Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Successful Treatment of Non-Langerhans Cell Histiocytosis With Topical Rapamycin in Two Pediatric Cases
Authors Effendi RMRA , Rizqandaru T , Yuliasari R , Gondokaryono SP , Diana IA, Dwiyana RF
Received 30 May 2022
Accepted for publication 28 July 2022
Published 6 August 2022 Volume 2022:15 Pages 1575—1582
DOI https://doi.org/10.2147/CCID.S375995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Raden Mohamad Rendy Ariezal Effendi, Trustia Rizqandaru, Renata Yuliasari, Srie Prihianti Gondokaryono, Inne Arline Diana, Reiva Farah Dwiyana
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, Indonesia
Correspondence: Raden Mohamad Rendy Ariezal Effendi, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur no. 38, Bandung, West Java, 40161, Indonesia, Tel +62 22 2032426, Email [email protected]
Abstract: Non-Langerhans cell histiocytosis (non-LCH) is a group of diseases characterized by the proliferation of histiocytes in tissues that is excluded from the diagnostic criteria for LCH. Juvenile xanthogranuloma (JXG) and benign cephalic histiocytosis (BCH) are the most common types of cutaneous non-LCH. These two diseases share similarities in both clinical and histological features, therefore, they can be difficult to differentiate. Thorough physical, dermoscopic, and histopathological examinations are required to distinguish between JXG and BCH. We hereby present two rare cases of non-LCH in pediatric patients, presented with JXG and BCH. The dermoscopic examination of both cases showed a setting-sun appearance, while the histopathological examination revealed Touton giant cells in the JXG case, and massive lymphocyte infiltration in the BCH case. Both patients were treated with 1% topical rapamycin in a split-side comparison for the first 12 weeks, followed by applications on both sides for a total duration of 24 weeks. As a result, there was a significant reduction in the size of the lesion, leading to patient’s satisfaction. Rapamycin is an immunosuppressive agent with antineoplastic activity. Rapamycin can be used as an alternative non-invasive topical treatment option for JXG and BCH. However, long-term observations are required to assess its effectiveness and side effects.
Keywords: benign cephalic histiocytosis, dermoscopy examination, histopathological examination, juvenile xanthogranuloma, non-Langerhans cell histiocytosis
Introduction
Histiocytoses are a rare disorder characterized by the accumulation of macrophage, dendritic cell, or monocyte-derived cells in various tissues.1,2 In a recent update published by the Histiocyte Society,3 the Langerhans and non-Langerhans dichotomy has been questioned due to frequent overlaps of their clinical manifestations in both groups. As a result, they introduced a new classification consist of 5 groups of diseases: Langerhans-related (L), cutaneous and mucocutaneous (C), malignant histiocytoses (M), Rosai-Dorfman disease (R), as well as hemophagocytic lymphohistiocytosis and macrophage activation syndrome (H). Juvenile xanthogranuloma (JXG) and benign cephalic histiocytosis (BCH) are classified into the group C. However, the incidence of JXG and BCH remains unclear.4 The lack of epidemiological data on both diseases may be attributed to underdiagnosis or even misdiagnosis, as well as its self-limiting nature.5 Both JXG and BCH are characterized by yellowish-red papules or nodules on the face, neck, and trunk.1,2,6 The clinical and histopathological features of these diseases are almost indistinguishable and may be overlapping.7–9 Differences in histopathological features include the presence of Touton giant cells in JXG, while histiocyte infiltration can be found in both diseases.8–10
Both JXG and BCH that are limited to the skin do not require specific treatment. The majority of patients have complete resolution (57.7%), while in some cases (6.6%) may leave a residual scar.11 Certain treatment can be considered for rapidly growing skin-limited cases that raise aesthetic concerns.12 However, this therapeutic approach may not always be consensual and should be evaluated on a case-by-case basis. Therapeutic options may include low-dose radiotherapy, intralesional steroid injections, cryotherapy, CO2 laser treatment, and surgical excision.1,2,6 At present, there is only one case report that utilizes non-invasive treatment for BCH using topical rapamycin that leads to a significant reduction in skin lesions.13 Unfortunately, there is no data for JXG case treated with topical rapamycin.
Rapamycin is an immunosuppressive agent with antineoplastic activity through the inhibition of angiogenesis and growth signal corrections in several tumours.14 In this article, we present two cases of cutaneous non-LCH: JXG in a 2-year-old boy and BCH in an 11-month-old boy. Both patients were treated with 1% topical rapamycin in a split-side comparison for the first 12 weeks, followed by applications on both sides for a total duration of 24 weeks. The result was based on photodocumentation and reduction in the size of the lesion.
Case Presentation
Case 1
A 2-year-old boy was presented to a pediatric dermatology clinic with a chief complaint of non-itchy and painless tan-orange colored papules on the face, trunk, both upper arms and legs. These lesions first appeared when the patient was one-year-old. They initially started on the right cheek, and gradually spread to other areas of the face, the trunk, and the legs. There were no abnormalities at birth or a history of any previous trauma. The patient’s familial and personal medical history were unremarkable.
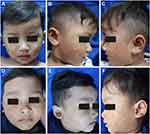
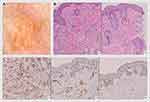
Dermatological examination revealed multiple lesions in the form of tan-orange papules and plaques on the face, ears (Figure 1A–C), trunk, both upper arms, and legs. Dermoscopy examination showed a brownish-yellow hue resembling “setting-sun appearance” (Figure 2A). The histopathological features of the lesions include infiltration of lymphocytes, foam cells, and histiocytes in the upper dermis. Additionally, histiocytes-forming Touton giant cells were also observed, supporting the diagnosis of JXG (Figure 2B). Furthermore, the immunohistochemistry analysis revealed that the cells were positive for CD68 and S100 (Langerhans cell and nerve cell), and negative for CD1a (Figure 2C).
The patient’s parents requested for the facial region to be prioritized due to its visibility, and they showed a preference towards non-invasive treatment. Therefore, topical rapamycin therapy (0.5 mg Certican® everolimus compounded into 1% ointment) was initiated. It was applied twice daily to the right cheek, as per the family’s preference, where the skin lesions first appeared. After 12 weeks of treatment, the lesions on the right cheek improved as it started to flatten and fade. The size of the largest lesion decreased from 25x10x3 mm to 10x8x1 mm. Encouraged by the results and lack of local side effects (such as irritation), the family opted to treat both of his cheeks. After 24 weeks of using topical rapamycin on both sides, most of the skin lesions on his face had flattened and faded (Figure 1D–F).
Case 2
An 11-month-old boy presented with multiple papules and nodules on his scalp, retro-auricular region, face, and chest, which were first noted at the age of 6 months. The number and size of the papules gradually increased over the past 5 months. His personal and family history were unremarkable, and there were no abnormalities reported at birth or previous history of any trauma.
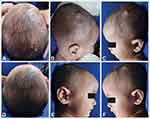
On physical examination, the lesions presented as multiple, discrete, asymptomatic, tan-orange nodules on the scalp and papules on the retroauricular region, face, and chest, with variable sizes, with the largest one being 15 mm in diameter (Figure 3A–C). Upon dermoscopy examination, a brownish-yellow structureless center with a pale erythematous halo lesion was observed, indicating a “setting-sun appearance” (Figure 4A). Histopathological examination revealed fibrocollagenous connective tissue on the dermis with massive histiocytic infiltration (Figure 4B). On immunohistochemistry analysis, the cells stained positive for CD68, but weak positive for S100 and negative for CD1a (Figure 4C). These findings were suggestive of BCH diagnosis.
Non-invasive treatment was considered by his family considering the patient’s age. Topical rapamycin (0.5 mg Certican® everolimus compounded into 1% cream) was initiated by applying twice daily to the scalp on the right side of the head, where the largest skin lesion appeared. After 12 weeks of treatment, the size of the lesion was reduced from 15x10x8 mm to 8x8x6 mm. No local side effects, such as irritation, were reported. Treatment was continued until 24 weeks for the rest of the BCH lesions on the scalp, where most of the skin lesions decreased in size and faded (Figure 3D–F).
Discussion
According to a 35-year tumor register data from the University of Kiel in Germany (1965–2001), the incidence of JXG was reported to be 129 out of 24,600 tumor cases in children (0.5%).5 Meanwhile, 2002–2019 Singaporean retrospective data reported 147 patients with JXG.11 The incidence of JXG15 and BCH4 was higher in boys compared to girls (1.3:1 and 1.2:1, respectively). Existing literature stated that JXG is a disease of the young child, with a median age of onset at 2 years, but lesions may present at birth.15 In BCH, skin lesions may develop at 3–36 months of age, most commonly before 6 months of age (45%).4 In the JXG patient reported in this article, cutaneous lesions first appeared at the age of 14 months, while in the BCH patient, the lesions first initiated at the age of 6 months.
Skin lesions of JXG and BCH can overlap. Both diseases may show solitary or multiple reddish-brown papules or nodules. Statistically, initial lesions of JXG can be found on the head and neck (44.2%), lower extremities (21.8%), upper extremities (9.5%), trunk (6.8%), and more than one side of the body can be affected (12.9%).11 In a report of 40 patients diagnosed with BCH, the most common affected sites are cheeks (22%), eyelids (13%), and ears (10%), and only one case (2.5%) at the scalp.16 Some evidence mentions that BCH is a histopathological variation or part of the JXG spectrum, as both diseases show infiltration of histiocytes. Nevertheless, BCH can be distinguished from JXG by the absence of Touton giant cells in histopathological examination.8,9 Both patients in our report had similar pathognomonic skin lesion, yet the histopathological examination results of Case 2 showed no findings of Touton Giant Cell, leading to a diagnosis of BCH.
Dermoscopy can be used as a supporting examination to aid in establishing the diagnosis of JXG.17,18 The dermoscopic appearance of JXG can vary depending on the evolution of the lesion. Initially, the characteristic dermoscopic feature of JXG lesions is the presence of xanthomatous cells, known as “setting-sun appearance”. In more advanced lesions, pale yellow to yellow-orange globules and white streaks may be seen,18 and branched or straight blood vessels may also be present around the skin lesion.17 To date, there are still limited data or reports regarding dermoscopic features of BCH; therefore, the characteristic dermoscopy feature of BCH remains unclear. In our case, “setting-sun appearance” was observed in both cases. The difference between JXG and BCH is shown in Table 1.
 |
Table 1 Differentiation Between Juvenile Xanthogranuloma and Benign Cephalic Histiocytosis |
JXG and BCH cases that are limited to the skin require no therapy, although diagnostic and therapeutic surgical excision is occasionally performed for cosmetic purposes.6 In one study reporting 33 cases of congenital JXG diagnosed at the age of 0–14 months in New York, United States, 22 cases was treated with placebo, while the rest received excision (8 cases), intralesional steroid injection (2 cases), and systemic steroid (one case – where the patient had congenital ophthalmic juvenile xanthogranuloma with thrombocytopenia).19 Ekinci et al4 reported eleven cases of BCH in children aged 9–72 months, all of which were left untreated and they achieved spontaneous resolution within a time span of 1–8 years.
To date, topical treatment for BCH and JXG is the preferred option by the patient’s parents and physician, although it has rarely been used. However, there is one case report of topical drug administration in BCH patients showing satisfactory results, as indicated by significant thinning of the skin lesions.13 There were no reports of JXG cases treated with rapamycin. Based on the understanding that BCH and JXG both belong to the same group of diseases and have identical pathogenesis, both patients were treated with 1% topical rapamycin.
Rapamycin is a fermented product of Streptomyces hygroscopicus with the ability to inhibit serine or threonine protein kinases in its mammalian target (mTOR).20 Its active ingredient has an antineoplastic effect that works by inhibiting angiogenesis and correcting growth signals that are pivotal in some tumors. Several studies have reported the efficacy of topical rapamycin administration for angiofibroma in patients with tuberous sclerosis complex (TSC).14,21,22
In non-LCH cases, systemic rapamycin has been utilized in Erdheim-Chester disease (ECD).13,23 Giafrenda et al23 reported treatment with systemic rapamycin (sirolimus) at an initial dose of 0.75 mg/kg and gradually reducing the dose to 2.5–5 mg/day for 6 months in 10 ECD patients. The result showed that 8 out of 10 ECD patients achieved a stable condition. Mutations in the mTOR gene were found in cases of ECD; therefore, the use of rapamycin played an important role in these cases.13 The pathogenesis of JXG and BCH remains uncertain, however, as both conditions being in the same histiocytosis group, there is a possibility for them to respond to Rapamycin.
Several studies have also reported the efficacy of topical rapamycin therapy for angiofibroma with different vehicle, concentration, and frequency of administration (0.003%–1%).14,21,22 Koenig et al24 reported a ratio of 1% to 0.1% rapamycin administration in 179 angiofibroma patients. The results showed that rapamycin at 1% concentration showed better results, indicated by a higher angiofibroma grading scale at the score of 16.7 and 11 for 1% and 0.1% concentrations, respectively. In 2 previous studies using topical rapamycin to treat BCH, the 1% concentration was used.13 Side effects occurring at this concentration were minimal in both studies.13,24 Therefore, both patients in our case received 1% rapamycin for all skin lesions.
In our study, the topical rapamycin (0.5 mg Certican® everolimus compound into 1% ointment) was initially applied twice daily for 12 weeks to the right check in the JXG patient, and to the right scalp for the BCH patient. The skin lesion improvements were assessed through clinical photographic monitoring and lesion measurement, due to the lack of an existing measurable scale for assessing the progression in JXG and BCH lesions. In both cases, we observed improvements on the treated side with a marked reduction in the size of the lesions, whereas on the untreated side, there was no change in the size of the lesions. In Case 1, the size of the largest lesion on the right side of the face was reduced from 25x10x3 mm to 10x8x1 mm after 12 weeks. In Case 2, skin lesions also significantly improved, with a reduction in the size of the largest lesion on the right scalp from 15x10x8 mm to 8x8x6 mm. Encouraged by the half-part results and lack of side effects, the treatment was resumed for both sides until 24 weeks. A high variability of evidence exists regarding the duration of treatment for topical rapamycin (4–24 weeks).22 We opted for 24 weeks as the duration of treatment for these two cases based on the longest duration that has been reported, while during these periods, evaluation for recurrence can be performed.
Koenig et al24 conducted a study on the efficacy and side effects of topical rapamycin in 59 TSC patients with angiofibroma. Side effects were found in 6 patients, which included pain (10.2%), itching (8.5%), acne (5.1%), redness (3.4%), and irritation (1.7%). Case reports on the use of topical rapamycin in BCH patients did not report any topical or systemic side effects.13 Systemic use of rapamycin can lead to side effects, such as hypersensitivity, increased risk of infection, and skin cancer. Research on the topical use of rapamycin in cases of TSC and BCH showed that no systemic absorption was found. Rapamycin can be used as an alternative topical treatment option in JXG and BCH cases that require therapy. Neither patient in this case reported any side effects from the use of topical rapamycin and their parents were satisfied with the end results.
Conclusion
The use of topical rapamycin in both cases gave satisfactory results. Rapamycin can be used as an alternative for non-invasive topical treatment options for JXG and BCH. However, long-term observation of effectiveness and side effects should be evaluated.
Ethics and Consent Statements
The parents’ consent and institutional approval were obtained in order to publish the case, including the case details and images.
The parents of the patients signed a consent form for the publication of the case details and images. The case report has been approved by the institutional ethics committee of Dr. Hasan Sadikin General Hospital, Bandung, Indonesia (Ethical Clearance Number: LB.02.01/X.6.5/27/2022).
Acknowledgments
The authors would like to thank the staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schmieder A, Goerdt S, Utikal J, et al. Histiocytosis. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, editors. Fitzpatrick’s Dermatology. 1,
2. Ratzinger G, Zelger BWH. Juvenile Xanthogranuloma and Other Non-Langerhans Cell Histiocytoses. In: Hoeger P, Kinsler V, Yan A, editors. Harper’s Textbook of Pediatric Dermatology. Juvenile Xanthogranuloma and Other Non-Langerhans Cell Histiocytoses. Pondicherry: Wiley-Blackwell; 2020:1078–1096.
3. Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood J Am Soc Hematol. 2016;127(22):2672–2681.
4. Polat Ekinci A, Buyukbabani N, Baykal C. Novel clinical observations on benign cephalic histiocytosis in a large series. Pediatr Dermatol. 2017;34(4):392–397. doi:10.1111/pde.13153
5. Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the Kiel pediatric tumor registry. Am J Surg Pathol. 2005;29(1):21–28. doi:10.1097/01.pas.0000147395.01229.06
6. Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology. Elsevier; 2016.
7. Koca R, Bektaş S, Altinyazar HC, Sezer T. Benign cephalic histiocytosis: a case report. Ann Dermatol. 2011;23(4):508–511. doi:10.5021/ad.2011.23.4.508
8. Rapini R. Non-Infectious Granuloma. In: Rapini R, editor. Practical Dermathopathology. Houston: Sanders; 2014. 115–116.
9. Patterson JW. Cutaneous infiltrates-nonlymphoid. In: Patterson JW, editor. Weedon’s Skin Pathology. Dallas: Churcill Livingstone; 2016:1149–1151.
10. Elder DE, Elenitsas R, Johnson B, Loffreda M, Miller J, Miller OF. Histopathology of the skin. In: Elder DE, Elenitsas R, Johnson B, Loffreda M, Miller J, Miller OF, editors. Histopathology of the skin. Philadelphia: Lippincot William and Wilkin; 2007.
11. Wee LWY, Ling HY, Ho VPY, Foong AYW, Koh MJA. Juvenile xanthogranulomas in Asian children. Dermatol Ther. 2022;35(2):e15224. doi:10.1111/dth.15224
12. Park YW, Koh EJ, Choi HY. Rapid-growing juvenile xanthogranuloma on the scalp in 18-month-old girl. J Korean Neurosurg Soc. 2011;50(3):271. doi:10.3340/jkns.2011.50.3.271
13. Habeshian K, Silverman RA, DeKlotz CM. Treatment of benign cephalic histiocytosis with topical 1% rapamycin ointment. Pediatr Dermatol. 2019;36(3):411–413. doi:10.1111/pde.13800
14. Haemel AK, O’Brian AL, Teng JM. Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol. 2010;146(7):715–718. doi:10.1001/archdermatol.2010.125
15. Dehner LP. Juvenile xanthogranulomas in the first two decades of life: a clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003;27(5):579–593. doi:10.1097/00000478-200305000-00003
16. Jih DM, Salcedo SL, Jaworsky C. Benign cephalic histiocytosis: a case report and review. J Am Acad Dermatol. 2002;47(6):908–913. doi:10.1067/mjd.2002.124602
17. Micali G, Verzl AE, Tedeschi A, Lacarruba F. Juvenile xanthogranuloma. In: Micali G, Lacarruba F, Stinco G, Argenziani G, Neri I, editors. Atlas Pediatric Dermatoscopy. Gewerbestrasse: Springer; 2018:205–212.
18. Bañuls J, Arribas P, Berbegal L, DeLeón F, Francés L, Zaballos P. Yellow and Orange in cutaneous lesions: clinical and dermoscopic data. J Eur Acad Dermatology Venereol. 2015;29(12):2317–2325. doi:10.1111/jdv.13249
19. Oza VS, Stringer T, Campbell C, et al. Congenital‐type juvenile xanthogranuloma: a case series and literature review. Pediatr Dermatol. 2018;35(5):582–587. doi:10.1111/pde.13544
20. Madke B. Topical rapamycin (sirolimus) for facial angiofibromas. Indian Dermatol Online J. 2013;4(1):54. doi:10.4103/2229-5178.105488
21. Mutizwa M, Berk D, Anadkat M. Treatment of facial angiofibromas with topical application of oral rapamycin solution (1 mg mL− 1) in two patients with tuberous sclerosis. Br J Dermatol. 2011;4(165):922–923. doi:10.1111/j.1365-2133.2011.10476.x
22. Vasani R. Topical sirolimus in the treatment of facial angiofibromas. Indian J Drugs Dermatol. 2018;4(2):49. doi:10.4103/ijdd.ijdd_34_18
23. Gianfreda D, Nicastro M, Galetti M, et al. Sirolimus plus prednisone for Erdheim-Chester disease: an open-label trial. Blood J Am Soc Hematol. 2015;126(10):1163–1171.
24. Koenig MK, Bell CS, Hebert AA, et al. Efficacy and safety of topical rapamycin in patients with facial angiofibromas secondary to tuberous sclerosis complex: the TREATMENT randomized clinical trial. JAMA dermatol. 2018;154(7):773–780. doi:10.1001/jamadermatol.2018.0464
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.