Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Superficial Acral Fibromyxoma: A Report of Two Cases with CD68 Expression
Authors Huang Q, Li Y , Niu M, Chen G
Received 19 September 2023
Accepted for publication 10 January 2024
Published 17 January 2024 Volume 2024:17 Pages 117—123
DOI https://doi.org/10.2147/CCID.S441055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Qingan Huang,1,* Yuan Li,2,3,* Mu Niu,2 Gaihe Chen4
1Department of General Ward, the Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 2Department of Cosmetic Dermatology, the Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 3Department of Dermatology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 4Department of Dermatology, the Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gaihe Chen, Department of Dermatology, the Fifth People’s Hospital of Hainan Province, No. 8 Longhua Road, Longhua District, Haikou, Hainan, 570100, People’s Republic of China, Email [email protected] Mu Niu, Department of Cosmetic Dermatology, the Fifth People’s Hospital of Hainan Province, No. 8 Longhua Road, Longhua District, Haikou, Hainan, 570100, People’s Republic of China, Email [email protected]
Abstract: Superficial Acral fibroma (SAF), also known as osteofibroma, is a rare fibromatous tumor primarily involving superficial soft tissues. Clinically, SAF typically manifests as a slow-growing, solitary, well-defined nodule or mass. Although these lesions are generally asymptomatic, some cases may present with associated pain, often linked to a history of trauma. SAF lesions commonly exhibit hemispherical, polypoid, or warty growths, with occasional occurrences of ulceration and bleeding.The majority of SAFs express CD34 and CD99, but in the two cases we report, there was diffuse expression of CD34 and focal positive expression of CD68. CD68 positivity suggests a propensity for tumor cells to metastasize to secondary sites. Notably, previously reported cases of single SAF did not display positive CD68 expression, indicating a potential association with other aggressive tumors. However, the current clinical and pathological manifestations do not clarify the diagnosis of additional malignant tumors. Consequently, regular postoperative monitoring of the patient from the aforementioned two cases is essential to detect the presence of other malignant tumors. The significance of CD68-positive expression in this case lies in its potential association with such tumors.
Keywords: superficial acral fibromyxoma, immunohistochemistry, CD34, CD68
Introduction
Superficial Acral Fibromyxoma (SAF), also known as fibromyxoma of the digits and toes, is a rare soft-tissue fibromyxomatous tumor. It was first reported by Fetsch et al in 2001.1 This tumor predominantly affects adult males and typically develops in the subungual and periungual areas of the digits and toes. Clinical manifestations include slow-growing, solitary, well-defined nodules or lumps, most of which are asymptomatic. Some patients may experience pain, and a few cases are associated with a history of trauma. These nodules often present a hemispherical, polypoid, or warty proliferative appearance; some may exhibit ulceration and bleeding. Clinicians should carefully differentiate SAF from other conditions, such as dermatocysts, neurofibromas, dermatocystadenomas, and fibromas.2 Histopathological examination reveals several distinctive features of SAF. The tumor comprises spindle-shaped to star-shaped fibroblast-like cells, irregularly distributed within a mucus-like stroma. In certain areas, the tumor cells may form bundles or loose mats. Abundant ciliated blood vessels are present within the mucus-like stroma, and scattered mast cells can be observed.
The abnormalities in the tumor cells were generally mild, featuring few instances of nuclear schizophrenia and minimal necrosis. Immunohistochemical analysis revealed the expression of waveform proteins, CD34, and CD99 in spindle and astrocytes. Additionally, there was focal expression of CD10,3 while no CD68 expression was observed. In contrast, we present two cases with diffuse expression of CD34 and positive focal expression of CD68.Positive expression of CD68 serves as a potential marker for tumor metastasis. Despite being a benign tumor that grows relatively slowly and rarely metastasizes, Superficial rostral fibroma (SAF) exhibited focal positive staining of CD68 in these two cases we report. This observation suggests a potential association of the tumor with other aggressive tumors. Therefore, regular postoperative follow-up of this patient is essential to monitor for the presence of other malignant tumors.
Materials and Methods
The patient received a diagnosis between November 2022 and March 2023 following an outpatient visit, histopathologic biopsy, and confirmation through immunohistochemistry for the suspected disease. The specimens were surgically excised and fixed in a 10% neutral formalin solution. Subsequently, they were embedded in paraffin, processed using routine procedures, and stained with Hematoxylin and Eosin (HE). The stained specimens were then examined using light microscopy. Immunohistochemistry was conducted using the EnVision method, and the antibodies were acquired from Shanghai Gene Technology Company. All steps of the immunohistochemistry procedure followed the instructions, including establishing both negative and positive controls.
Results
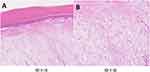
One female and one male, aged 49 and 24, respectively. The location of the masses was as follows: one on the ventral side of the second finger of the left middle finger and the other on the ventral side of the terminal phalanx of the left middle finger (Figure 1A and B). Notably, neither mass involved the nails. One of the lesions caused pain, while the other was asymptomatic. The growth of these masses was gradual, with disease durations of 5 and 2 years, respectively. Both cases exhibited rapid growth within the last year. One of the cases had a history of trauma preceding the onset of the disease, whereas the other had no history of trauma. Upon visual inspection, all lesions appeared as white cutaneous nodules with well-defined boundaries, measuring 6 to 7 mm in size (Figure 2A and B). Histologically, both cases were found to affect the dermis and subcutaneous tissues. Treatment for both cases involved surgical excision, and both have been under follow-up for one year without any signs of recurrence. Currently, they continue to be monitored closely.
 |
Figure 2 (A and B) Intraoperative white skin nodule, 6–7 mm in size, well defined. |
Histological examination at low magnification reveals a well-defined, non-enveloped tumor with the lesion mainly concentrated within the dermis and subcutaneous tissue. Under high magnification, the tumor comprises bundles of short spindle, ovoid, and stellate shapes with more branching small to medium thin-walled blood vessels. Large deposits of mucus-like material and scattered mast cells were seen in the tumor tissue. There was no apparent heterogeneity of the tumor cells (Figure 3A and B). Immunohistochemical studies showed diffuse expression of CD34, focal positivity for CD68, and only scattered positivity for Ki-67 immunostaining in both cases.S-100 protein was negative in both patients (Figure 4A-D).
 |
Figure 4 (A) Immunohistochemistry:Tumor spindle cells with diffuse positive CD34, (B) focal positive CD68, (C) negative S-100 and (D) KI-67 2%. (IHC * 40). |
Discussion
Superficial Acral Fibromyxoma (SAF) is a rare soft tissue tumor that primarily affects the fingers and toes of adults. It was first reported by Fetsch et al in 2001.1 Diagnosis of SAF relies on conventional pathological examination and immunohistochemical phenotyping.4 SAF typically presents as a slow-growing, isolated nodule or mass, and in some cases, it may be associated with pain, often linked to a history of traumatic injury. Occasional instances of ulceration have also been reported.5 SAF is more commonly observed in males than females, with an age of onset typically between 43 and 52 years. While fingers and toes are the most common sites of occurrence, SAF can also manifest in the palms, heels, wrists, and soles of the feet. The duration of the tumor can vary significantly, ranging from 3 months to 30 years. Tumor diameter typically falls within the range of 0.6 to 5.0 cm, with an average size of 1.5 cm.6
In some cases, imaging studies have shown SAF invading bone tissue.7 Due to its non-specific clinical presentation, SAF is susceptible to misdiagnosis as other conditions. In the two cases presented in this paper, one patient was notably younger, at 24 years, than the average age of onset for SAF. The tumor was located at the end of the left middle finger and the abdomen of the second finger of the left middle finger, with a maximum diameter of 0.7 cm. Similar to typical SAF cases, it exhibited slow growth and was associated with pain. Other clinical characteristics observed in these cases align with previously reported findings in the literature.
The pathological features of SAF exhibit several distinctive characteristics: Gross Manifestation: SAF often displays exophytic growth, appearing as rounded, polypoid, or warty isolated lesions. The texture can vary, with some lesions being soft and others having a harder or mucous jelly-like consistency. The cut surface typically appears grayish-white. In some instances, the tumor boundaries are well-defined and may appear nodular or lobulated; in others, they are irregular or unclear.8 Low Magnification Observation: SAF tumors are primarily located in the dermis and may extend into the subcutaneous tissue and, occasionally, bone tissue. They lack a distinct capsule and grow in a pushing or infiltrative pattern. In most instances, the tumors are bordered by the epidermis.9 High Magnification Observation: Tumor cells in SAF are predominantly composed of star-shaped or spindle-shaped fibroblast-like cells. A mucus-like, mucus collagen-like or collagen-like appearance characterizes the interstitium. Abundant slender, thin-walled blood vessels and varying numbers of mast cells are found within the mucus-like areas. Some cases exhibit erythrocyte extravasation. The arrangement of tumor cells in the mesenchyme can be loose and mat-like or disorganized.10 Cellular Characteristics: Most SAF tumors are mildly cellular, with mild to moderate cellular heterogeneity. They display homogeneous and fine nuclear chromatin with inconspicuous nucleoli. The cytoplasm is pale eosinophilic, and nuclear schizophrenia is rare.11 However, a few tumors may show moderate to severe nuclear pleomorphism, increased nuclear schizonts (up to 7/50 high-power fields), and occasional multinucleated giant cells. The prognostic significance of these features is unclear.12 Immunohistochemistry: Immunohistochemistry analysis reveals that SAF tumor cells typically express CD34 and CD99, and in some cases, they may also express EMA, CD10, and Nestin, albeit focally. However, they do not express a-SMA, MSA, desmin, AE1/AE3, S-100, HMB45, apolipoprotein D, Claudin, or MUC4. This lack of expression suggests the absence of myofibroblastic differentiation and the absence of epithelial, melanocytic, or certain other specific markers in SAF tumor cells.13
The cases presented in this report exhibit clinical, morphological, and immunohistochemical characteristics that align with the typical features of SAF. Additionally, an exciting finding in these cases was the focal expression of CD68 in two instances. The interpretation of CD68 expression was based on the percentage of positive cells: cases with 0–5% of positive cells were considered negative, those with 6%-50% were regarded as having focal positive expression, and those with 51%-100% were classified as having diffuse positive expression.14 Notably, previously reported cases of single SAFs did not demonstrate positive expression of CD68. CD68 binds to tissue- and organ-specific lectins or selectins, homing specific macrophage subpopulations to particular sites. In addition, it is expressed in many tumor cell lines and can facilitate their attachment to selectins on vascular endothelium, potentially promoting their spread to secondary sites.15
Therefore, CD68 is regarded as a potential marker for tumor metastasis. Superficial Rostral Fibroma (SAF), typically a slow-growing and rarely metastasizing benign tumor, usually exhibits tumor cells expressing CD34 and CD99. However, the focal positive staining of CD68 observed in the two cases we reported suggests a potential association of the tumor with other invasive tumors. Regular postoperative follow-up for this patient is essential to monitor for the presence of other malignant tumors, emphasizing the significance of positive CD68 expression in this case.
Indeed, distinguishing Superficial Acral Fibromyxoma (SAF) from other diseases with different clinical presentations and pathological features is crucial for accurate diagnosis and appropriate treatment. Some diseases may require differentiation from SAF, key differentiating points, and immunohistochemical markers. Pilomatricoma: Pilomatricoma is a tumor originating from hair follicles, typically located beneath the skin. In contrast to SAF, dermatoid cysts may exhibit hair follicle-like structures and keratinized material on pathological sections. Immunohistochemically, SAF may express markers like CD34 or CD10, distinct from hair follicle structures, whereas dermatoid cysts generally do not.16 Neurofibroma: Neurofibromas are tumors composed of nerve sheath cells and often found near nerve fibers. Unlike SAF, neurofibromas may display the presence of nerve fibers and nerve sheath cells. Immunohistochemically, neurofibromas may show positive expression of the S100 protein, which is typically not observed in SAF.17 Pilomatrix Adenoma: Pilomatrix adenoma originates from hair follicles and can manifest as a complex cystic-glandular structure on pathological sections. In contrast to SAF, Pilomatrix Adenoma may demonstrate specific markers in immunohistochemical staining, such as CK20.18 Fibroma: Fibromas are tumors composed of mature fibroblasts and may exhibit abundant fibrous tissue on pathological sections. Unlike SAF, fibromas generally do not contain mucus-like material. Immunohistochemically, fibromas may not express markers specific to SAF. These distinguishing characteristics and immunohistochemical markers are crucial in accurately identifying and differentiating SAF from similar conditions, aiding in proper diagnosis and subsequent clinical management.19 LG-FMS (Low-Grade Fibromyxoid Sarcoma) typically manifests as a slowly growing deep soft tissue mass, often accompanied by pain or pressure symptoms. These lesions primarily occur in the deep connective tissues of the limbs.20 In contrast, SAF (Superficial Rostral Fibroma) is more common in young and middle-aged males, with lesions predominantly appearing on the fingers, toes, and palms.1 Nail damage is a common associated feature.1 SAF tumors are characterized by their small size, slow growth, asymptomatic nature, and rarity of local recurrence following excision2. Histologically, LG-FMS displays a fibrous and mucinous component, featuring well-arranged spindle cells, collagen fibers, and a mucinous matrix. SAF, on the other hand, exhibits a typical benign cystic structure comprising cystic lumens and blood vessels, with tumor cells commonly expressing CD34 and CD99. Immunohistochemical findings in LG-FMS often reveal strong positive expression of Vimentin, MUC4, and CD99, while expression of S100 protein, Desmin, and Smooth Muscle Actin (SMA) is usually negative.21 In SAF cases, immunohistochemistry typically demonstrates positive expression of CD34 and CD99, while S100 protein may be negative. These distinctive features in both LG-FMS and SAF aid in their differentiation and contribute to accurate diagnostic assessments.3
For the non-invasive detection of Superficial Rostral Fibroma (SAF), the optical cross-sectional imaging technology of reflectance confocal microscopy enables real-time observation of the three-dimensional structure of SAF lesions. This in-depth observation facilitates a comprehensive analysis of the tissue architecture in the lesion area, encompassing voids, cystic cavity structures, and the interface with surrounding normal tissues. Such an approach allows for a more thorough and accurate assessment of the tissue characteristics of SAF, providing robust support for its clinical diagnosis.22,23 Additionally, low-consistency optical coherence tomography (LC-OCT) demonstrates considerable potential in SAF diagnosis. Guided by LC-OCT, we can obtain high-resolution images of SAF lesions and delve into the microstructure of the lesion area. LC-OCT can clearly delineate the hierarchical structure of tissues, including bone tissues, blood vessels, cystic cavities, and other microstructures, thus offering detailed anatomical information of SAF lesions.24 This non-invasive imaging technique presents the opportunity to gain an in-depth understanding of SAF without the need for a biopsy, thereby reducing the necessity for invasive examinations for patients.
In subsequent encounters with relevant cases, employing non-invasive testing methods as an initial step can aid in clarifying the diagnosis without resorting to invasive procedures.
Conclusions
In conclusion, while superficial fibromyxoma of the extremities is generally considered a benign tumor with no documented cases of malignant transformation or metastasis,25 the observation of CD68-positive expression in the two cases we report raises concerns. CD68 positivity implies a tendency for tumor cells to spread to secondary sites. Notably, previously reported cases of single Superficial Rostral Fibroma (SAF) did not exhibit CD68 positive expression, suggesting a potential association of the disease with other aggressive tumors.However, the current clinical and pathological manifestations do not provide clarity on the diagnosis of other malignant tumors. As a result, regular postoperative follow-up for this patient is recommended after the operations in the two cases mentioned above to monitor the potential emergence of other malignant tumors. The significance of CD68-positive expression in this case underscores the need for vigilant monitoring and early detection in such instances. In the cases presented here, the patients were followed up for one year after surgery, and no recurrence of the mass was observed. In conclusion, SAF possesses distinct characteristics regarding its site of occurrence, histological features, and immunophenotype. Recognizing and accurately diagnosing this tumor is essential for effective clinical management and ensuring patient outcomes.
Ethics Statement
The publications of images were included in the patient’s consent for publication of the case. The Hospital Ethics Committees of the Fifth People’s Hospital of Hainan Province approved to publish the case details.
Consent Statement
Informed consent was provided by the patient for publication of the case.
Funding
This project is supported by Hainan Province Clinical Medical Center.
Disclosure
Qingan Huang and Yuan Li are the co-first authors of this study. Gaihe Chen andMu Niu are Co-corresponding authors for this study. The authors have no conflicts of interest to declare.
References
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32(7):704–714. doi:10.1053/hupa.2001.25903
2. Chiheb S, Mouradi M, Hali F. Superficial Acral Fibromyxoma: clinicopathologic Analysis of Five Cases. Skin Appendage Disord. 2021;7(6):468–474. doi:10.1159/000516302
3. Crepaldi BE, Soares RD, Silveira FD, Taira RI, Hirakawa CK, Matsumoto MH. Superficial Acral Fibromyxoma: literature Review. Rev Bras Ortop. 2019;54(5):491–496. doi:10.1016/j.rbo.2017.10.011
4. Campbell V, Machnikowski N, Houghton J, Murphy B, Kerr O. Rare plantar heel presentation of superficial acral fibromyxoma. Dermatol Ther. 2020;33(6):e14517. doi:10.1111/dth.14517
5. Agaimy A, Michal M, Giedl J, Hadravsky L, Michal M. Superficial acral fibromyxoma: clinicopathological, immunohistochemical, and molecular study of 11 cases highlighting frequent Rb1 loss/deletions. Hum Pathol. 2017;60:192–198. doi:10.1016/j.humpath.2016.10.016
6. Braga JM, Bartosch I, Baldaia H, Oliveira I, Canelhas A, Silva Á. Superficial Acral Fibromyxoma: a Rare Soft Tissue Tumor. J Foot Ankle Surg. 2017;56(3):653–655. doi:10.1053/j.jfas.2017.01.003
7. Mejía Rodríguez SA, Maza de Franco A. Dermatoscopy of subungual digital fibromyxoma (superficial acral fibromyxoma). JAAD Case Rep. 2023;33:109–111. doi:10.1016/j.jdcr.2023.01.006
8. Ashby-Richardson H, Rogers GS, Stadecker MJ. Superficial acral fibromyxoma: an overview. Arch Pathol Lab Med. 2011;135(8):1064–1066.
9. DeFroda SF, Starr A, Katarincic JA. Superficial acral fibromyxoma: a case report. J Orthop. 2016;14(1):23–25. doi:10.1016/j.jor.2016.10.018
10. Fanti PA, Dika E, Piraccini BM, Infusino SD, Baraldi C, Misciali C. Superficial acral fibromyxoma: a clinicopathological and immunohistochemical analysis of 12 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. G Ital Dermatol Venereol. 2011;146(4):283–287.
11. Lavery MJ, Vasile C, Bakshi A, Azurdia RM. A Superficial Acral Fibromyxoma Presenting as an Exquisitely Painful Lesion on the Finger. Skinmed. 2021;19(5):399–400.
12. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35(11):1020–1026. doi:10.1111/j.1600-0560.2007.00954.x
13. Pasquinelli G, Foroni L, Papadopoulos F, Dicandia L, Bisceglia M. Superficial acral fibromyxoma: immunohistochemical and ultrastructural analysis of a case, with literature review. Ultrastruct Pathol. 2009;33(6):293–301. doi:10.3109/01913120903359768
14. Stingeni L, Covarelli P, Simonetti S, et al. Superficial acral fibromyxoma with CD10 expression: an underrecognized feature. G Ital Dermatol Venereol. 2017;152(4):404–406. doi:10.23736/S0392-0488.16.05349-9
15. Zhang J, Li S, Liu F, Yang K. Role of CD68 in tumor immunity and prognosis prediction in pan-cancer. Sci Rep. 2022;12(1):7844. doi:10.1038/s41598-022-11503-2
16. Ramya C, Nayak C, Tambe S. Superficial Acral Fibromyxoma. Indian J Dermatol. 2016;61(4):457–459. doi:10.4103/0019-5154.185734
17. Akçay Çelik M, Erdem H, Turhan Haktanır N. Superficial Acral Fibromyxoma: a case report. Int J Surg Case Rep. 2020;77:531–533. doi:10.1016/j.ijscr.2020.11.048
18. Cabete J, Campos S, Lencastre A. Residents’corner June 2015. And next… Adnexa: superficial Acral Fibromyxoma. Eur J Dermatol. 2015;25(3):283–285. doi:10.1684/ejd.2015.2612
19. Sood N, Soin N. Superficial acral fibromyxoma: a cytohistopathological correlation in a recurrent, nonperiungual, acral lesion. Indian J Pathol Microbiol. 2019;62(2):348–350. doi:10.4103/IJPM.IJPM_238_18
20. Tian K, Johnstone K, Lambie D, Frankel A. Low-grade fibromyxoid sarcoma with high-grade features, a rare finding. ANZ J Surg. 2022;92(6):1519–1521. doi:10.1111/ans.17308
21. Oh AJ, Singh P, Pirakitikulr N, Roelofs K, Glasgow BJ, Rootman DB. Low-grade fibromyxoid sarcoma of the orbit. Orbit. 2022;28:1–5. doi:10.1080/01676830.2022.2149820
22. Verzì AE, Broggi G, Micali G, Sorci F, Caltabiano R, Lacarrubba F. Line-field confocal optical coherence tomography of psoriasis, eczema and lichen planus: a case series with histopathological correlation. J Eur Acad Dermatol Venereol. 2022;36(10):1884–1889. doi:10.1111/jdv.18293
23. Lacarrubba F, Verzì AE, Caltabiano R, Broggi G, Di Natale A, Micali G. Discoid lupus erythematosus: reflectance confocal microscopy features correlate with horizontal histopathological sections. Skin Res Technol. 2019;25(2):242–244. doi:10.1111/srt.12636
24. Verzì AE, Lacarrubba F, Caltabiano R, Broggi G, Musumeci ML, Micali G. Reflectance Confocal Microscopy Features of Plaque Psoriasis Overlap With Horizontal Histopathological Sections: a Case Series. Am J Dermatopathol. 2019;41(5):355–357. doi:10.1097/DAD.0000000000001297
25. An MK, Hong EH, Cho EB, Park EJ, Kim KH, Kim KJ. A Case of Solitary Fibrous Tumor of Subungual Region. Ann Dermatol. 2020;32(2):146–150. doi:10.5021/ad.2020.32.2.146
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.