Back to Journals » Drug Design, Development and Therapy » Volume 14
Sugar Codes Conjugated Alginate: An Innovative Platform to Make a Strategic Breakthrough in Simultaneous Prophylaxis of GERD and Helicobacter pylori Infection
Authors Moayedi S, Yadegar A , Balalaie S, Yarmohammadi M, Zali MR, Suzuki H , Fricker G, Haririan I
Received 26 March 2020
Accepted for publication 10 June 2020
Published 17 June 2020 Volume 2020:14 Pages 2405—2412
DOI https://doi.org/10.2147/DDDT.S255611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Saeed Moayedi,1 Abbas Yadegar,2 Saeed Balalaie,3 Mahdiyeh Yarmohammadi,2 Mohammad Reza Zali,4 Hidekazu Suzuki,5 Gert Fricker,6 Ismaeil Haririan1
1Department of Pharmaceutical Biomaterials, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran; 2Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Peptide Chemistry Research Center, K. N. Toosi University of Technology, Tehran, Iran; 4Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 5Division of Gastroenterology and Hepatology, Department of Internal Medicine, Tokai University School of Medicine, Isehara, Kanagawa 259-1193, Japan; 6Institute of Pharmacy and Molecular Biotechnology, Ruprecht-Karls-University, Heidelberg, Germany
Correspondence: Ismaeil Haririan
Department of Pharmaceutical Biomaterials, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Email [email protected]
Introduction: Currently, gastroesophageal reflux disease (GERD) is one of the most ubiquitous problems in clinical practice. An antacid-alginate combination (under the trade name Gaviscon) is a natural-based product that effectively suppresses GERD. This product acts via the formation of viscous gel that floats on the top of the gastric content. On the other hand, efficient management of Helicobacter pylori infection with minimal side effects is an important goal for gastroenterologists. Furthermore, some H. pylori-positive patients suffer from GERD.
Methods: Here, we present the results of investigations on alginate conjugated to sugar codes in order to find initial clues regarding the potential ability of this conjugate in the simultaneous prophylaxis of GERD and H. pylori infection in an in vitro assay.
Results: It is noteworthy that our results reveal that sugar codes conjugated alginate considerably decrease (approximately 74%) the adhesion of H. pylori to gastric epithelial cells in vitro. Moreover, surprisingly after conjugation of sugar codes, alginate can maintain its ability to create gel. Our results demonstrate that alginate conjugated to sugar codes is not cytotoxic.
Conclusion: The preparation of these conjugates can be regarded as the first step to establish a new roadmap for the simultaneous prevention of GERD and H. pylori infection in future studies on in vivo models.
Keywords: GERD, Helicobacter pylori infection, sugar codes, alginate, conjugation
Introduction
GERD is one of the most common issues in clinical practice.1 The prevalence of GERD is approximately 8.8–25.9% in Europe, 2.5–7.8% in East Asia, 8.7–33.1% in the Middle East, 11.6% in Australia, 23.0% in South America, and 18.1–27.8% in North America.2 GERD is the retrograde movement of gastric content into the esophagus. This physiological phenomenon becomes pathological when it is associated with symptoms or mucosal complications. The most typical symptoms of GERD are heartburn and acid regurgitation. Additional esophageal symptoms such as dysphagia and chest pain are also common. Increased intra-abdominal pressure and transient relaxations of the lower esophageal sphincter (LES) are among the most common reasons for GERD.3 Pharmaceutical products, especially antacids, play a central role in the management of GERD. Antacids neutralize acid without affecting subsequent acid secretion. Until recently, they were considered as products that have limited effects on GERD symptoms.
However, recently the acid pocket has been considered as an interesting target for the management of GERD by the oral administration of antacids combined with alginates. The acid pocket corresponds to the newly secreted acid layer on top of the ingested meal that is evident about 17 min after eating a meal.4 Neutralizing this acid layer might be associated with the reduction of postprandial GERD symptoms. Alginates are natural polysaccharide polymers. On contact with gastric acid, sodium alginate combined with antacid (such as calcium carbonate) forms a gel, and the antacid releases carbon dioxide, which is trapped in the alginate gel. Consequently, this gel floats on the top of the gastric content and helps prevent acid coming back up into the esophagus. An antacid–alginate combination (under the trade name Gaviscon), eliminates or displaces the “acid pocket” in GERD patients, hence being beneficial in the prophylaxis of GERD.3–6
On the other hand, Helicobacter pylori (H. pylori) infection is a common worldwide infection that is an important cause of peptic ulcer disease and gastric cancer. H. pylori is a Gram-negative spiral bacterium. The human stomach is considered as an ecological niche for H. pylori.7–10 Resistance to antibiotics is a serious concern for gastroenterologists during the management of H. pylori infection.11–13 Furthermore, lectins are considered as one of the most important types of proteins in biological sciences. They recognize carbohydrates covalently linked to proteins and lipids on the cell surface and within the extracellular matrix. These carbohydrates are called sugar codes. Many cellular functions ranging from cell adhesion to pathogen recognition are mediated via lectins.14,15 H. pylori in stomach via its outer membrane proteins, namely BabA and SabA, adheres to Lewis b and Sialyl Lewis x antigens (sugar codes), respectively, found on gastric epithelial cells in order to achieve colonization.9,10 Lewis b antigen is a hexasaccharide. Five residues of Lewis b antigen, namely Fuc1, Gal2, GlcNAc3, Fuc4 and Gal5 are required for interaction between BabA and Lewis b antigen. Free anomeric carbon of Glc6 is not required for interaction between BabA and Lewis b antigen.9
Sialyl Lewis x antigen is a tetrasaccharide. Two residues of Sialyl Lewis x antigen, namely Neu5Ac1 and Gal2 are required for interaction between SabA and Sialyl Lewis x antigen. Free anomeric carbon of GlcNAc3 is not required for the interaction between SabA and Sialyl Lewis x antigen.10,16 Gonçalves et al17 developed chitosan microspheres coated with Lewis b and Sialyl Lewis x and demonstrated that these microspheres are able to prevent H. pylori adhesion to gastric epithelial cells in an in vitro and ex vivo model. Parreira et al18 demonstrated that Lewis b and Sialyl Lewis x immobilized on self-assembled monolayers appropriately interact with H. pylori.
Furthermore, some H. pylori positive patients suffer from GERD.19,20 It is worth mentioning that the binding affinity of H. pylori to gastric epithelial cells is significantly decreased at low pH (for example at pH 2). For this reason, H. pylori positive patients (hidden and undiagnosed H. pylori infection) with GERD undergoing treatment with antacids, proton-pump inhibitors and H2 receptor antagonists may be predisposed to PUD and gastric cancer.21 It is noteworthy that some studies confirm that long-term use of proton-pump inhibitors may increase the risk of gastric cancer.22,23 If there is a natural-based product which not only help in the prophylaxis of H. pylori infection, but also has great benefit to prophylaxis of GERD, it may result in a strategic breakthrough in the simultaneous prophylaxis of GERD and H. pylori infection. Based on our current knowledge, the preparation of sugar codes conjugated alginate with the potential ability of help in the simultaneous prophylaxis of GERD and H. pylori infection has not been reported before. Here we report alginate conjugated to Lewis b and Sialyl Lewis x with the potential ability of help in the simultaneous prophylaxis of GERD and H. pylori infection.
Materials and Methods
Chemicals, H. pylori Strain and Cell Line
Biotin alginate was purchased from Carbomer, Inc (San Diego, USA). Lewis B hexasaccharide-sp-biotin and Sialyl Lewis X-biotin were purchased from Carbosynth (Newbury, UK). Biotin-4-fluorescein, sodium alginate, avidin from egg white, saponin and Methylthiazolyldiphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (Munich, Germany). Clinical H. pylori strain HC 168 isolated from a PUD patient (BabA+ and SabA+) and AGS cell line were obtained from Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.24,25
Preparation of Sugar Codes Conjugated Alginate
Preparation of Avidin-Alginate Conjugates
50 µL of avidin with concentrations of 300, 500 and 700 µg/mL was added individually to 500 µL of biotin alginate solution of 0.5% w/v. All samples were incubated at 25°C for 1 h. After washing via Amicon Ultra centrifugal filter (MWCO 100,000) in order to remove unbound avidin, 50 µL of biotin-4-fluorescein (75 µg/mL) was added to prepared solutions. Assessment of the biotin alginate’ binding to the avidin was carried out using fluorescence spectroscopy (λex 494 nm; λem 522 nm) to identify the best conjugation condition. All experiments were performed in triplicates.
Preparation of Sugar Codes-Alginate Conjugates
50 µL of sugar codes (30 µL of Lewis b and 20 µL of Sialyl Lewis x) with concentrations of 10, 25 and 50 µg/mL was added individually to 500 µL of optimized avidin-alginate conjugates. All samples were incubated at 25°C for 1 h. After washing via Amicon Ultra centrifugal filter (MWCO 10,000) in order to remove unbound sugar codes, 50 µL of biotin-4-fluorescein (75 µg/mL) was added to prepared solutions. Assessment of the avidin-alginate conjugates’ binding to the sugar codes was carried out using fluorescence spectroscopy (λex 494 nm; λem 522 nm) to identify the best conjugation condition. All experiments were performed in triplicates.
Investigation of the Stability of Conjugates in the pH Range of 2.5–8
The pH of 500 µL of optimized sugar codes-alginate conjugates was set individually to 2.5, 4.5, 7 and 8 by HCl. All samples were incubated at 25°C for 3 h. After washing via Amicon Ultra centrifugal filters (MWCO 100,000) in order to remove unbound sugar codes and avidin, 50 µL of avidin with concentration of 500 µg/mL (as additional avidin) was added to all samples in order to bind to the remaining active sites on the surface of biotin alginate. Then, all samples were incubated at 25°C for 1 h. After washing via Amicon Ultra centrifugal filter (MWCO 100,000) in order to remove unbound avidin, 50 µL of biotin-4-fluorescein (75 µg/mL) was added to prepared solutions. Assessment of the biotin alginate’ binding to the additional avidin was carried out using fluorescence spectroscopy (λex 494 nm; λem 522 nm) to identify the stability of optimized sugar codes-alginate conjugates in the pH range of 2.5–8. All experiments were performed in triplicates.
Preparation of Sugar Codes Conjugated Alginate Gel
The pH of 2 mL of optimized sugar codes-alginate conjugates (0.5% w/v) was set to 4.5 by HCl and then, 400 mg calcium carbonate was added to prepared solution under stirring. Furthermore, as control group, the pH of 2 mL of sodium alginate (0.5% w/v) was set to 4.5 by HCl and in the next step, 400 mg calcium carbonate was added to prepared solution under stirring. Photography was applied to identify gel formation. All experiments were performed in triplicates.
Adhesion Assay
H. pylori strain (BabA+ and SabA+) was recovered from the stocks on Brucella agar plates containing fetal calf serum (10%), horse blood (7%), Campylobacter-selective supplement (vancomycin 2.0 mg, polymyxin B 0.05 mg and trimethoprim 1.0 mg), and amphotericin B (3 mg/l). The plates were incubated in a CO2 incubator (Innova® CO-170; New Brunswick Scientific, USA) under microaerophilic atmosphere (5% O2, 10% CO2 and 85% N2) at 37°C for 3–5 days. Moreover, AGS cells were seeded into 24-well plates at a density of 1×105 cells per well and incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% nonessential amino acid, 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37°C in a humidified incubator (Memmert, Dusseldorf, Germany) containing 5% CO2 for 2 days before adhesion assay. In the next step, AGS cells were treated with 200 µL of sugar codes conjugated alginate (1 mg/mL) and incubated for 30 min. Then, 100 µL of bacterial suspension with MOI 100 (1×107 CFU/mL) were used to infect AGS cells and incubated for 3 h. Saponin, which lyses the AGS cells without affecting H. pylori, was used to evaluate the efficiency of sugar codes conjugated alginate in preventing H. pylori adhesion to AGS cells. Saponin is a natural amphiphilic material that is able to interfere with AGS cell membrane via loss of the cell membrane integrity.26 After washing with PBS, AGS cells were treated with 100 µL of saponin (0.05% w/v) and incubated for 15 min. Then, H. pylori was cultured on Brucella agar plates and colonies were counted via the measurement of OD at 600 nm after 5 days of growth. AGS cells plus H. pylori with and without sodium alginate were considered as controls.
Effect of Sugar Codes Conjugated Alginate on AGS Cells Viability
AGS cells were seeded into 24-well plates (1×105 cells per well) and after 24 h, treated with 200 µL of sugar codes conjugated alginate (1 mg/mL) and incubated at 37°C in a humidified incubator containing 5% CO2 for 24 h. Biocompatibility was evaluated by measurement of the cell metabolic activity using the MTT Assay Kit. Briefly, 50 µL of MTT solution (5 mg/mL) was added to each well and incubated for the 4 h. The culture medium was removed and replaced with 500 µL DMSO solution. Finally, the absorbance value was measured at 570 and 630 nm using a micro-plate reader.
Results and Discussion
Assessment of the Binding of Alginate to Avidin
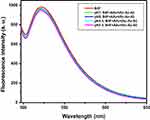
The fluorescence emission spectra of biotin-4-fluorescein following the addition of various concentrations of avidin are presented in Figure 1A. Biotin-4-fluorescein was used to assess the biotin alginate’ binding to the avidin. Biotin-4-fluorescein is used for detecting and quantifying biotin-binding sites by fluorescence spectroscopy. Fluorescence of this molecule is quenched upon binding to avidin.27–31 The criteria of selecting the optimized conjugates included the sample with minimum concentration of avidin, which has maximum decreased fluorescence intensity at 522 nm. Thus, based on the spectra presented in Figure 1A, the sample prepared by avidin with concentration of 500 µg/mL was selected as optimized sample.
Assessment of the Binding of Alginate to Sugar Codes
The fluorescence emission spectra of biotin-4-fluorescein after the addition of various concentrations of sugar codes are presented in Figure 1B. The criteria of selecting the optimized conjugates included the sample with minimum concentration of sugar codes, which has minimum decreased fluorescence intensity at 522 nm. Thus, based on the spectra presented in Figure 1B, the sample prepared by sugar codes with concentration of 25 µg/mL was selected as an optimized sample.
Assessment of the Stability of Conjugates in the pH Range of 2.5–8
The fluorescence emission spectra of biotin-4-fluorescein for final optimized sample in the pH range of 2.5–8 are presented in Figure 2. Avidin, a glycoprotein from egg white, binds biotin with high affinity. The avidin–biotin complex is the strongest known non-covalent interaction (Kd = 10−15 M) between a protein and a ligand. The bond formation between biotin and avidin is very rapid, and once formed, is unaffected by extremes of pH, temperature, organic solvents and other denaturing agents. Furthermore, avidin is resistant to enzymatic proteolysis and biotin cannot be released from avidin binding in the gastrointestinal tract.32,33 Therefore, optimized sugar codes-alginate conjugates are stable in the pH range of gastrointestinal tract. However, in this study, the stability of optimized sugar codes-alginate conjugates in the pH range of 2.5–8 was investigated in order to confirm this rationale. It is worth mentioning that after a meal, intragastric pH increases about 4.5. As it could be seen in Figure 2, all samples had good stability in the pH range of 2.5–8.
The Formation of Sugar Codes Conjugated Alginate Gel
Surprisingly, upon mixing sugar codes-alginate conjugates with calcium carbonate, hydrogel formed within 2 to 4 min. Furthermore, after mixing sodium alginate with calcium carbonate, hydrogel formed within 1 to 3 min. These results indicated that sugar codes-alginate conjugates preserved its ability to create gel-like alginate (Figure 3). Currently, the efficient treatments for GERD infection rely on proton-pump inhibitors, H2 receptor antagonists and antacids. Long-term treatment with these pharmaceutical products has side effects. An antacid–alginate combination has a beneficial effect on typical symptoms of GERD such as heartburn and acid regurgitation since this combination forms a gel and then floats in the interface between lower esophageal sphincter and meal that helps prevent acid coming back up into the esophagus. It is worth noting that our results indicate that sugar codes-alginate can create gel-like alginate. Consequently, calcium carbonate-sugar codes conjugated alginate combination can be taken into consideration in future therapeutic strategies for prophylaxis of GERD.
 |
Figure 3 The photographic images of alginate gel and sugar codes conjugated alginate gel. |
Efficiency of Conjugates in Preventing H. pylori Adhesion to Gastric Epithelial Cells
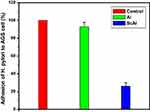
Based on results of optical density (OD), the percentage of adhesion of H. pylori to AGS cell is presented in Figure 4. As it could be seen in Figure 4, sugar codes conjugated alginate considerably decreased (approximately 74%) the adhesion of H. pylori to AGS cells in vitro. Sodium alginate did not influence the adhesion of H. pylori to AGS cells. Currently, the management of H. pylori infection relies on antibiotics, but rapid emergence of drug resistance has hindered the efficacy of treatment regimens. For this reason, there is a clinical need for novel alternatives to the available conventional therapeutic strategies. Bacterial adhesion to gastric epithelial cells is an essential event in the development of H. pylori infection. Thus, targeting the bacterial binding process and colonization is a strategic vision. This study shows the proof of concept for works aimed at the development of alternatives to the available conventional therapeutic platforms that can both decrease the adhesion of H. pylori to the host gastric epithelial cells via introducing a competitive decoy for bacterial binding, and help prevent GERD. At present, the association between H. pylori infection and GERD is a controversial issue because some studies indicate that eradication of H. pylori can increase the risk of GERD19,34 and some other studies demonstrate that H. pylori eradication is not associated with development of new cases of GERD.20,35 Moreover, some H. pylori positive patients suffer from GERD. Here, a clinical H. pylori strain expressing BabA and SabA was used for studies of bacterial adhesion to alginate conjugated to Lewis b and Sialyl Lewis x. Although other bacterial biomolecules may be engaged in adhesion to the host gastric epithelial cells, only BabA and SabA have been well investigated regarding the host receptors. Our results indicate that conjugated sugar codes maintain their ability to specifically bind H. pylori and thus sugar codes conjugated alginate appropriately interact with H. pylori, which are in agreement with previously reported studies.17,18 From a therapeutic point of view, these conjugates can be used as both H. pylori binders and natural-based products with the ability of prophylaxis of GERD. It is expected that after oral use, calcium carbonate-sugar codes conjugated alginate combination forms a gel that both helps prevent acid coming back up into the esophagus and competes with the gastric epithelial cells and decreases the number of H. pylori in the stomach of infected patients (Figure 5). Therefore, this combination improves the efficacy of conventional therapeutic strategies in the simultaneous management of GERD and H. pylori infection. Here, adhesion assay was performed under in vitro condition, which was the main limitation of our study. For this reason, further studies on in vivo models are needed before the clinical benefits of sugar codes conjugated alginate can be evaluated in humans.
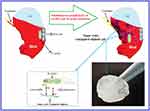 |
Figure 5 Potential ability of sugar codes conjugated alginate gel in simultaneous prophylaxis of GERD and H. pylori infection. |
In vitro Cell Viability Assessment
The cytotoxicity of sugar codes conjugated alginate is presented in Figure 6. As it can be observed in Figure 6, a reduction of ~11% in cell metabolic activity was observed after incubation with sugar codes conjugated alginate. Based on ISO standard, a reduction in cell viability of more than 30% is considered a cytotoxic effect.36 Thus, sugar codes conjugated alginate is not cytotoxic.
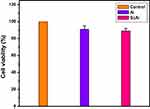 |
Figure 6 The percentage of cell viability. Control (AGS cell), Al (alginate+AGS cell), ScAl (sugar codes conjugated alginate+AGS cell). All experiments were performed in triplicates. |
Conclusion
Our results represent this important fact that after conjugation, sugar codes maintain their ability to specifically bind H. pylori and consequently alginate conjugated to sugar codes appropriately interact with H. pylori. Sugar codes conjugated alginate can be considered as an interesting platform to biomimic the gastric mucosa and gastric epithelial cells. Furthermore, sugar codes conjugated alginate has ability to create gel. As a result, calcium carbonate–sugar codes conjugated alginate combination can be taken into consideration in future therapeutic options for simultaneous prophylaxis of GERD and H. pylori infection. We believe that our research will serve as a base for further studies on in vivo models.
Acknowledgment
The authors wish to thank all laboratory staff of Department of Pharmaceutical Biomaterials in Tehran University of Medical Sciences. Furthermore, the authors would like to thank all laboratory staff of Food Borne and Waterborne Diseases Research Center in Shahid Beheshti University of Medical Sciences. This study was financially supported by Tehran University of Medical Sciences (Grant No. 35740).
Disclosure
Professor Hidekazu Suzuki reports grants, personal fees from Takeda, personal fees from Astellas, grants, personal fees from Otsuka, personal fees from Mylan, personal fees from EA Pharma, personal fees from AstraZeneca, grants, personal fees from Daiichi Sankyo, grants from Tsumura, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kandulski A, Malfertheiner P. Gastroesophageal reflux disease—from reflux episodes to mucosal inflammation. Nat Rev Gastroenterol Hepatol. 2012;9(1):15. doi:10.1038/nrgastro.2011.210
2. Yamasaki T, Hemond C, Eisa M, Ganocy S, Fass R. The changing epidemiology of gastroesophageal reflux disease: are patients getting younger? J Neurogastroenterol Motil. 2018;24(4):559. doi:10.5056/jnm18140
3. Koda-Kimble MA. Koda-Kimble and Young’s Applied Therapeutics: The Clinical Use of Drugs. Lippincott Williams & Wilkins; 2012.
4. Kahrilas PJ, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol. 2013;108(7):1058. doi:10.1038/ajg.2013.132
5. De Ruigh A, Roman S, Chen J, Pandolfino JE, Kahrilas PJ. Gaviscon double action liquid (antacid & alginate) is more effective than antacid in controlling post‐prandial oesophageal acid exposure in GERD patients: a double‐blind crossover study. Aliment Pharmacol Ther. 2014;40(5):531–537.
6. Thomas E, Wade A, Crawford G, Jenner B, Levinson N, Wilkinson J. Randomised clinical trial: relief of upper gastrointestinal symptoms by an acid pocket‐targeting alginate–antacid (gaviscon double action)–a double‐blind, placebo‐controlled, pilot study in gastro‐oesophageal reflux disease. Aliment Pharmacol Ther. 2014;39(6):595–602. doi:10.1111/apt.12640
7. Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629. doi:10.1038/nrgastro.2010.154
8. Polk DB, Peek RM
9. Hage N, Howard T, Phillips C, et al. Structural basis of Lewisb antigen binding by the Helicobacter pylori adhesin BabA. Sci Adv. 2015;1(7):e1500315. doi:10.1126/sciadv.1500315
10. Pang SS, Nguyen STS, Perry AJ, et al. The three-dimensional structure of the extracellular adhesion domain of the sialic acid-binding adhesin SabA from Helicobacter pylori. J Biol Chem. 2014;289(10):6332–6340.
11. Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5(2):103–109. doi:10.1177/1756283X11432492
12. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):34–42. doi:10.1136/gutjnl-2012-302254
13. Thung I, Aramin H, Vavinskaya V, et al. The global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi:10.1111/apt.13497
14. St-Pierre C, Ouellet M, Tremblay MJ, Sato S. Galectin-1 and HIV-1 infection. In: Fukuda M, editor. Methods in Enzymology. Vol. 480. Elsevier; 2010:267–294.
15. Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7(6):424. doi:10.1038/nrmicro2146
16. Trinchera M, Aronica A, Dall’Olio F. Selectin ligands sialyl-Lewis a and sialyl-Lewis x in gastrointestinal cancers. Biology. 2017;6(1):16. doi:10.3390/biology6010016
17. Gonçalves IC, Magalhães A, Costa AM, et al. Bacteria-targeted biomaterials: glycan-coated microspheres to bind Helicobacter pylori. Acta Biomater. 2016;33:40–50. doi:10.1016/j.actbio.2016.01.029
18. Parreira P, Magalhães A, Reis C, Borén T, Leckband D, Martins M. Bioengineered surfaces promote specific protein–glycan mediated binding of the gastric pathogen Helicobacter pylori. Acta Biomater. 2013;9(11):8885–8893. doi:10.1016/j.actbio.2013.06.042
19. Nam SY, Choi IJ, Ryu KH, Kim BC, Kim CG, Nam B-H. Effect of Helicobacter pylori infection and its eradication on reflux esophagitis and reflux symptoms. Am J Gastroenterol. 2010;105(10):2153. doi:10.1038/ajg.2010.251
20. Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol. 2010;105(5):1007. doi:10.1038/ajg.2009.734
21. Bugaytsova JA, Björnham O, Chernov YA, et al. Helicobacter pylori adapts to chronic infection and gastric disease via pH-responsive BabA-mediated adherence. Cell Host Microbe. 2017;21(3):376–389. doi:10.1016/j.chom.2017.02.013
22. Lai S-W, Lai H-C, Lin C-L, Liao K-F. Proton pump inhibitors and risk of gastric cancer in a case–control study. Gut. 2019;68(4):765–767. doi:10.1136/gutjnl-2018-316371
23. Poulsen A, Christensen S, McLaughlin J, et al. Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer. 2009;100(9):1503–1507. doi:10.1038/sj.bjc.6605024
24. Yadegar A, Mohabati Mobarez A, Zali MR. Genetic diversity and amino acid sequence polymorphism in Helicobacter pylori CagL hypervariable motif and its association with virulence markers and gastroduodenal diseases. Cancer Med. 2019;8(4):1619–1632. doi:10.1002/cam4.1941
25. Farzi N, Yadegar A, Aghdaei HA, Yamaoka Y, Zali MR. Genetic diversity and functional analysis of oipA gene in association with other virulence factors among Helicobacter pylori isolates from Iranian patients with different gastric diseases. Infect Genet Evol. 2018;60:26–34. doi:10.1016/j.meegid.2018.02.017
26. Böttger S, Melzig MF. The influence of saponins on cell membrane cholesterol. Bioorg Med Chem. 2013;21(22):7118–7124. doi:10.1016/j.bmc.2013.09.008
27. McMahon RJ. Avidin-Biotin Interactions: Methods and Applications. Springer Science & Business Media; 2008.
28. Chivers CE, Crozat E, Chu C, Moy VT, Sherratt DJ, Howarth M. A streptavidin variant with slower biotin dissociation and increased mechanostability. Nat Methods. 2010;7(5):391. doi:10.1038/nmeth.1450
29. Mittal R, Bruchez MP. Biotin-4-fluorescein based fluorescence quenching assay for determination of biotin binding capacity of streptavidin conjugated quantum dots. Bioconjug Chem. 2011;22(3):362–368. doi:10.1021/bc100321c
30. Sun S, Huang X, Ma M, et al. Systematic evaluation of avidin–biotin interaction by fluorescence spectrophotometry. Spectrochim Acta A Mol Biomol Spectrosc. 2012;89:99–104. doi:10.1016/j.saa.2011.12.003
31. Waner MJ, Mascotti DP. A simple spectrophotometric streptavidin–biotin binding assay utilizing biotin-4-fluorescein. J Biochem Biophys Methods. 2008;70(6):873–877. doi:10.1016/j.jbbm.2007.06.001
32. Bender DA. Nutritional Biochemistry of the Vitamins. Cambridge university press; 2003.
33. Liebesa Y, Marksa RS. Optical fiber immunosensors and genosensors for the detection of viruses. Viral Diagn. 2014;2:343.
34. Zhao Y, Li Y, Hu J, et al. The effect of Helicobacter pylori eradication in patients with gastroesophageal reflux disease: a meta-analysis of randomized controlled studies. Dig Dis. 2020;1–8. doi:10.1159/000504086
35. Na HK, Lee JH, Park SJ, et al. Effect of Helicobacter pylori eradication on reflux esophagitis and GERD symptoms after endoscopic resection of gastric neoplasm: a single-center prospective study. BMC Gastroenterol. 2020;20(1):1–7. doi:10.1186/s12876-020-01276-1
36. International Standards Organization. ISO 10993-5. Biological Evaluation of Medical Devices. Tests for in vitro Cytotoxicity. Geneva: International Standards Organization; 2009.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.