<0.003) and BE (Control group vs LCT group, P=0.029; Control group vs LCT/MCT group, P=0.005), and lower lactate (Control group vs LCT group, P=0.011; Control group vs LCT/MCT group, P=0.021), when compared with the Control group. In regard to hemodynamic metrics, the LCT and LCT/MCT groups had higher HR (for 120 minutes, Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; for 72 hours, Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001), MAP (for 120 minutes, Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; for 72 hours, Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001) and RPP (for 120 minutes, Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001; for 72 hours, Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001) compared with the Control group 120 minutes and 72 hours after onset of resuscitation. No significant differences were observed among the three groups in PCO2 at 120 minutes or 72 hours after onset of resuscitation.
 |
Table 5 Blood Gas Values and Hemodynamic Metrics for Control, LCT and LCT/MCT Groups 120 Minutes and 72 Hours After Onset of Resuscitation in Experiment A |
Neurological Assessment Score
Neurological assessment score in the three groups is graphically demonstrated in Figure 3. The LCT and LCT/MCT groups had lower neurological assessment score (Control group vs LCT group, P=0.001; Control group vs LCT/MCT group, P<0.001) compared with the Control group, and the LCT/MCT group had lower neurological assessment score (LCT group vs LCT/MCT group, P<0.001) compared with the LCT group.
 |
Figure 3 Neurological assessment score for rats that survived to 120 minutes in experiment A. |
Lung Permeability Index and Wet-to-Dry Ratio
Lung permeability index and wet-to-dry ratio in the three groups are graphically demonstrated in Figure 4. The LCT and LCT/MCT groups had decreased lung permeability index compared with the Control group (Control group vs LCT group, P<0.001; Control group vs LCT/MCT group, P<0.001). The LCT and LCT/MCT groups had decreased wet-to-dry ratio compared with the Control group (Control group vs LCT group, P=0.002; Control group vs LCT/MCT group, P=0.033).
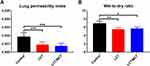 |
Figure 4 (A) Lung permeability index for rats that survived to 120 minutes in experiment A. |
Energy Charge
Energy charge in the three groups is graphically demonstrated in Figure 5. The LCT/MCT group resulted in superior energy charge at 20 (P<0.001) and 40 minutes (P<0.001) compared with the Control group. The LCT group resulted in superior energy charge at 40 (P<0.001) and 60 minutes (P<0.001) compared with the Control group. The LCT/MCT group resulted in superior energy charge at 20 (P<0.001) and 40 minutes (P<0.001), and inferior energy charge at 60 minutes (P<0.001) compared with the LCT group. The comparison of energy charge among the three groups demonstrated no statistical significance at 0, 80, 100 or 120 minutes.
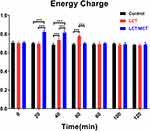 |
Figure 5 Energy charge at 0, 20, 40, 60, 80, 100 and 120 minutes. |
Discussion
This aged rodent model demonstrated that epinephrine supplemented with lipid emulsions resulted in significantly shorter time to ROSC and higher survival rate. The combination of epinephrine and lipid emulsions also exhibited significant improvement in hemodynamic status, pulmonary gas exchange, and neurological performance. In addition, LCT/MCT emulsion provides more benefits over LCT emulsion. These changes might be attributed to ATP generated by lipid emulsions and the different levels of energy supply between them.
Epinephrine is a first-line medication in resuscitation of CA because it can exert an α-adrenergic effect which increases diastolic blood pressure and CPP by peripheral vascular constriction, and a β-adrenergic effect which produces cardiac positive inotropic action.10 However, a high dose of epinephrine is associated with augmented myocardial oxygen consumption, severe metabolic acidosis, pulmonary edema and even unsuccessful resuscitation.11,12 Degenerated myocardium and impaired myocardial microcirculation make senior patients more vulnerable to the side effects exerted by epinephrine.27 Lipid emulsion plays a crucial role in multi-organ protection. Dong et al28 reported that ω-3 fish oil lipid emulsion preconditioning mitigates myocardial damage from aldehyde stress.28 Chen et al29 reported that 15- hydroxyeicosatetraenoic acid promotes angiogenesis and improves neurological function in a mouse model of focal ischemia.29 In a rat model of limb ischemia-reperfusion, LCT lipid emulsion mitigates impaired pulmonary function through attenuation of systemic inflammation.30 At the background of epinephrine-based therapy, lipid emulsion was demonstrated to be effective in improving resuscitation outcomes of lipophilic toxin-induced cardiac arrest.16,17 The main mechanisms behind it include the “lipid sink” hypothesis:19 whereby lipid-soluble compartments in the blood provide a medium for lipophilic toxins to partition in, thus allowing the toxin to be transferred from heart to organs that can store them with high blood flow, and the “lipid flux” theory:20 in which lipids supply the mitochondria with sufficient free fatty acids (FFA) to generate energy the heart needs to restore regular heartbeat under ischemia and hypoxia conditions. Different to local anesthetic-induced cardiac arrest, which is induced by directly interfering with myocardial iron channels and inhibition of myocardial contraction, asphyxia-induced cardiac arrest is associated with myocardial hypoxia and low supply of energy. The resuscitation effect of lipid emulsion on asphyxia-induced cardiac arrest in aged animal has not been reported so far.
In the current study, the combination of lipid emulsions (LCT or LCT/MCT) and epinephrine resulted in a significantly shorter time to ROSC and higher survival rate compared with epinephrine alone. What is more, the LCT and LCT/MCT groups showed much higher RPP and RPP recovery ratio than the Control group during the initial 120 minutes observation period. These results are consistent with the findings of Van de Velde et al31 where infusion of LCT lipid emulsion improved recovery from myocardial ischemia. These authors reconfirm this conclusion in an isolated heart model of myocardial stunning, where contractile function was improved with intralipid (a kind of LCT lipid emulsion) perfusion.32 In addition, Fettiplace et al33 demonstrated that lipid emulsion could exert a direct positive inotropic effect in both intact animal and isolated heart models.33 These data from animal studies concur with clinical evidence showing that lipid emulsion increases blood pressure when applied for total parental nutrition.34,35 All the observed beneficial effects of lipid emulsions here might partially correlate with increased energy supply from FFA oxidation. Fatty acids are the preferred fuel for cardiomyocyte under normal aerobic conditions and cycled from the intracellular triglyceride pool into mitochondria for oxidation and production of high-energy phosphates.36,37 Lipid emulsions' administration could theoretically increase intracellular fatty acid content, and impede inhibition of ATP synthesis resulted from myocardial ischemia in the cardiomyocyte, thereby improving ATP production and cardiac function.20,38 In this study, we found higher energy charge during the first 80 minutes of the observation period in the lipid-containing groups when compared with that in the Control group. This finding is consistent with Van de Velde et al’s31 discovery, in which they reversely verified the existence of exogenous energy supply from lipid emulsion by importing oxfenicine, a carnitine palmitoyltransferase-1 (CPT-1) inhibitor, which could block myocardial FFA metabolism effectively.31 Independent of the cardiac effect, lipid emulsion was also confirmed to moderate blood pressure by increasing vascular resistance.39 The vasoconstriction driven by elevated FFA is possibly due to modifying adrenergic sensitivity40 and interfering with the nitric oxide signal pathway.41 However, we were unable to test the vascular effect in such an intact animal model applied in the current study.
Not all studies testing the response of lipid in animal models of CA have reported positive results. Increased serum and myocardial triglyceride have been shown to suppress ischemic myocardial performance both in animal models42,43 and human subjects.44,45 Under conditions of hypoxia and ischemia, myocardial substrate preference shifts to Beta oxidation of FFA, which consumes approximately 11% larger phosphate-to-oxygen ratios compared to glucose.46 In addition, the increased oxygen consumption of FFA oxidation resulted in a relatively low efficiency in ATP generation, and high FFA loads may activate cycling of FFA in and out of the triglyceride pool, which is futile and energy-consuming.47,48 These authors believe that augmenting inefficient FFA metabolism makes the already low-perfused cardiomyocyte even worse. CPP, calculated as simultaneously recorded diastolic arterial pressure minus central venous pressure, is a crucial index reflecting myocardial perfusion, and is strongly associated with resuscitation outcomes.49 Epinephrine is effective in increasing diastolic arterial pressure via peripheral vasoconstriction, thus elevating CPP. The combination of epinephrine and lipid has shown its superiority to lipid or epinephrine alone in improving CPP and cardiac function, both in in vivo and ex vivo animal models.50 In the current study, lipid emulsions were administered with epinephrine. We speculate that epinephrine rapidly and temporarily elevates the CPP, which increases oxygen supply for cardiomyocyte, thus affording the high oxygen consumption of FFA oxidation, and the sufficient oxidation of FFA provides higher ATP content for cardiac contraction, thereby forming a virtuous cycle. In addition, the bolus of 10 μg/kg epinephrine used in this study was shown to be an appropriate dosage which elevates CPP without increasing oxygen consumption. This may explain the higher CPP and hemodynamic variables in the lipid-ontaining groups, and the positive results of lipid therapy in the current study.
Carnitine is essential for long-chain fatty acids to be transported into the mitochondria.51 Instead, medium-chain fatty acid can flux into the mitochondria via simple diffusion independent of the enzyme.52 What is more, medium-chain fatty acid could be rapidly oxidized without deposition of fat stores and supply energy with higher ketone bodies.21 Accordingly, we found that the LCT/MCT group showed earlier increase of energy charge and higher increase of CPP, thus leading to earlier and better recovery of hemodynamics compared with LCT group. Meanwhile we note that the increase of energy charge in the LCT group lasted for a longer time. The half-life may have accounted for this observation: 33 minutes for LCT and 17 minutes for MCT,53 which indicates that eliminating of the plasma LCT/MCT is quicker than LCT.
We found that the results of blood gas analysis, lung permeability index and wet-to-dry ratio showed a parallel trend to the hemodynamics. We supposed that prolonged time of resuscitation and delayed recovery of effective circulation contribute to pulmonary transudation, deterioration of pulmonary gas exchange and insufficient perfusion of peripheral tissues, which manifested as low PaO2 and metabolic acidosis. Recovery of neurological function is also a main concern after successful resuscitation of CA in aged subjects.27 In this study, the LCT/MCT group showed a lower score compared with the other two groups. Neurocyte depends on glucose uptake as a major source of energy, and is sensitive to hypoxia and ischemia, where a few minutes of complete ischemia would lead to irreversible neuronal apoptosis.54 The rats receiving LCT/MCT emulsion showing earliest recovery of RPP among the three groups, were considered to have the shortest period of hypoxia injury to neurons, thus having relatively minor damage to neurological function. Mechanisms underlying neuroprotection and lung protection reported include interfering with vascular resistance, vasopermeability change, anti-oxidation, and anti-inflammation.55,56 However, we consider that improvement of hemodynamics plays the major role in reservation of neurological and pulmonary function due to their consistency of trends.
Our study has several shortcomings. 1) The regimen of lipid emulsions and epinephrine may not be the optimal. However, the dosage was determined in preliminary experiments for the purpose of optimizing recovery. 2) Large doses of lipids may lead to eg, intravascular hemolysis, pulmonary arterial hypertension, hypersensitivity, deep venous thrombosis, and pancreatitis.,57,58 however we were unable to estimate these negative effects in the current study. 3) General anesthesia and mechanical ventilation may have an effect on the pharmacokinetics of study drugs and the responses of the cardiovascular systems in the setting of asphyxia-induced cardiac arrest, although it is indispensable for both practical and ethical considerations. 4) The current study tentatively tested the effect of lipid emulsions in a rodent model, however massive efforts in both ethical and practical aspects should be made to translate the animal study into clinical human trials.
In conclusion, the supplement of lipid emulsion to epinephrine improves resuscitation outcomes of asphyxia-induced cardiac arrest as it provides a higher survival rate after resuscitation, more energy charge, superior hemodynamic recovery, and more reserved pulmonary and neurological function than did epinephrine alone in an in vivo model of aged rat. Moreover, LCT/MCT emulsion provides benefits over LCT emulsion in epinephrine-based resuscitation. Further studies are needed to investigate the optimal regimen of lipid emulsion and the possible application of lipid resuscitation besides existing treatment in the clinical scenario of asphyxia-induced cardiac arrest in senior populations.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Meyer L, Stubbs B, Fahrenbruch C, et al. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30-year review. Circulation. 2012;126(11):1363–1372. doi:10.1161/CIRCULATIONAHA.111.076810
2. Nishio S, Matsuo K, Shibata T, et al. Changes in the clinicopathological demographics of vulvar cancer in Japan: increasing oldest-old, stage shifting, and decreasing cohort-level survival (†). J Clin Med. 2019;8(12):2081. doi:10.3390/jcm8122081
3. Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S729–67. doi:10.1161/CIRCULATIONAHA.110.970988
4. Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302(20):2222–2229. doi:10.1001/jama.2009.1729
5. Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. 2007;33(2):237–245. doi:10.1007/s00134-006-0326-z
6. Bloom HL, Shukrullah I, Cuellar JR, Lloyd MS, Dudley SC, Zafari AM. Long-term survival after successful inhospital cardiac arrest resuscitation. Am Heart J. 2007;153(5):831–836. doi:10.1016/j.ahj.2007.02.011
7. Yuan J, Yang MC, Wu MJ, Gou YS. Sedative depth on neurological outcomes in a juvenile rat model of cardiopulmonary resuscitation. Med Hypotheses. 2019;132:109233. doi:10.1016/j.mehy.2019.109233
8. Quillinan N, Dingman AL, Deng G, et al. Single dose of 17β-estradiol provides transient neuroprotection in female juvenile mice after cardiac-arrest and cardiopulmonary resuscitation. Neurochem Int. 2019;127:80–86. doi:10.1016/j.neuint.2018.11.013
9. Panchal AR, Berg KM, Hirsch KG, et al. 2019 American Heart Association focused update on advanced cardiovascular life support: use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2019;140(24):e881–e94. doi:10.1161/CIR.0000000000000732
10. Krishnamoorthy V, Hiller DB, Ripper R, et al. Epinephrine induces rapid deterioration in pulmonary oxygen exchange in intact, anesthetized rats: a flow and pulmonary capillary pressure-dependent phenomenon. Anesthesiology. 2012;117(4):745–754. doi:10.1097/ALN.0b013e31826a7da7
11. Mishra S, Chattopadhyay A, Naaz S, Ghosh AK, Das AR, Bandyopadhyay D. Oleic acid ameliorates adrenaline induced dysfunction of rat heart mitochondria by binding with adrenaline: an isothermal titration calorimetry study. Life Sci. 2019;218:96–111. doi:10.1016/j.lfs.2018.12.035
12. Wang QG, Wu C, Xia Y, et al. Epinephrine deteriorates pulmonary gas exchange in a rat model of bupivacaine-induced cardiotoxicity: a threshold dose of epinephrine. Reg Anesth Pain Med. 2017;42(3):342–350. doi:10.1097/AAP.0000000000000541
13. Crowley CP, Salciccioli JD, Kim EY. The association between ACLS guideline deviations and outcomes from in-hospital cardiac arrest. Resuscitation. 2020;153:65–70. doi:10.1016/j.resuscitation.2020.05.042
14. Li Z, Xia Y, Dong X, et al. Lipid resuscitation of bupivacaine toxicity: long-chain triglyceride emulsion provides benefits over long- and medium-chain triglyceride emulsion. Anesthesiology. 2011;115(6):1219–1228. doi:10.1097/ALN.0b013e318238be73
15. Weinberg G. Lipid infusion resuscitation for local anesthetic toxicity: proof of clinical efficacy. Anesthesiology. 2006;105(1):7–8. doi:10.1097/00000542-200607000-00005
16. Tebbutt S, Harvey M, Nicholson T, Cave G. Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med T. 2006;13(2):134–139. doi:10.1197/j.aem.2005.08.016
17. Cave G, Harvey MG, Castle CD. The role of fat emulsion therapy in a rodent model of propranolol toxicity: a preliminary study. J Med Toxicol. 2006;2(1):4–7. doi:10.1007/BF03161005
18. Harvey MG, Cave GR. Intralipid infusion ameliorates propranolol-induced hypotension in rabbits. J Med Toxicol. 2008;4(2):71–76. doi:10.1007/BF03160958
19. Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88(4):1071–1075. doi:10.1097/00000542-199804000-00028
20. Weinberg GL, Palmer JW, VadeBoncouer TR, Zuechner MB, Edelman G, Hoppel CL. Bupivacaine inhibits acylcarnitine exchange in cardiac mitochondria. Anesthesiology. 2000;92(2):523–528. doi:10.1097/00000542-200002000-00036
21. Johnson RC, Young SK, Cotter R, Lin L, Rowe WB. Medium-chain-triglyceride lipid emulsion: metabolism and tissue distribution. Am J Clin Nutr. 1990;52(3):502–508. doi:10.1093/ajcn/52.3.502
22. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi:10.1016/0003-2697(85)90442-7
23. Hendrickx HH, Safar P, Miller A. Delayed recovery of behavior after anesthesia in rats. Resuscitation. 1984;12(3):213–221. doi:10.1016/0300-9572(84)90008-X
24. Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15(6):1032–1039. doi:10.1038/jcbfm.1995.129
25. Noppens RR, Kelm RF, Lindemann R, Engelhard K, Werner C, Kempski O. Effects of a single-dose hypertonic saline hydroxyethyl starch on cerebral blood flow, long-term outcome, neurogenesis, and neuronal survival after cardiac arrest and cardiopulmonary resuscitation in rats*. Crit Care Med. 2012;40(7):2149–2156. doi:10.1097/CCM.0b013e31824e6750
26. Stehr SN, Ziegeler JC, Pexa A, et al. The effects of lipid infusion on myocardial function and bioenergetics in l-bupivacaine toxicity in the isolated rat heart. Anesth Analg. 2007;104(1):186–192. doi:10.1213/01.ane.0000248220.01320.58
27. Ibitoye SE, Rawlinson S, Cavanagh A, Phillips V, Shipway DJH. Frailty status predicts futility of cardiopulmonary resuscitation in older adults. Age Ageing. 2020. doi:10.1093/ageing/afaa104
28. Dong J, Feng X, Zhang J, et al. ω-3 fish oil fat emulsion preconditioning mitigates myocardial oxidative damage in rats through aldehydes stress. Biomed Pharmacother. 2019;118:109198.
29. Chen L, Zhu Y-M, Li Y-N, et al. The 15-LO-1/15-HETE system promotes angiogenesis by upregulating VEGF in ischemic brains. Neurol Res. 2017;39(9):795–802. doi:10.1080/01616412.2017.1321710
30. Xia F, Xia Y, Chen S, et al. Lipid emulsion mitigates impaired pulmonary function induced by limb I/R in rats through attenuation of local cellular injury and the subsequent systemic inflammatory response/inflammation. BMC Anesthesiol. 2017;17(1):83. doi:10.1186/s12871-017-0375-6
31. Van de Velde M, Wouters PF, Rolf N, Van Aken H, Flameng W, Vandermeersch E. Long-chain triglycerides improve recovery from myocardial stunning in conscious dogs. Cardiovasc Res. 1996;32(6):1008–1015. doi:10.1016/S0008-6363(96)00165-4
32. Van de Velde M, DeWolff M, Leather HA, Wouters PF. Effects of lipids on the functional and metabolic recovery from global myocardial stunning in isolated rabbit hearts. Cardiovasc Res. 2000;48(1):129–137. doi:10.1016/S0008-6363(00)00151-6
33. Fettiplace MR, Ripper R, Lis K, et al. Rapid cardiotonic effects of lipid emulsion infusion*. Crit Care Med. 2013;41(8):e156–62. doi:10.1097/CCM.0b013e318287f874
34. Siqueira J, Smiley D, Newton C, et al. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab. 2011;96(10):3207–3216. doi:10.1210/jc.2011-0480
35. Umpierrez GE, Smiley D, Robalino G, et al. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(2):609–614. doi:10.1210/jc.2008-1590
36. Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114(4):717–729. doi:10.1161/CIRCRESAHA.114.301863
37. Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15(6):805–812. doi:10.1016/j.cmet.2012.04.006
38. Weinberg GL, Ripper R, Murphy P, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med. 2006;31(4):296–303. doi:10.1097/00115550-200607000-00004
39. Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100(5):1230–1239. doi:10.1172/JCI119636
40. Stepniakowski KT, Goodfriend TL, Egan BM. Fatty acids enhance vascular α-adrenergic sensitivity. Hypertension. 1995;25(4):774–778. doi:10.1161/01.HYP.25.4.774
41. Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49(7):1231–1238. doi:10.2337/diabetes.49.7.1231
42. Pittner H. [Intrinsic sympathomimetic action and its special features as demonstrated by the beta-1-receptor blocker celiprolol]. Wien Klin Wochenschr Suppl. 1985;162:1–21.
43. Hexeberg S, Hessevik I, Hexeberg E. Intravenous lipid infusion results in myocardial lipid droplet accumulation combined with reduced myocardial performance in heparinized rabbits. Acta Physiol Scand. 1995;153(2):159–168. doi:10.1111/j.1748-1716.1995.tb09847.x
44. Isenstein DA. Cardiopulmonary effects of Intralipid infusion in critically ill patients. Crit Care Med. 1989;17(7):710. doi:10.1097/00003246-198907000-00028
45. Tansey MJ, Opie LH. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet. 1983;322(8347):419–422. doi:10.1016/S0140-6736(83)90388-4
46. Suga H. Ventricular energetics. Physiol Rev. 1990;70(2):247–277. doi:10.1152/physrev.1990.70.2.247
47. Borst P, Loos JA, Christ EJ, Slater EC. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962;62:509–518. doi:10.1016/0006-3002(62)90232-9
48. Myrmel T, Forsdahl K, Larsen TS. Triacylglycerol metabolism in hypoxic, glucose-deprived rat cardiomyocytes. J Mol Cell Cardiol. 1992;24(8):855–868. doi:10.1016/0022-2828(92)91099-Q
49. Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14(1):78–84. doi:10.3109/10903120903349796
50. Liu L, Xia Y, Chen Y, et al. The comparative effects of lipid, epinephrine, and their combination in the reversal of bupivacaine-induced asystole in the isolated rat heart. Anesth Analg. 2012;114(4):886–893. doi:10.1213/ANE.0b013e3182166a0a
51. Mitchell ME. Carnitine metabolism in human subjects. I. Normal metabolism. Am J Clin Nutr. 1978;31(2):293–306. doi:10.1093/ajcn/31.2.293
52. Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. 1. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr. 2005;41(Suppl 2):S1–87. doi:10.1097/01.mpg.0000181841.07090.f4
53. Sailer D, Müller M. Medium chain triglycerides in parenteral nutrition. JPEN J Parenter Enteral Nutr. 1981;5(2):115–119. doi:10.1177/0148607181005002115
54. Wang CH, Chang WT, Huang CH, Tsai MS, Liu SH, Chen WJ. Cerebral blood flow-guided manipulation of arterial blood pressure attenuates hippocampal apoptosis after asphyxia-induced cardiac arrest in rats. J Am Heart Assoc. 2020;9:e016513.
55. Kloska A, Malinowska M, Gabig-Cimińska M, Jakóbkiewicz-Banecka J. Lipids and lipid mediators associated with the risk and pathology of ischemic stroke. Int J Mol Sci. 2020;21(10):3618. doi:10.3390/ijms21103618
56. Cañadas O, Olmeda B, Alonso A, Pérez-Gil J. Lipid-protein and protein-protein interactions in the pulmonary surfactant system and their role in lung homeostasis. Int J Mol Sci. 2020;21(10):3708. doi:10.3390/ijms21103708
57. El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018;11:35–44. doi:10.2147/LRA.S154512
58. Harvey M, Cave G. Lipid emulsion in local anesthetic toxicity. Curr Opin Anaesthesiol. 2017;30(5):632–638. doi:10.1097/ACO.0000000000000498
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
