Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Successful Treatment of Recurrent Adult-Onset Still’s Disease with Tocilizumab: A Case Report and Literature Review
Authors Zhong X , Xu T , Li T, Luo N , Luo N , Hao P
Received 20 July 2023
Accepted for publication 14 October 2023
Published 2 November 2023 Volume 2023:16 Pages 3157—3163
DOI https://doi.org/10.2147/CCID.S431605
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xiaojing Zhong,* Tongtong Xu,* Tianhao Li,* Nana Luo, Nan Luo, Pingsheng Hao
Author Affiliations Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pingsheng Hao, Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, 39 Shi-Er-Qiao Road, Jinniu District, Chengdu, Sichuan, 610072, People’s Republic of China, Tel +86138 8196 5024, Fax +86-28-87732407, Email [email protected]
Abstract: Adult-onset Still’s disease (AOSD) is considered a rare autoimmune inflammatory disorder with an unclear etiology and pathogenesis.The main clinical manifestations of this disease are high fever, joint pain, and transient skin lesions. Physical examination may reveal hepatomegaly, splenomegaly, and lymphadenopathy, while laboratory tests show abnormalities such as elevated white blood cell count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and serum ferritin (SF). The lack of specific diagnostic markers contributes to a relatively high rate of clinical misdiagnosis and missed diagnoses.In terms of treatment, glucocorticoids have always been the cornerstone medication, but some patients exhibit suboptimal responses to conventional drug therapy, making disease control challenging. However, as our understanding of the pathogenesis continues to grow, novel therapeutic approaches targeting various cytokines have been gradually identified. In this report, we present a case of successful treatment of recurrent AOSD with tocilizumab (TCZ), along with a concise review of innovative treatment strategies for AOSD based on literature retrieval.
Keywords: AOSD, tocilizumab, treatment, interleukin-6 inhibitors
Introduction
Adult-onset Still’s disease (AOSD) is a rare systemic disorder that lies within the spectrum between autoinflammatory syndromes and autoimmune diseases.1 The etiology of AOSD remains incompletely elucidated. The estimated incidence rate ranges from 1 to 34 per million individuals. The average age of onset is typically around the age of 35, with a slightly higher prevalence observed in females.2 The typical presentation of AOSD encompasses fever, evanescent rash, and arthritis. The evanescent rash is characterized by transient erythematous macules that intensify with rising body temperature and fade as the fever subsides. In recent years, atypical skin manifestations of AOSD have also been reported. The clinical features of AOSD are nonspecific and can mimic other infectious, rheumatic, autoimmune, and hematologic malignancies. Diagnostic markers specific to AOSD are lacking, making it a diagnosis of exclusion. Glucocorticoids are the mainstay of treatment for AOSD. However, as understanding of the disease deepens, novel therapeutic modalities, such as tumor necrosis factor-alpha (TNF-α) inhibitors and interleukin inhibitors, have emerged.3 This paper aims to present a case report highlighting the successful treatment of recurrent AOSD with tocilizumab (TCZ) and provide a brief review of innovative treatment strategies for AOSD based on literature retrieval.
Case Presentation
The patient is a 37-year-old Chinese female who has been experiencing recurrent erythema, itching, joint pains in the extremities, and fever for 5 years, with exacerbation over the past two weeks. Five years ago, she presented with recurrent fever, skin erythema, itching, and joint pains in the extremities along with enlarged lymph nodes in the left posterior neck. She sought medical assistance from the rheumatology and immunology departments of the local hospital, where lymph node biopsy showed no significant abnormalities. Subsequently, her symptoms improved with oral administration of traditional Chinese medicine. However, over the past two weeks, her condition has recurred and worsened, manifesting as symmetrical whiplash lesions on the chest, abdomen, and back of the shoulders, as well as high fever, sore throat, generalized aches, and pain in the joints of the extremities, with more pronounced symptoms in the upper extremities. The laboratory examination conducted at the local hospital indicates that the patient has developed inflammatory syndrome, specifically with a white blood cell count (WBC) of 14.39 × 109/L, erythrocyte sedimentation rate (ESR) is 66mm/h, C-reactive protein (CRP) level is 91mg/L, serum ferritin (SF) level is greater than 20000ng/mL, and interleukin-6 (IL-6) level is 151pg/mL. Multiple enlarged lymph nodes were also observed. The patient received an unspecified dose of methylprednisolone and celecoxib, which resulted in partial improvement of her generalized body aches. However, she still had recurrent fever, occurring daily during the patients’ active phase.Her temperature reached its peak in the afternoon every day, with a peak of up to 39.5°C.Additionally, she developed persistent new erythema and rashes, some of which formed confluent patches.
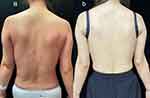
The patient walked into the ward on their own and was in an average mental state. The values of vital signs measured at admission were: T 39 °C, R 23 times/min, P 105 times/min, and BP 110/80 mmHg. The patient’s physical examination showed that significantly enlarged lymph nodes were palpable in the right supraclavicular fossa, bilateral armpits, and bilateral inguinal areas. The size of the lymph nodes in the right supraclavicular fossa is about 15 * 8mm, the larger lymph nodes in the left armpit are about 15 * 8mm, the larger lymph nodes in the right armpit are about 14 * 6mm, the larger lymph nodes in the left inguinal area are about 14 * 7mm, and the larger lymph nodes in the right inguinal area are about 9 * 5mm. These swollen lymph nodes feel hard to the touch and have clear boundaries. The patient did not experience significant pain when pressing on these lymph nodes.Her upper limb muscle strength was slightly lower than normal, while the lower limb muscle strength was at normal levels.The liver can be palpable 1cm below the costal margin, and no other abnormalities were found on physical examination of the heart, lungs, and abdomen.The patient exhibited a diffuse red rash on the skin, accompanied by whip-like lesions present on the shoulder, back, chest, and abdomen regions (Figure 1). The lesions were accompanied by intense itching. Symmetric erythema was observed on the face, without evidence of eyelid swelling.
The auxiliary examinations revealed the following results: WBC 10.58×109/L, ESR 90 mm/h, CRP 151.17 mg/L, SF level greater than 2000 ng/mL, and IL-6 level of 162.3 pg/mL (Table 1). The result of testing the patient’s immunoglobulin E was 190IU/mL, alanine aminotransferase was 80U/L, and aspartate aminotransferase was 119U/L.The triglyceride level is 2.21mmol/L, the high-density lipoprotein cholesterol level is 0.71mmol/L, and there are no significant abnormalities in the low-density lipoprotein cholesterol level, rheumatoid factor test, anti CCP antibody, and blood culture.The patient’s neutrophil count is 9.24×109/L and platelet count is normal. The patient tested negative for anti-myositis antibody spectrum, autoimmune antibody spectrum, ANCA, ACE, TORCH, EB virus, T-spot, viral hepatitis, AIDS, and syphilis. Lymph node ultrasonography indicated enlarged lymph nodes with abnormal morphological structure in the right supraclavicular fossa, bilateral axillae, and bilateral inguinal regions. Lymph node biopsy showed no significant abnormalities. Nerve conduction studies showed reduced amplitude of the left peroneal nerve, while no significant abnormalities were detected in other examined nerves. Electromyography revealed myogenic damage in the examined muscles. Magnetic resonance imaging (MRI) scans of both upper arms showed possible damage to the patient’s bilateral rotator cuff, as well as fluid accumulation and exudation around the left biceps tendon long head sheath, subacromial deltoid sac, shoulder and elbow joints. In addition, the skin biopsy of the cutaneous lesion on the left calf revealed basket-weave keratinization in the epidermis, occasional necrotic keratinocytes in the upper spinous layer, and interface vacuolar degeneration. The superficial dermis exhibited infiltration of lymphocytes, a few eosinophils, and neutrophils around small blood vessels (Figure 2).
 |
Table 1 Alterations in Laboratory Examinations Pre- and Post-Treatment |
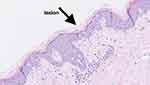 |
Figure 2 Histopathologic images of the patient’s skin lesions (HE × 200).The arrow in the figure indicates the precise lesion of the patient. |
The patient met the Yamaguchi criteria4 and was given the diagnosis of AOSD due to her clinical presentation, which included daily fevers, sore throats, polyarthralgia, and workup significant for elevated SF in the absence of another definite cause, including infectious, autoimmune, and hematological etiology.
The patient was administered intravenous methylprednisolone succinate at a daily dose of 40 mg.Simultaneously take ibuprofen sustained-release capsules (0.3g bid) orally to alleviate symptoms. As a result, the patient’s fever subsided, with no significant improvement in skin lesions, joint and muscle aches, or sore throat.On the 6th day of treatment, she received a single intravenous infusion of 400 mg of TCZ (8 mg/kg).After using TCZ, the patient’s symptoms improved. Subsequently, on the 10th day of treatment, the patient switched to oral prednisone at a dose of 40 mg to provide ongoing treatment. During the treatment of this patient, we routinely chose oral polyene phosphatidylcholine capsules (456mg tid) combined with dicyclol tablets (25mg tid) to alleviate her liver injury.Ultimately, the patient exhibited significant improvement in both skin rash and joint pain symptoms, leading to her subsequent discharge.
During the 4-week follow-up after discharge, the patient’s skin lesions, fever, and joint pain gradually disappeared. Repeat testing showed a SF level of 1356 ng/mL and an IL-6 level of 10.82 pg/mL (Table 1). In the 4th week of treatment, the patient received another intravenous infusion of 400 mg of TCZ (8 mg/kg). Throughout the 4-week follow-up period, the patient remained afebrile and reported an absence of discomfort such as headache, fatigue, or sore throat. The skin rash and joint pain were completely resolved (Figure 1). On the 9th week of treatment, repeat testing showed the following levels: IL-6 2.89 pg/mL, SF 195.8 ng/mL, WBC 7.8×109/L, and CRP 20.01 mg/L (Table 1 and Figure 3). Additionally, the patient’s prednisone dose was tapered to 15 mg.We monitored the patient for six months, and throughout that time she experienced no further fever, rash, joint pain, or discomfort. Liver function and SF were not markedly abnormal, according to ancillary assays.With no relapse of the patient’s illness during this time, we lowered the prednisone dose until discontinuance and gradually increased the TCZ dosing interval.
 |
Figure 3 Dynamic changes of inflammatory markers. |
Discussion
AOSD is a systemic inflammatory disorder characterized by an unclear etiology and pathogenesis. Its principal clinical symptoms encompass fever, transient polymorphic rash, and arthritis or joint pain. At present, there is no unified treatment plan for AOSD. Commonly used drugs include NSAIDs, glucocorticoids, and disease-modifying anti-rheumatic drugs (DMARDs). Due to the varying degrees of AOSD, different organ injuries are often combined. Therefore, treatment should be based on the progression of the disease, alleviating existing symptoms, and preventing recurrence and complications as the main treatment principles.Glucocorticoids are the first-line therapeutic approach for AOSD and demonstrate a certain clinical efficacy in approximately 60% of patients.5 For AOSD, when the efficacy of NSAIDs alone is not satisfactory, hormones can be started. The initial dose of hormones should be between 0.5~1mg/kg/d, and in clinical practice, prednisone with a dose of 0.8~1mg /kg/d is more effective.However, their usage may lead to steroid dependence and adverse reactions. Interleukin-1β (IL-1β) and IL-18 play crucial roles in the inflammatory factors of AOSD and exhibit a close association with disease activity and severity.6 As initial cytokines involved in the pathogenesis of AOSD, IL-1β and IL-18 stimulate the production of IL-6, TNF-α, and IFN-γ through distinct signaling pathways, thereby triggering onset of the disease.7 Consequently, blocking the transmission of these cytokines has emerged as a novel therapeutic strategy for the treatment of AOSD.
In cases of recurrent refractory episodes or severe life-threatening systemic symptoms in AOSD, the utilization of biologic agents may be considered for treatment.8 Various categories of biologic agents are available for the management of AOSD, including TNF-α inhibitors, IL-1 inhibitors, IL-6 inhibitors, and other biologics.9 Among these, TNF-α inhibitors were the earliest biologic agents employed in AOSD treatment and have demonstrated efficacy in patients with chronic arthritis.10 A retrospective study comparing the effectiveness of different biologic agents in treating AOSD revealed that TNF-α inhibitors exhibited lower efficacy in compared to IL-1 inhibitors and IL-6 inhibitors, suggesting a potential requirement for switching to the other two biologics to sustain disease remission.11 However, the specific mechanisms remain incompletely elucidated, implying that the significance of IL-1 and IL-6 exceeds that of TNF-α in the pathogenesis of AOSD. In clinical trials, Anakinra, an IL-1 inhibitor, has been investigated and demonstrated significant efficacy in alleviating clinical and serological manifestations, with discernible therapeutic benefits observed within a short timeframe of 3 months.12 Nonetheless, a subset of patients still exhibits a suboptimal response to anti-TNF-α and IL-1 receptor blockers.
Research has demonstrated a significant upregulation of IL-6 and TNF-α in the skin histopathology and plasma of patients with AOSD, which is one of the reasons contributing to the clinical symptoms of inflammatory disease and arthritis.13 Meanwhile, TNF-α exhibits increased levels in the plasma and skin tissue of patients, albeit lacking a significant association with disease activity. Notably, the administration of TNF-α antagonists has not demonstrated substantial symptom relief in patients. In untreated AOSD patients, elevated levels of IL-6 have been observed in both the erythematous rash and serum, and it is closely related to disease activity.14 IL-6 is intimately associated with the clinical symptoms of AOSD, including fever and rash, and is implicated in the hepatic production of acute-phase proteins.3 These studies provide a prerequisite for the clinical application of IL-6 inhibitors.
TCZ is a humanized monoclonal antibody that specifically targets the IL-6 receptor, which selectively recognizes both membrane-bound and soluble forms of the IL-6 receptor, resulting in the inhibition of IL-6 activity. Clinical studies have demonstrated that TCZ exerts favorable effects on systemic and articular symptoms in refractory AOSD patients, leading to a substantial reduction in corticosteroid requirements. Notably, patients show a rapid response to TCZ treatment and achieve sustained clinical remission, intriguingly persisting for at least 6 months even following discontinuation of treatment. Additionally, TCZ has demonstrated a favorable safety profile15 and tolerability.13 Typically, it is administered intravenously at a dosage of 5 to 8 mg/kg every 2 to 4 weeks. In order to refine and determine the optimal therapeutic regimen for TCZ in AOSD, it is essential to conduct larger-scale randomized controlled studies in the future. In addition, it is worth paying special attention to the fact that patients with allergies to TCZ and active infections should not use this drug. Patients with liver function damage, a neutrophil count below 2×109/L, a platelet count below 100×103/μL, and elderly patients should use TCZ with caution. Meanwhile, the successful use of tocilizumab has encouraged the development of other biologic agents specifically targeting the IL-6 pathway, either directed against IL-6 cytokine (sirukumab, olokizumab, and clazakizumab) or IL-6 receptor (sarilumab).
As shown in this case, TCZ can rapidly control the symptoms of skin lesions, joint and muscle pain, and sore throat in adult patients with recurrent AOSD, effectively reduce all inflammatory indexes, and facilitate the reduction of glucocorticoid dose. When the glucocorticoid dose is reduced to a lower level, the interval of TCZ administration can be gradually extended.However, the US Food and Drug Administration has not yet approved TCZ as a therapeutic drug for AOSD. Therefore, more multicenter collaborative research is needed as a basis to enhance the persuasiveness of TCZ as a treatment choice for AOSD patients, especially in low- and middle-income countries and regions.
Conclusions
We report the case of a 37-year-old Chinese female patient with AOSD presenting with recurrent fever, skin erythema, itching, and joint pains in the extremities, successfully treated with TCZ. In addition, TCZ can also be used in refractory AOSD patients with macrophage activation syndrome. In clinical practice, TCZ is usually administered subcutaneously every 2–4 weeks at a dose of 5–8mg/kg. However, the exact position of tocilizumab in the treatment of AOSD requires confirmation through extensive, large-scale clinical trials, and the optimal timing for its administration remains uncertain. Consequently, extensive randomized controlled trials are essential in future work to further substantiate the efficacy and safety of TCZ in AOSD treatment, as well as to establish treatment guidelines.
Ethics Approval
The Declaration of Helsinki’s guiding principles were followed in the creation of this article. In compliance with local law and institutional norms, publishing the case information did not require ethical review or clearance. The authors attest that the patient’s written consent has been received for the submission and publishing of this case report, including the data and photographs.
Acknowledgments
We are incredibly appreciative to our kind patient for enabling us present her case.
Funding
This study was funded by the Research Capacity Enhancement Hundred Talents Program of Hospital of Chengdu University of Traditional Chinese Medicine (20B04).
Disclosure
Xiaojing Zhong, Tongtong Xu and Tianhao Li are co-first authors for this study. The authors have no conflicts of interest to declare in this work.
References
1. Rao S, Tsang LSL, Zhao M, et al. Adult-onset Still’s disease: a disease at the crossroad of innate immunity and autoimmunity. Front Med. 2022;9:881431. doi:10.3389/fmed.2022.881431
2. Macovei LA, Burlui A, Bratoiu I, et al. Adult-onset still’s disease-a complex disease, a challenging treatment. Int J Mol Sci. 2022;23(21):12810. doi:10.3390/ijms232112810
3. Maranini B, Ciancio G, Govoni M. Adult-onset still’s disease: novel biomarkers of specific subsets, disease activity, and relapsing forms. Int J Mol Sci. 2021;22(24):13320. doi:10.3390/ijms222413320
4. Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19(3):424–430.
5. Siddiqui M, Putman MS, Dua AB. Adult-onset Still’s disease: current challenges and future prospects. Open Access Rheumatol. 2016;8:17–22. doi:10.2147/OARRR.S83948
6. Giampietro C, Fautrel B. Anti-Interleukin-1 agents in adult onset still’s disease. Int J Inflam. 2012;2012:317820. doi:10.1155/2012/317820
7. Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult-onset Still’s disease. Autoimmun Rev. 2014;13(7):708–722. doi:10.1016/j.autrev.2014.01.058
8. Ma Y, Meng J, Jia J, et al. Current and emerging biological therapy in adult-onset Still’s disease. Rheumatology. 2021;60(9):3986–4000. doi:10.1093/rheumatology/keab485
9. Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi:10.1038/s41584-020-0419-z
10. Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol. 2018;14(10):603–618. doi:10.1038/s41584-018-0081-x
11. Zhou S, Qiao J, Bai J, et al. Biological therapy of traditional therapy-resistant adult-onset Still’s disease: an evidence-based review. Ther Clin Risk Manag. 2018;14:167–171. doi:10.2147/TCRM.S155488
12. Vastert SJ, Jamilloux Y, Quartier P, et al. Anakinra in children and adults with Still’s disease. Rheumatology. 2019;58(Suppl6):vi9–vi22. doi:10.1093/rheumatology/kez350
13. Castañeda S, Martínez-Quintanilla D, Martín-Varillas JL, et al. Tocilizumab for the treatment of adult-onset Still’s disease. Expert Opin Biol Ther. 2019;19(4):273–286. doi:10.1080/14712598.2019.1590334
14. Chen DY, Lan JL, Lin FJ, et al. Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still’s disease. Ann Rheum Dis. 2004;63(10):1300–1306. doi:10.1136/ard.2003.013680
15. Ortiz-Sanjuán F, Blanco R, Calvo-Rio V, et al. Efficacy of tocilizumab in conventional treatment-refractory adult-onset Still’s disease: multicenter retrospective open-label study of thirty-four patients. Arthritis Rheumatol. 2014;66(6):1659–1665. doi:10.1002/art.38398
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.