Back to Journals » Drug Design, Development and Therapy » Volume 14
Super Activated Platelet Lysate, a Novel Autologous Platelet Lysate, Regulates the Expression of Inflammasome and Cytokine in the Experimental Periodontitis in Rats
Authors Zhang Y, Zhuang D, Zhang Y, Lu H, Zhang H, Li T, Bi L
Received 3 November 2020
Accepted for publication 7 December 2020
Published 15 December 2020 Volume 2020:14 Pages 5535—5543
DOI https://doi.org/10.2147/DDDT.S289753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yi Zhang,1 Deshu Zhuang,1,2 Yi Zhang,3 Huiying Lu,3 Haijiao Zhang,3 Tingting Li,3 Liangjia Bi1
1Department of Stomatology, The Fourth Affiliated Hospital, Harbin Medical University, Harbin 150001, People’s Republic of China; 2Faculty of Dentistry, Department of Oral Biological and Medical Sciences, University of British Columbia, Vancouver BC V6T 1Z3, Canada; 3National and Local Joint Stem Cell Research & Engineering Center for Aging Diseases, Tian Qing Stem Cell Co., Ltd., Harbin 150028, People’s Republic of China
Correspondence: Liangjia Bi
Department of Stomatology, The Fourth Affiliated Hospital, Harbin Medical University, Harbin 150001, People’s Republic of China
Tel/Fax +86-451-82576565
Email [email protected]
Purpose: The aim of the present study was to evaluate the expression of inflammasome and cytokine on experimental periodontitis with super activated platelet lysate (SPL) in rats.
Methods: Periodontitis was induced by submerging cotton ligatures on the right side of the maxillary second molar in 36 Wistar rats. The rats were divided into 3 groups randomly: the rats received no treatment (control group); local injection with sterile saline (ligature+saline group) and local injection with SPL (ligature+SPL group). After treatments, the alveolar bone level and inflammation of periodontal tissue were evaluated by micro-computed tomography (micro-CT) scanning and histological examination, respectively. The expression of inflammasome and cytokine was evaluated by real-time quantitative polymerase chain reaction (RT-qPCR) assay.
Results: Compared with the control group, the bone loss significantly increased by 0.9 mm in the ligature+saline group and 0.4 mm in the ligature+SPL group (P < 0.001). 0.5 mm reduction in the bone loss was founded in the ligature+SPL group compared with the ligature+saline group (P < 0.001). The gene expression of CCL2, CXCL2, IL-6, IL-18, IL-1α, IL-1β, CXCL10, CXCL16, CCL5 was significantly reduced in the ligature+SPL group compared with the ligature+saline group (P < 0.05). Compared with the ligature+saline group, the expression for inflammasome NLRP3, AIM2, CASP1 was both downregulated in the ligature+SPL group (P < 0.05).
Conclusion: Our present study demonstrated local injection of SPL regulated the expression of inflammasome and cytokine and had a visible effect of relieving inflammation in the experimental periodontitis in rats.
Keywords: periodontitis, super activated platelet lysate, inflammasome, cytokine
Introduction
Periodontal disease is a complex chronic inflammatory disease, which is triggered by periodontal pathogens in subgingival periodontal tissue.1,2 Microorganism virulence factors initiate a host response, which stimulates the generation of inflammatory cells and cytokines in the gingiva.3 Dysregulation of adaptive and innate immune systems in periodontal tissue may induce the occurrence of periodontal disease.4 Lipopolysaccharide in periodontal pathogens activates immune cells and stimulates the toll-like receptor pathways, which lead to the expression of cytokines such as interleukin-1α (IL-1α), IL-1β, IL-6, CXC chemokine ligand 10 (CXCL10).5 Inflammasome is a large multiprotein complex located in the cytoplasm which can promote the maturation of pro-inflammatory cytokines such as IL-1β and IL-18. It plays an important role in the initiation of periodontal disease and the relative systemic diseases.6 It is well recognized that nucleotide-binding domain-like receptor protein inflammasome3 (NLRP3) and absent in melanoma 2 (AIM2) are crucial factors in the defending of invading pathogens in periodontal disease.7
Platelet lysate (PL) is one of the autologous hemoderivative materials which is obtained by the cryogenic disruption of platelets.8 It contains many growth factors such as platelet derived growth factor (PDGF), fibroblast growth factor (FGF), insulin like growth factor (IGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and so on.9 Tan et al demonstrated that local injection of PL could effectively treat lateral epicondylitis.10 Tenci et al found that PL along with pectin/chitosan exhibits had a positive effect in chronic skin ulcer treatment in rats.11 Del Fante et al demonstrated that PL could be used as an effective ingredient to treat oral mucositis.12 However, to the best of our knowledge, PL in treating experimental periodontitis in vivo has not been reported up to date.
Scaling and root planing (SRP) is used as a basic application in the traditional treatment of periodontal disease.13 The complementary treatment for SRP is antibiotics, such as metronidazole, cephalosporin, and minocycline hydrochloride.14 Although traditional treatment can obtain a satisfactory effect, the problem of bacteria resistance during the therapeutic process remains to be solved.15 Therefore, to explore an anti-inflammatory and non-resistant therapeutic method of periodontitis is imminent. Base on the characteristic of autologous platelets, PL has many advantages such as no drug resistance, easy availability, and no side effect.16
In the present study, a novel PL was applied in the treatment of experimental periodontitis in rats, which was named super activated platelet lysate (SPL). Based on original PL, CaCl2 and low molecular weight sodium heparin were added to activate platelets and release growth factors during the preparation process. The purpose of this study is to evaluate the expression of the inflammasome and cytokine in the experimental periodontitis in rats after using local injection of SPL treatment. We attempt to provide a novel experimental basis for future clinical application of SPL in the treatment of periodontal disease in humans.
Materials and Methods
Animal
Forty-eight female Wistar rats were used in the present study. All rats weighted 200–250 g and at an average of 8 weeks’ age group. There were 12 rats for the preparation of SPL and 36 rats for periodontal therapy. The rats were obtained from the animal experimental center of the Second Affiliated Hospital of Harbin Medical University (Harbin, People’s Republic of China). They were kept in polyethylene plastic cages and were fed with free access to water and rat feedstuff. All rats had been kept to acclimatize to the housing conditions for 5 days before the operative procedures. Animal care and experimental procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications Number 8023, revised 1978). The protocol was approved by the Experimental Animal Ethics Committee of Harbin Medical University.
Preparation of SPL
SPL was provided by Tian Qing Stem Cell Co., Ltd. (Harbin, People’s Republic of China). A total of 60 mL blood was harvested from twelve healthy Wistar rats through cardiac punctures under anesthesia. The blood was anticoagulated with 3.8% sodium citrate at a blood/citrate ratio of 9:1. The blood sample was gently agitated to thoroughly mix the sodium citrate with the blood. Subsequently, the sample was centrifuged at 1000 rpm for 10 min at room temperature. The plasma and buffy coat layer were then transferred to another centrifuge tube and were centrifuged with ultra-filtration centrifuge tubes at 4000 rpm for 10 min. Platelet rich plasma (PRP) was obtained after discarding 3/4 of the upper layer of plasma. To activate platelets and release growth factors, PRP was incubated with CaCl2 and low molecular weight sodium heparin for 1–2 h at 37 °C in a CO2 incubator (the final concentrations of CaCl2 and heparin were 20 mM and 2 U/mL, respectively), and then the mixture was frozen at −80 °C for 1–2 h. The mixture was finally thawed at 37 °C for 5 min and cooled at 4 °C for 1 h to allow fibrin coalescence. After centrifugation at 2000 rpm and 4 °C for 10 min, the supernatant was filtered through a 0.22 μm filter to obtain SPL. Approximately 6 mL of SPL was obtained from this preparation.
Establishment of Periodontitis Model in Rats
The rats were anesthetized by administering ketamine (80 mg/kg) and dexmedetomidine (0.6 mg/kg) via intraperitoneal injection. The right side of the maxillary second molar of each rat was selected to obtain cotton ligatures (3–0). The above process was operated by the same operator (Yi Zhang). After 4 weeks, ligatures were removed from rats.
Periodontal Therapy
The 36 rats were randomly divided into 3 groups (12 rats in each group): the rats received no treatment (control group); local injection with sterile saline (ligature+saline group); local injection with SPL (ligature+SPL group). The rats in the ligature+saline group and ligature+SPL group were locally injected into the alveolar bone area, 2 mm below the gingival margin, between the right side of the first and second maxillary molars, using a micro syringe. Each rat was given 50 μL saline or SPL injection treatment every other day, totaling 8 times, and last for 2 weeks (As shown in Figure 1). The animals were sacrificed after the treatment. Their jaws were collected and used for bone loss assessment, histological assessment, and gene expression studies (see below).
Micro-Computed Tomography (Micro-CT) Scanning
Six rats in each group were used to assess alveolar bone loss. The alveolar bone resorption of all the samples was evaluated using micro-CT (Sedecal, SuperArgus, Spain). Maxilla samples were fixed with 4% paraformaldehyde for micro-CT scanning. 3D images were constructed using SuperArgus visualization and analysis (Version 5.0, Sedecal, Spain). Alveolar bone resorption was assessed from coronal planes micro-CT image by calculating the linear distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) in both mesial and distal sites of the maxillary second molar. The image was analyzed by ImageJ (Version 1.51r; National Institute of Health; USA).
Histology
Another 6 rats in each group were implemented for the histological examination. Samples were fixed in 4% paraformaldehyde for 48 h and then transferred into 10% ethylenediaminetetraacetic acid solution for 40 days. Serial 5 μm paraffin sections were made in a mesiodistal direction and stained with hematoxylin and eosin.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Assay
Gingival tissue was removed from the rats before the micro-CT scanning. 15c surgical blade (swann-morton; UK) and tissue tweezers (Hu-Friedy; USA) were used to cut and collected the gingival tissue in the lingual aspect area around the right maxillary second molars. Total mRNA was isolated using TRIzol reagent method (Invitrogen; USA) as described by the manufacturers. According to the manufacturer’s instructions, 1 µg of total mRNA was reverse-transcribed using First Strand cDNA Synthesis Kit ReverTra Ace -α- (TOYOBO; JAPAN). RT-qPCR was performed via LightCycler®480 system (Roche; USA). Each sample was analyzed in three replicates. β-actin was used as the reference gene. Reference genes and primers sequences are listed in Table 1. Reactions were performed with 20 μL of a mixture containing 10 μL of TB Green Fast qPCR Mix (2×; Takara; Japan), 8 μL of sterile distilled water, 1 μL of each gene-specific primer, and 1 μL of the cDNA template. Real-time PCR amplification was performed by the CFX96 system (Bio-Rad; USA). Data were analyzed using the comparative Ct method (CFX Manager Software Version 2.1; Bio-Rad; USA).
 |
Table 1 Primer List |
Statistical Analysis
Using one‑way ANOVA followed by Tukey’s post‑hoc test to analyze the differences between multiple groups. Statistical analyses were performed by Graphpad Prism 6 (San Diego; California; USA). P<0.05 was considered to indicate a statistically significant.
Results
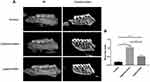
Compared with the control group, alveolar bone in both mesial and distal sites of the maxillary second molar were demonstrated resorption in other groups, especially in the ligature+saline group (Figure 2A). In the coronal plane, the distal sites of bone loss extended to the apical of the roots, and the mesial sites of bone loss extended to half of the roots of the second molar in the ligature+saline group. Although bone resorption was founded in the ligature+SPL group, the alveolar bone lost half of the roots in the distal sites and one-third of the roots in the mesial sites of the second molar. As shown in Figure 2B, compared with the control group, the bone loss significantly increased by 0.9 mm in the ligature+saline group and 0.4 mm in the ligature+SPL group (P < 0.001). 0.5 mm reduction in bone loss was founded in the ligature+SPL group compared with the ligature+saline group (P < 0.001).
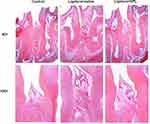
The homologous results could also be found in the histological section (Figure 3). ABC in the mesial sites of the second molar located in the apical third of the roots in the ligature+saline group. And an intensive inflammatory infiltrate could be found in both gingival connective tissue and epithelium. However, in the ligature+SPL group, the alveolar bone lost one-third of the roots in the mesial sites of the second molar and the thick bone trabeculae organization was founded in bone tissue. The periodontal ligament was basically intact and the fiber connective tissue was arranged regularly. The proliferation of the epithelial spike process was not obvious and a faint inflammatory infiltrate throughout the periodontal ligament was shown in both epithelium and gingival connective tissue.
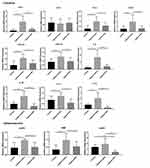
To search for further differences between different groups in the inflammatory response, the gene expression of periodontitis associated cytokine markers were investigated. As shown in Figure 4, the expression of CC chemokine ligand 2 (CCL2), CXCL2, IL-6, IL-18, IL-1α, IL-1β was significantly reduced in the ligature+SPL group compared with the ligature+saline group (P < 0.001). The level of CXCL10, CXCL16 was also reduced in the ligature+SPL group compared with the ligature+saline group (P < 0.01). The expression of CCL5 in the ligature+SPL group had statistically significant compared with the ligature+saline group (P < 0.05). For the above-mentioned cytokines, compared with the control group, the expression of cytokines was both elevated in the ligature+saline group (P < 0.05). However, the level of expression of CCL3 did not significantly differ in different groups (P > 0.05). Interestingly, for cytokines IL-18 and Il-1β, the expression level is even less in the ligature+SPL group than that of the control group.
Compared with the ligature+saline group, the expression for inflammasome AIM2, was downregulated in the ligature+SPL group (P < 0.05). Expression levels of NLRP3 were also decreased in the ligature+SPL group compared with the ligature+saline group (P < 0.01). In the ligature+SPL group, the expression of caspases1 (CASP1) was significantly reduced compared with the ligature+saline group (P < 0.001). Moreover, the level of CASP1 in the ligature+SPL group was significantly decreased compared with the control group (P < 0.001).
Discussion
Since 1990, the global age-standardized incidence and prevalence of periodontal disease have remained stable.17 And in 2010, severe periodontitis affecting 10.8% of people, nearly 743 million worldwide, and periodontitis was the sixth-most prevalent health disease.18 PL was previously used in relieving inflammation because of multi-growth factors ingredient.19–21 In the present study, according to the micro-CT measurement, the experimental periodontitis was successfully established in rats after ligation. The results of the linear distance from the CEJ to the ABC demonstrated that local injection of SPL could have the potential to improve the decrease in alveolar crest height in experimental periodontitis in rats. Consistent with this finding, histology observation also indicated that after the treatment of SPL, inflammation was alleviated no matter in gingival connective tissue or epithelium. The observation of thick bone trabeculae and the intact periodontal ligament in the ligature+SPL group also indicated the efficacy of SPL local injection treatment. The relief of inflammation may due to a large number of growth factors in SPL.
Platelets are small blood cells that play an important role in both thrombosis and hemostasis.22 Autologous hemoderivative materials contain human serum, PRP, and PL, etc.9 To obtain growth factors from the platelet, a physical method which is freezing-thawing the PRP can be used.23 Alternatively, growth factors can be obtained by disrupting the platelets using ultrasound.24 Growth factors can be also released by physiological external stimuli as thrombin, collagen, adenosine diphosphate, epinephrine, tri-n-butyl phosphate, and CaCl2, etc.25 In this study, CaCl2 was used as the external stimulus to activate the platelets. However, thrombocyte-derived factors, fibrinogen, and clotting factors may also be produced during the SPL production process which may lead to gel formation.26 Low molecular weight sodium heparin was added as an anticoagulant. Platelets contain growth factors that are released from α-granules after the destruction by physical or physiological stimulation.27
In periodontal disease, pathobionts invaded host periodontal cells which led to the secretion of cytokines such as CCL2, CCL5, CXCL2, CXCL10, etc.28 The main role of pro-inflammatory cytokine was to act as a chemotactic agent for leukocytes, attracted monocytes, neutrophils, and other effector cells from the blood to the site of infected tissue.29 In the meanwhile, the recognized pro-inflammatory cytokines from IL-1 and IL-6 families were secreted by host immune cells and recruited a subset of specific immune cells which caused direct tissue damage.30 Inflammasome, especially NLRP3 and AIM2 are particularly characterized for their role in the recognition of bacteria.6 They also played a critical part in initiating innate immune responses by acting as a platform to activate inflammatory caspases.28 Among inflammasome, CASP1 initiated the innate immune response by specifically cleaving pro-IL-1β and pro-IL-18, and then mediated their maturation and release.6 In the present study, the expression of IL-1 family (IL-1α, IL-1β, IL-18), IL-6 family (IL-6), CCL2, CCL5, CXCL2, CXCL10, CXCL16, NLRP3, AIM2, CASP1 were elevated in the experimental periodontitis in rats compared to the control group (P < 0.05). When using SPL as a treatment of periodontitis, the expression of the above pro-inflammatory cytokines and inflammasomes were reduced significantly compared to the ligature+saline group (P < 0.05). These may be due to the ability in promoting tissue recovery and the healing of growth factors. As several studies mentioned, FGF-2 and PDGF showed a significant effect on periodontal regeneration.31 Furfaro et al found that EGF did not improve periodontal or alveolar bone healing, but it could improve vascularisation.32 Giannobile et al reported that the concurrent use of IGF-I and PDGF increased new attachment formation of periodontal tissue in both the primate and canine models.33
Our study may provide useful information for the local injection of SPL in experimental periodontal disease in rats. However, further relative studies on the regulation mechanism of many inflammatory cytokines after SPL treatment and the effect of clinical trials by SPL are greatly needed. Our present study demonstrates that local injection of SPL regulates the expression of inflammasome and cytokine and has a visible effect of relieving inflammation in the experimental periodontitis in rats. These encouraging results indicated that SPL may provide a novel promising treatment against periodontal disease in the future.
Ethics Approval Statement
Animal care and experimental procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications Number 8023, revised 1978). The protocol was approved by the Experimental Animal Ethics Committee of Harbin Medical University.
Acknowledgments
This work was supported by the Natural Science Foundation, Heilongjiang Province of China [grant number H2017022]; Natural Science Foundation of China [grant number 81670994].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75(1):7–23.
2. Bui FQ, Almeida-da-Silva CLC, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35. doi:10.1016/j.bj.2018.12.001
3. Okano T, Ashida H, Suzuki S, Shoji M, Nakayama K, Suzuki T. Porphyromonas gingivalis triggers NLRP3-mediated inflammasome activation in macrophages in a bacterial gingipains-independent manner. Eur J Immunol. 2018;48(12):1965–1974. doi:10.1002/eji.201847658
4. Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394. doi:10.3390/ijms20143394
5. Diomede F, Zingariello M, Cavalcanti MFXB, et al. MyD88/ERK/NFkB pathways and pro-inflammatory cytokines release in periodontal ligament stem cells stimulated by Porphyromonas gingivalis. Eur J Histochem. 2017;61(2):2791.
6. Olsen I, Yilmaz Ö. Modulation of inflammasome activity by Porphyromonas gingivalis in periodontitis and associated systemic diseases. J Oral Microbiol. 2016;8:30385. doi:10.3402/jom.v8.30385
7. Shibata K. Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases. Mol Oral Microbiol. 2018;33(3):203–211. doi:10.1111/omi.12217
8. Babo PS, Cai X, Plachokova AS, et al. Evaluation of a platelet lysate bilayered system for periodontal regeneration in a rat intrabony three-wall periodontal defect. J Tissue Eng Regen Med. 2018;12(2):e1277–e1288.
9. Zamani M, Yaghoubi Y, Movassaghpour A, et al. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: are we ready for clinical use? J Cell Physiol. 2019;234(10):17172–17186. doi:10.1002/jcp.28496
10. Tan X, Ju H, Yan W, et al. Autologous platelet lysate local injections for the treatment of refractory lateral epicondylitis. J Orthop Surg Res. 2016;11:17. doi:10.1186/s13018-016-0349-2
11. Tenci M, Rossi S, Bonferoni MC, et al. Particulate systems based on pectin/chitosan association for the delivery of manuka honey components and platelet lysate in chronic skin ulcers. Int J Pharm. 2016;509(1–2):59–70. doi:10.1016/j.ijpharm.2016.05.035
12. Del Fante C, Perotti C, Bonferoni MC, et al. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS PharmSciTech. 2011;12(3):893–899. doi:10.1208/s12249-011-9649-3
13. Zhuang D, Han J, Bi L, et al. Sonodynamic effect of hematoporphyrin monomethyl ether on ligature-induced periodontitis in rats. Drug Des Devel Ther. 2015;9:2545–2551. doi:10.2147/DDDT.S82347
14. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038.
15. Zhang Y, Zhang H, Zhuang D, Bi L, Hu Z, Cao W. Hematoporphyrin monomethyl ether mediated sonodynamic antimicrobial chemotherapy on porphyromonas gingivalis in vitro. Microb Pathog. 2020;144:104192. doi:10.1016/j.micpath.2020.104192
16. Astori G, Amati E, Bambi F, et al. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7(1):93. doi:10.1186/s13287-016-0352-x
17. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. doi:10.1177/0022034514552491
18. Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–260. doi:10.1016/S0140-6736(19)31146-8
19. Malgarim Cordenonsi L, Faccendini A, Rossi S, et al. Platelet lysate loaded electrospun scaffolds: effect of nanofiber types on wound healing. Eur J Pharm Biopharm. 2019;142:247–257. doi:10.1016/j.ejpb.2019.06.030
20. Bonferoni MC, Sandri G, Rossi S, et al. Association of alpha tocopherol and Ag sulfadiazine chitosan oleate nanocarriers in bioactive dressings supporting platelet lysate application to skin wounds. Mar Drugs. 2018;16(2):56. doi:10.3390/md16020056
21. Ito R, Morimoto N, Pham LH, Taira T, Kawai K, Suzuki S. Efficacy of the controlled release of concentrated platelet lysate from a collagen/gelatin scaffold for dermis-like tissue regeneration. Tissue Eng Part A. 2013;19(11–12):1398–1405. doi:10.1089/ten.tea.2012.0375
22. Thon JN, Italiano JE. Platelets: production, morphology and ultrastructure. Handb Exp Pharmacol. 2012;210:3–22.
23. Bernardi M, Agostini F, Chieregato K, et al. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J Transl Med. 2017;15(1):90. doi:10.1186/s12967-017-1185-9
24. Hara Y, Steiner M, Baldini MG. Platelets as a source of growth-promoting factor(s) for tumor cells. Cancer Res. 1980;40(4):1212–1216.
25. Bieback K. Platelet lysate as a replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother. 2013;40(5):326–335. doi:10.1159/000354061
26. Laner-Plamberger S, Lener T, Schmid D, et al. Mechanical fibrinogen-depletion supports heparin-free mesenchymal stem cell propagation in human platelet lysate. J Transl Med. 2015;13:354. doi:10.1186/s12967-015-0717-4
27. Badran Z, Abdallah MN, Torres J, Tamimi F. Platelet concentrates for bone regeneration: current evidence and future challenges. Platelets. 2018;29(2):105–112. doi:10.1080/09537104.2017.1327656
28. Bi J, Dai J, Koivisto L, et al. Inflammasome and cytokine expression profiling in experimental periodontitis in the integrin β6 null mouse. Cytokine. 2019;114:135–142. doi:10.1016/j.cyto.2018.11.011
29. Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11(3):30.
30. Araya AV, Pavez V, Perez C, et al. Ex vivo lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2 secretion in whole blood from Type 1 diabetes mellitus patients with or without aggressive periodontitis. Eur Cytokine Netw. 2003;14(3):128–133.
31. Li F, Yu F, Xu X, et al. Evaluation of recombinant human FGF-2 and PDGF-BB in periodontal regeneration: a systematic review and meta-analysis. Sci Rep. 2017;7(1):65. doi:10.1038/s41598-017-00113-y
32. Furfaro F, Ang ES, Lareu RR, Murray K, Goonewardene M. A histological and micro-CT investigation in to the effect of NGF and EGF on the periodontal, alveolar bone, root and pulpal healing of replanted molars in a rat model - a pilot study. Prog Orthod. 2014;15(1):2. doi:10.1186/2196-1042-15-2
33. Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol. 1994;65(12):1158–1168. doi:10.1902/jop.1994.65.12.1158
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.