Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects
Authors Aravinthan A, Govarthanan M , Selvam K, Praburaman L, Selvankumar T, Balamurugan R, Kamala-Kannan S, Kim JH, Koildhasan M
Received 11 December 2014
Accepted for publication 5 February 2015
Published 11 March 2015 Volume 2015:10(1) Pages 1977—1983
DOI https://doi.org/10.2147/IJN.S79106
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Adithan Aravinthan,1,* Muthusamy Govarthanan,2,3,* Kandasamy Selvam,4 Loganathan Praburaman,2,3 Thangasamy Selvankumar,3 Rangachari Balamurugan,1 Seralathan Kamala-Kannan,2 Jong-Hoon Kim1
1College of Veterinary Medicine, Biosafety Research Institute, Chonbuk National University, Jeonju, South Korea; 2Division of Biotechnology, Advanced Institute of Environment and Bioscience, College of Environmental and Bioresource Sciences, Chonbuk National University, Iksan, South Korea; 3PG and Research Department of Biotechnology, Mahendra Arts and Science College, Kalippatti, Namakkal, Tamil Nadu, India; 4Centre for Biotechnology, Muthayammal College of Arts and Science, Rasipuram, Namakkal, Tamil Nadu, India
*These authors contributed equally to this work
Abstract: A rapid, green phytosynthesis of silver nanoparticles (AgNPs) using the aqueous extract of Helianthus tuberosus (sunroot tuber) was reported in this study. The morphology of the AgNPs was determined by transmission electron microscopy (TEM). Scanning electron microscopy–energy-dispersive spectroscopy (SEM–EDS) and X-ray powder diffraction (XRD) analysis confirmed the presence of AgNPs. Fourier transform infrared spectroscopy (FTIR) analysis revealed that biomolecules in the tuber extract were involved in the reduction and capping of AgNPs. The energy-dispersive spectroscopy (EDS) analysis of the AgNPs, using an energy range of 2–4 keV, confirmed the presence of elemental silver without any contamination. Further, the synthesized AgNPs were evaluated against phytopathogens such as Ralstonia solanacearum and Xanthomonas axonopodis. The AgNPs (1–4 mM) extensively reduced the growth rate of the phytopathogens. In addition, the cytotoxic effect of the synthesized AgNPs was analyzed using rat splenocytes. The cell viability was decreased according to the increasing concentration of AgNPs and 67% of cell death was observed at 100 µg/mL.
Keywords: cytotoxicity, Helianthus tuberosus, nanobiotechnology, phytosynthesis, splenocytes
Introduction
Nanobiotechnology is a promising interdisciplinary field of biotechnology and nanoscience that offers novel nanoscale materials with interesting applications in different scientific fields such as medicine, biotechnology, chemistry, physics, and material sciences.1,2 Metallic nanoparticles are attractive due to their potential applications in novel technologies.3 Among the metallic nanoparticles, silver nanoparticles (AgNPs) have attracted the attention of researchers in the field of science and technology due to their vast biological applications. Several studies have reported the synthesis of AgNPs by physicochemical methods.4,5 The chemical synthesis of AgNPs may lead to the presence of some toxic chemicals on the surface that may have adverse effects in its application. Also, the chemical used in the synthesis may pollute the environment.6 Hence, there is an urgent need to develop eco-friendly biological methods of AgNPs synthesis instead of using toxic chemicals.
Nowadays, biological synthesis of AgNPs has been proposed as an emerging technology, and offers several advantages compared to conventional methods. Several studies reported AgNPs synthesis using biological materials, such as microorganisms, plant extracts, milk, and panchakavya.7–10 Phytosynthesis of AgNPs using plant materials has an edge over microbial mediated synthesis owing to their immediate and large-scale production.11,12 Thus, considerable attention is given to exploit the synthesis of AgNPs using plant-based products. Several studies reported on the synthesis of AgNPs using various plant extracts.13–15 It has been established that proteins present in the plant extracts were involved in the reduction of Ag+ ions to nanocrystallites. Helianthus tuberosus L., a perennial herb, is a species of sunflower native to eastern North America and widely cultivated across the temperate zone for its edible tuber. Extracts of H. tuberosus L. tubers are aperient, cholagogue, and diuretic and have long been used in folk medicine to treat stomach problems, diabetes, and rheumatism.16,17 However, to our knowledge, the sunroot (H. tuberosus L.) tuber extract has never been used for the synthesis of AgNPs.
In vitro cytotoxicity study is an important assay to evaluate the mechanisms of toxicity caused by nanoparticles. AgNP-induced toxicity is related with mitochondrial damage, oxidative stress, DNA damage, and induction of apoptopsis.18 Previous studies reported the cytotoxicity of AgNPs against NIH 3T3 fibroblast cells, HeLa cells, human glioblastoma cells, and human breast cancer cells (MCF-7).19–22 However, to our knowledge, cytotoxicity of AgNPs in rat splenocytes have never been explored.
Plant disease control is an important requirement for agriculture in the 21st century. Microorganisms are associated with several devastating diseases in economically important crops worldwide. Phytopathogenic bacteria cause enormous problems in agriculture, resulting in severe economic losses, since plants are the main nutrient sources of these pathogens.23 Ralstonia solanacearum and Xanthomonas axonopodis are the most extensively studied phytopathogens in potato (Solanum tuberosum), tobacco (Nicotiana tabacum), and tomato (Lycopersicon esculentum) plant systems. The increasing population of these phytopathogens causes degradation of the occluded xylem vessels and the death of the plants. Hence, the synthesis of AgNPs and their antibacterial properties are emerging as fields of great interest among researchers. Hence, the objectives of the present study were (i) to synthesize AgNPs using H. tuberosus tuber extract, (ii) to characterize the synthesized AgNPs, and (iii) to assess the cytotoxicity of AgNPs synthesis against freshly isolated rat splenocytes, and (iv) to evaluate the bactericidal activities of the synthesized AgNPs.
Materials and methods
Plant material
The dried tuber of H. tuberosus was purchased from a local shop in Iksan, South Korea. One kilogram of tuber powder was soaked in 2.5 L methanol for 78 hours with occasional stirring. The solvent was removed by using Rotovac below 70°C. The solvent-free aqueous extract was used for the synthesis of AgNPs.
Synthesis of AgNPs
Silver nitrate (AgNO3) was purchased from Sigma-Aldrich (St Louis, MO, USA) and the synthesis of AgNPs was carried out according to Lee et al.8 Briefly, 4 mL of the extract was mixed with 96 mL of 1 mM AgNO3 solution and the resulting greenish white mixture was incubated for 8 hours in a rotary shaker (200 rpm) at 26°C. The reduction of Ag+ ions to Ag nanocrystals was monitored by the change in the color of the reaction mixture from greenish white to dark brown.
Characterization of AgNPs
The morphology of the synthesized AgNPs was examined using transmission electron microscopy (Bio-TEM) (H-7650; Hitachi Ltd., Tokyo, Japan). The elemental composition of the synthesized AgNPs was confirmed by scanning electron microscopy–energy-dispersive spectra (SEM–EDS) (JEOL-64000; Tokyo, Japan). The X-ray powder diffraction (XRD) was carried out using Rigaku X-ray diffractometer (Rigaku, Japan). The scanning was performed in the region of 2θ=30°–80° at 0.041°/min with a time constant of 2 seconds. The Fourier transform infrared spectrum (FTIR) of the AgNPs was obtained on a PerkinElmer FTIR spectrophotometer (Waltham, MA, USA) in the diffuse reflectance mode at a resolution of 4 cm−1 in KBr pellets.
Antibacterial activity of AgNPs
The phytopathogenic bacterial strains R. solanacearum and X. axonopodis were procured from the Korean Agriculture Culture Collection (KACC), South Korea. The freshly cultured bacterial strains from the Luria-Bertani (LB) agar plates were inoculated into LB broth and incubated at 37°C in a shaking incubator. After appropriate growth, the cultures were used for further experiments. The cultures were allowed to grow in 100 mL of LB broth containing the synthesized AgNPs at different concentrations in the range 1–4 mM. The optical density was measured every 4 hours to determine the growth of the bacteria using the Shimadzu UV-1800 spectrophotometer. The culture without AgNPs was used as a control.
Isolation and propagation of rat splenocytes
Adult (Sprague dawley, 8–12 week old) rats were purchased from Koatech, South Korea. The rats were maintained in a specific pathogen-free facility. Fresh splenocytes of the rat was obtained by teasing the spleen under aseptic conditions according to Lu et al.24 Single-cell suspensions were prepared from rat spleen by pressing the tissues through a sterile wire mesh and washing the cells in Roswell Park Memorial Institute (RPMI) medium containing 1% antibiotic (Anti–Anti; Gibco, South Korea). The animal experiments were carried out in accordance with the institutional animal care and use committee at Chonbuk National University.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The isolated rat splenocytes were treated with series of 10–100 μg/mL of phytosynthesized AgNPs in 96-well tissue culture plates. The treated cells were incubated for 24 hours at 37°C with 5% CO2 for cytotoxicity analysis. The cells were then subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The stock concentration (5 mg/mL) of MTT, a yellow tetrazole, was prepared and 20 μL of MTT was added in each AgNP-treated well and incubated for 4 hours. Purple colored formazan crystals were observed in the bottom of the well and these crystals were dissolved with 200 μL of dimethyl sulfoxide (DMSO), and read at 595 nm18,22 in a multi-well plate reader (Epoch microplate spectrophotometer; Biotek, Winooski, USA).
Results and discussion
Characterization studies
The addition of the tuber extract to 1 mM-solution of AgNO3 changed the color from greenish white to dark brown in about 1 hour. The intensity of the color increased after 8 hours of incubation and the reduction of pure Ag+ ions to Ag0 was monitored by measuring the UV–Vis spectrum of the reaction media (Figure 1). The UV–Vis spectra of the silver surface plasmon resonance band occurs near 430 nm. Previous studies have reported the presence of AgNPs exhibiting yellowish brown color in solution due to the excitation of surface Plasmon vibrations.25,26 However, the color change was not observed in the control flask. The biomolecules present in the tuber extract may reduce the Ag ions present in the solution. This was supported by the results from FTIR studies where stretching vibrations of amines, alkanoids, and alkaloids were observed.
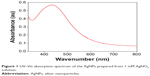  | Figure 1 UV–Vis absorption spectrum of the AgNPs prepared from 1 mM AgNO3 solution. |
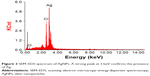
The morphology and size of the phytosynthesized AgNPs were observed by TEM images (Figure 2). The particles were spherical in shape, monodisperse, and the size of the particles varied from 10–70 nm. To confirm the presence of Ag, the samples were analyzed in SEM–EDS, and the results are shown in Figure 3. The results showed strong silver signals (3 keV), along with weak oxygen and carbon peaks, which might have originated from the tuber extract. The results are consistent with previous studies that reported the strong peak for AgNPs at 3 keV.7,8 The EDS quantitative analysis showed the presence of silver (100%) without any contaminants.
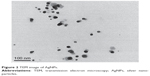  | Figure 2 TEM image of AgNPs. |
XRD was used for the identification of the crystal nature of the AgNPs. The XRD patterns of the synthesized AgNPs are shown in Figure 4. The peaks at 2θ values of 38, 46, 67.4, and 78 correspond to 111, 200, 220, and 311, indicating the cubic nature of AgNPs. The results are in agreement with several studies that reported the cubic nature of biologically synthesized AgNPs.27,28 The FTIR spectrum of the AgNPs is shown in Figure 5. Some pronounced peaks were observed at 3,329, 2,941, 1,759, 1,067, and 731 cm−1 in the 4,000–400 cm−1 region. The corresponding peaks were associated with the stretching vibrations of −C–O, C–H, C=C, CH2, and O–H, respectively. The absorbance peaks could be attributed to the phytochemicals present in the tuber extract, such as reducing sugars, flavonoids, saccharides, and proteins.29 Pan et al16 reported the presence of coumarins, unsaturated fatty acids, and phenols in the H. tuberosus extract.
  | Figure 4 XRD pattern of AgNPs synthesized using tuber extract. |
  | Figure 5 FTIR spectra of AgNPs synthesized using tuber extract. |
Antibacterial activity
Numerous studies reported that biologically synthesized AgNPs have significant antibacterial and antifungal activities, and could be used for the treatment of bacterial and fungal diseases in biotic communities.7,8 However, the adverse effects of AgNPs need to be carefully evaluated before they could be used in antimicrobial products. Hence, phytosynthesized AgNPs were studied for antibacterial activity against phytopathogenic bacteria, namely, R. solanacearum and X. axonopodis. The bacterial growth measurements at different concentrations (1–4 mM) of the AgNPs were determined 0–24 hours at regular time intervals (Figure 6A and B). The observed results indicated that 4 mM concentrations of the AgNPs effectively encountered the bacterial population in the medium. The results are consistent with our previous study reporting the antibacterial activity of AgNPs against antibiotic resistant strains.7
  | Figure 6 Effect of AgNPs on growth of phytopathogenic bacteria. |
Cytotoxicity
The cytotoxic potential of the biologically synthesized AgNPs was assessed using MTT assay. Figure 7A shows the viability of splenocytes with the increasing concentration (10–100 μg/mL) of AgNPs, and the reduction of color compared to untreated cells observed at 595 nm revealed the cytotoxicity. Thus, in vitro cytotoxicity of the AgNPs was evaluated against rat splenocytes at different concentrations (10, 20, 40, 80 and 100 μg/mL), and the results are shown in Figure 7B. The results clearly demonstrated that the splenocytes viability was directly proportional to the concentration of the AgNPs. The cell death (10%) was reported at 10 μg/mL and gradually increased according to the concentration and reached the maximum (67%) at 100 μg/mL. Hackenberg et al30 reported that 10 μg/mL of AgNPs reduced human mesenchymal stem cells viability within 1 hour exposure. Vivek et al22 reported that the cytotoxicity of AgNPs was significantly increased according to the concentration of nanoparticles. Moreover, several studies reported that AgNPs may induce reactive oxygen species and cause damage to cellular components leading to cell death.31–33
Conclusion
To the best of our knowledge, this is the first study to report the synthesis of AgNPs using H. tuberosus tuber extract. The proposed method is a simple, green, and cost-effective method for the synthesis of AgNPs without any harmful chemicals. The analytical results confirmed the presence of AgNPs without any contamination. The green synthesized AgNPs had a significant bactericidal property against phytopathogenic bacteria. The cytotoxicity of the synthesized AgNPs indicates that further studies are needed before using these nanoparticles in antimicrobial products.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A4A01011658).
Disclosure
The authors declare no conflict of interest in this work.
References
Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–13941. | ||
Prow T, Smith JN, Grebe R, et al. Construction, gene delivery, and expression of DNA tethered nanoparticle. Mol Vis. 2006;12:606–615. | ||
Dubey SP, Lahtinen M, Sarkka H, Sillanpaa M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf B Biointerfaces. 2010;80(1):26–33. | ||
Huang H, Yang Y. Preparation of silver nanoparticles in inorganic clay suspensions. Compos Sci Technol. 2008;68:2948–2953. | ||
Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–170. | ||
Suman TY, Radhika Rajasree SR, Kanchana A, Beena E. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf B Biointerfaces. 2013;106:74–78. | ||
Govarthanan M, Selvankumar T, Manoharan K, et al. Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int J Nanomedicine. 2014;9:1593–1599. | ||
Lee KJ, Park SH, Govarthanan M, et al. Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater Lett. 2013;105:128–131. | ||
Ananda Babu S, Gurumallesh Prabu S. Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Mater Lett. 2011;65:1675–1677. | ||
Otari SV, Patil RM, Nadaf NH, Ghosh SJ, Pawar SH. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater Lett. 2012;72:92–94. | ||
Shanker SS, Rai A, Ahmed A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496–502. | ||
Ramteke C, Chakrabarti T, Sarangi BK, Pandey R-A. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J Chem. 2013;2013:1–7. | ||
Zayed MF, Eisa WH, Shabaka AA. Malya parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta A. 2012;98:423–428. | ||
Shankar S, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19(6):1627–1631. | ||
Chandran SP, Chadudhary M, Pasricha R, Ahamad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583. | ||
Pan L, Sinden MR, Kennedy AH, Chai H, Watson LE, Graham TL. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke). Phytochem Lett. 2009;2(1):15–18. | ||
Talipova M. Lipids of Helianthus tuberosus L. Chem Nat Comp. 2001;37(3):213–215. | ||
Sukirtha R, Priyanka K, Antony JJ, et al. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012;47(2):273–279. | ||
Miura N, Shinohara Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem Biophys Res Commun. 2009;390:733–737. | ||
Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130–139. | ||
Asha Rani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. | ||
Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47:2405–2410. | ||
Pelegrini PB, Noronha EF, Muniz MA, et al. An antifungal peptide from passion fruit (Passiflora edulis) seeds with similarities to 2S albumin proteins. Biochim Biophys Acta. 2006;1764(6):1141–1146. | ||
Lu L, Hsieh M, Oriss TB, et al. Generation of DC from mouse spleen cell cultures in response to GM-CSF: immunophenotypic and functional analyses. Immunology. 1995;84:127–134. | ||
Vidhu VK, Aromal SS, Philip D. Green synthesis of silver nanoparticles using Macrotyloma uniorum. Spectrochim Acta A Mol Biomol Spectrosc. 2011;83:392–397. | ||
Ashok Kumar D, Palanichamy V, Mohana Roopan S. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;127:168–171. | ||
Shaligram NS, Bule M, Bhambure R, et al. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain process. Process Biochem. 2009;44:939–943. | ||
Balaji DS, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar BK, Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B Biointerfaces. 2009;68(1):88–92. | ||
Muniyappan N, Nagarajan NS. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem. 2014;49(6):1054–1061. | ||
Hackenberg S, Scherzed A, Kessler M, et al. Silver nanoparticle: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201(1):27–33. | ||
Jacob SJ, Finub JS, Narayanan A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf B Biointerfaces. 2012;91:212–214. | ||
Patel B, Shah VR, Bavadekar SA. Anti-proliferative effects of carvacrol on human prostate cancer cell line, LNCaP. FASEB J. 2012;26:1037. 5. | ||
Sankar R, Karthik A, Prabu A, Karthik S, Shivashankari KS, Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces. 2013;108:80–84. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.