Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Successful Treatment of Eruptive Pruritic Papular Porokeratosis in the Elderly with Tofacitinib: A Case Report
Authors Mu X, Li W, Zhang M, Yang C, Yang X, Li D, Ding Y
Received 15 March 2023
Accepted for publication 8 June 2023
Published 6 July 2023 Volume 2023:16 Pages 1741—1747
DOI https://doi.org/10.2147/CCID.S412495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xingyu Mu,1,* Wen Li,2,* Ming Zhang,2 Changxiao Yang,2 Xianxu Yang,2 Dan Li,2 Yan Ding3
1The First Clinical College of Hainan Medical University, Haikou, Hainan, People’s Republic of China; 2Department of Dermatology, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 3Department of Dermatology, Affiliated Dermatology Hospital of Hainan Medical University, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dan Li, Department of Dermatology, The Fifth People’s Hospital of Hainan Province, No. 8 Longhua Road, Longhua District, Haikou, Hainan, People’s Republic of China, Tel +8613807656023, Email [email protected] Yan Ding, Department of Dermatology, Affiliated Dermatology Hospital of Hainan Medical University, The Fifth People’s Hospital of Hainan Province, No. 8 Longhua Road, Longhua District, Haikou, Hainan, People’s Republic of China, Tel +8618689980361, Email [email protected]
Abstract: Eruptive pruritic papular porokeratosis (EPPP) is a rare subtype of porokeratosis that presents as an acute exacerbation of an annular papule with a distinct peripheral hyperkeratotic ridge border and severe pruritus. EPPP is mainly reported in elderly East Asian men. Its etiology and pathogenesis are unknown. We hereby present a case report of EPPP in a 68-year-old Chinese male with persistent circumscribed papules on the extremities, accompanied by severe pruritus for one year. After the patient was given conventional medication, a new rash appeared on the patient’s extremities and he felt intense itching in the area of the rash. The patient was switched to oral tofacitinib treatment. The patient felt that the pruritus had largely disappeared after one month of oral dosing, leaving only brown pigmentation on the erythema of the extremities. The patient has been off the drug for 2 months. There was no pruritus or new rash during the follow-up period.
Keywords: eruptive pruritic papular porokeratosis, porokeratosis, tofacitinib
Introduction
Porokeratosis is a group of diseases that manifest clinically as an annular keratotic papule with raised margins and a distinctive histological keratin-like laminae. There are several common types of porokeratosis: porokeratosis of Mibelli, disseminated superficial porokeratosis (DSP), disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, disseminated palmoplantar porokeratosis. there are also some rare types such as porokeratosis ptychotropica, porokeratoma, and pruritic papular porokeratosis.1–5 The exact incidence and prevalence of porokeratosis is unknown. EPPP predominantly occurs in older men in East Asia.6 Porokeratosis development may be associated with genetic factors, immunosuppression, ultraviolet radiation, electron beam therapy, chronic systemic diseases, infectious diseases and neoplastic diseases.3–5 Although porokeratosis is thought to be caused by abnormal clonal proliferation of porokeratosis, its pathogenesis is unclear. A suggested hypothesis is that inflammation in EPPP may be an immune response to tumourigenic keratinocyte clones leading to altered maturation of keratinocytes or accelerated epidermal proliferation.6 Here we report a case of a patient who presented with EPPP on the extremities and trunk with no known cause, and was treated in our dermatology department with oral “antihistamines, avobenzone and hydroxychloroquine” and topical “tretinoin cream, glucocorticoid ointment and allantoin cream”. He still felt itchy occasionally and the rash on the extremities partially subsided. Scattered corn to soy-sized pale dark red and pale brown macules with clear borders and no fusion were visible on the extremities, with some slightly elevated margins and keratosis or mild atrophy in the middle. After being discharged from the hospital, the patient was treated at our dermatology outpatient clinic with regular daily oral doses of Acetaminophen and topical rubbing of Tretinoin A cream for 3 months, new rashes appeared on the buttocks and both lower extremities, gradually increasing, with intense itching. After one month of treatment with tolvatinib, the pruritus largely disappeared and only brown pigmentation remained on the erythematous areas of the extremities. A review of the literature shows that this case is the first successful case of EPPP treated with tofacitinib.2–10
Case Presentation
A 68-year-old Chinese male patient with no known trigger developed a small erythematous, maculopapular rash on both lower limbs in May 2021, accompanied by pruritus. He was not concerned and was not treated. Subsequently, a new rash appeared on his trunk and both upper limbs and the itching worsened. He came to the Department of Dermatology at the Fifth People’s Hospital of Hainan Province and after treatment with oral antihistamines and topical hormone creams, his itching was relieved but the rash could not subside completely. In May 2022, his condition recurred with a gradually increasing rash on his trunk and extremities, mainly on his extremities, with significant itching. He used topical creams on his own without significant improvement. He again visited the Department of Dermatology at the Fifth People’s Hospital of Hainan Province. He was admitted to our inpatient dermatology unit on 9 June 2022. The patient had no history of chronic disease and there was no family history of genetic disorders, psychiatric disorders, tumours and similar conditions to this disease.
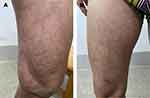
On physical examination, many pale red macules and papules (2–5 mm in diameter) with clear borders without fusion were found on the extremities. The rash was mildly atrophic in the middle, with brown ring-like elevations around the edges and a few adherent scales (Figure 1A and B). The rash did not involve the palms of the hands, soles of the feet or the oral mucosa.
In June 2022, the patient’s blood tests revealed total immunoglobulin E: 757.1 IU/mL, alanine aminotransferase 48 U/L, total cholesterol 5.89 mmol/L and Low density lipoprotein cholesterol 4 mmol/L. Other tests such as Electrocardiogram, Chest CT, Blood routine, C-reactive protein, Urine routine, Stool routine, Faecal occult blood, Blood glucose, Cardiac enzymes, Electrolytes, renal function, Immunoassays, Blood sedimentation, Treponema pallidum particle agglutination test, Hepatitis B virus, HIV antibodies and Tumour screening did not show any significant abnormalities.
Biopsy specimens were taken from the patient’s right upper and left lower extremity skin papules in June and July 2022 respectively. Pathological examination shows epidermal hyperplasia with focal hyperkeratosis and the formation of a plate of hyperkeratosis (ie conical plate) (Figure 2). Below this plate the epithelial cells were vacuolated and the granular layer was partially absent. The superficial dermal layer was pigmented and incontinent, with focal lymphocytic and eosinophilic infiltration around the blood vessels in the dermis (Figure 3). Diagnosis: consistent with porokeratosis. Direct immunofluorescence was negative for C3, IgG, IgM and IgA.
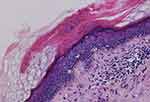 |
Figure 2 Epidermal hyperplasia with focal dyskeratosis, visible as a plate of dyskeratosis (HE, x100). |
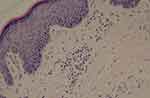 |
Figure 3 Lymphocytic and eosinophilic infiltration around blood vessels in the dermis (HE, x200). |
Based on the history, characteristics of the lesions, clinical manifestations and pathological findings, the disease was clearly diagnosed as “eruptive pruritic papular porokeratosis”. After hospitalisation, the patient was given intravenous sodium thiosulphate and vitamin C injections, oral antihistamines, etretinate 20mg twice a day and hydroxychloroquine 200mg twice a day, and topical tretinoin A cream once a day, glucocorticoid ointment twice a day and allantoin cream twice a day. After 14 days of hospitalization his rash partially subsided and the erythema darkened with occasional itching. He was discharged from hospital and attended our outpatient clinic and took regular oral etretinate 20mg twice a day and topical tretinoin A cream once a day. After 3 months of continuous treatment, the patient developed many new rashes on the buttocks and both lower limbs, which gradually increased and felt intensely itchy. Considering the widespread use of tofacitinib in pruritic inflammatory skin diseases, the efficacy is excellent. We therefore used tofacitinib for the first time in the treatment of this disease. The patient was given tofacitinib 5mg orally twice a day. At a follow-up visit half a month later, the patient’s pruritus had decreased, the erythema on the extremities had darkened and the papular ring of elevations had flattened (Figure 4). The patient continued to take tofacitinib 5mg orally twice a day and at the follow-up visit half a month later. Only light brown pigmentation was left on the erythema of the extremities and the pruritus had largely disappeared (Figure 5). In October 2022, the patient had stopped medication and no pruritus or new rash appeared during the follow-up period.
 |
Figure 4 Darkening of erythema on both lower extremities and flattening of the papular ring elevation. |
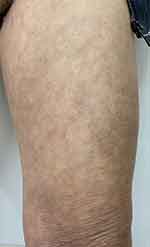 |
Figure 5 Only brown pigmentation was left on the erythema of the left thigh. |
Discussion
The patient had two pathological examinations at our hospital. The clinical consideration at the time was porokeratosis, but the patient’s rash has a distinctive erythema in the centre, accompanied by intense itching. The first pathological section showed the typical column of keratosis imperfecta in the epidermis and the second pathological section showed an eosinophilic infiltration in the dermis. In combination with the clinical presentation of a chronic persistent rash and recurrent acute pruritic episodes, the final diagnosis was EPPP.
Initially EPPP was thought to be a form of superficial DSP, but whereas DSP is generally asymptomatic, EPPP is associated with intense pruritus.2 Kanzaki first reported three cases of EPPP in 1992.6 EPPP is characterized by a history of DSP, superficially disseminated eruptive papular porokeratosis followed by the sudden appearance of scattered papules with intense pruritus in most patients. These papules may fade over several months, leaving brown spots or rings of damage, and histopathology shows a reduction or loss of the keratin-like laminae and granular layer, often with an intra-dermal eosinophilic infiltrate.1,5
This disease is also known as eruptive disseminated porokeratosis (EDP). Shoimer et al have classified EDP into four subtypes: paraneoplastic, immunosuppressive, inflammatory and other types.11 The patient’s rash was considered to be an inflammatory subtype of EDP according to the classification proposed by Shoimer. Histologically, the most common manifestations are horn-like plates and inflammatory skin infiltrates. In patients with EPPP, additional eosinophilic infiltrates are present near the upper dermal vessels in approximately 25–50% of patients.5,6,13
Porokeratosis may be triggered by several irritants, including genetic factors, exposure to electron beam radiation therapy, immunosuppressive therapy (eg chemotherapy, immunosuppressive drugs or systemic corticosteroids), ultraviolet radiation, chronic systemic diseases, infectious diseases and neoplastic diseases.3–5
Although porokeratosis is thought to be caused by abnormal clonal proliferation of keratinocytes, its pathogenesis is unclear. Inflammation in EPPP may be an immune response to tumourigenic keratinocyte clones leading to altered maturation of keratinocytes or accelerated epidermal proliferation.5–11,12 Premature apoptosis of keratinocytes underlying the cornoid lamella has been observed together with loss of the granular layer and altered loricrin and filaggrin expression.14 For some authors, DSAP is inherited as an autosomal dominant condition with reduced penetrance at younger ages.9 However, there are many cases where no positive family history of porokeratosis can be found, as in our patient. It is difficult to say whether genetic predisposition is being unmasked by the immune modulation or if an altered immune status will initiate the process of porokeratosis. Reed and Leone have suggested that in a genetically predisposed individual, the disease results from the proliferation of abnormal clones of epidermal cells.14 EPPP may be an immune response against an abnormal keratinocyte clone.4 The etiology of this immune response remains elusive, although in some cases it may be secondary to a tumour or viral infection.5 Viral infectious and neoplastic diseases have been largely excluded in the case we report, but follow-up is still required.
There is no standard treatment for EPPP. Topical corticosteroids and oral antihistamines are the most commonly used treatments. The majority of cases respond poorly to treatment.9 Complete spontaneous regression of the lesions has also been reported following changes in the patient’s immune status.7 Although a retrospective study of EPPP found that 75% of cases resolved spontaneously, with a median time to remission of 6 months (range 1–24).5,6 However, patients with EPPP are desperate for a solution to their intense itching and therefore require clinicians to choose medications that are more effective in stopping itching. Tofacitinib is an oral JAK inhibitor. It binds to the adenosine triphosphate (ATP) binding site in the catalytic cleft of the JAK kinase domain of JAK. Tofacitinib’s structure mimics that of ATP without the triphosphate group. As a result of the binding to the ATP site, tofacitinib inhibits the phosphorylation and activation of JAK, thereby preventing the phosphorylation and activation of STATs, and thus the activation of gene transcription. This leads to decreased cytokine production and modulation of the immune response.15 Anti-inflammatory and antipruritic at the root. Other therapies that have been tried in other types of porokeratosis are topical 5-fluorouracil, retinoids, calcipotriol, cryotherapy, systemic retinoids and corticosteroids.2–4,7 Topical vitamin D3 analogues, marsupialol, may be effective for pruritic papules.9
Conclusion
We first attempt to use tofacitinib was successful in treating an elderly male with EPPP. A review of the national and international literature did not reveal any reports.2–10 Although no specific studies have clarified the specific mechanism of tofacitinib in the treatment of EPPP, it could give clinicians new options and thoughts on the use of the drug. The specific therapeutic mechanism of tofacitinib still needs further research.
Ethics Statement
The publications of images were included in the patient’s consent for publication of the case. The Hospital Ethics Committees of the Fifth People’s Hospital of Hainan Province approved to publish the case details.
Consent Statements
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required for this case study.
Acknowledgments
We thank the patient for his permission to publish this information.
Funding
This project supported by Hainan Province Clinical Medical Center.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bian Z. Chinese Clinical Dermatology.
2. Tee SI, Chong WS. Eruptive pruritic papular porokeratosis. Indian J Dermatol Venereol Leprol. 2012;78(6):758–760. doi:10.4103/0378-6323.102384
3. Vargas-Mora P, Morgado-Carrasco D, Fustà-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111(7):545–560. doi:10.1016/j.ad.2020.03.005
4. Das A, Vasudevan B, Talwar A. Porokeratosis: an enigma beginning to unravel. Indian J Dermatol Venereol Leprol. 2022;88(3):291–299. doi:10.25259/IJDVL_806_20
5. Morgado-Carrasco D, Feola H, Fustà-Novell X. Eruptive pruritic papular porokeratosis or inflammatory form of disseminated superficial porokeratosis: a new case and review of the literature. Dermatol Online J. 2020;26(4):13030. doi:10.5070/D3264048345
6. Thomas L, Fatah S, Nagarajan S, Taylor WD. An intensely itchy papular eruption. Clin Exp Dermatol. 2016;41(7):834–836. doi:10.1111/ced.12905
7. Ricci C, Rosset A, Panizzon RG. Bullous and pruritic variant of disseminated superficial actinic porokeratosis: successful treatment with grenz rays. Dermatology. 1999;199(4):328–331. doi:10.1159/000018284
8. Kanzaki T, Miwa N, Kobayashi T, et al. Eruptive pruritic papular porokeratosis. J Dermatol. 1992;19:109–112. doi:10.1111/j.1346-8138.1992.tb03190.x
9. Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review. J Dermatolog Treat. 2012;23(1):52–56. doi:10.3109/09546634.2010.495381
10. Wu Q. A case of follicular sweat pore keratosis and review of literature. Med Health Technol. 2018;2018:1.
11. Shoimer I, Robertson L, Storwick G, et al. Eruptive disseminated porokeratosis: a new classification system. J Am Acad Dermatol. 2014;71(2):398–400. doi:10.1016/j.jaad.2014.04.034
12. Shen C-S, Tabata K, Matsuki M, et al. Premature apoptosis of keratinocytes and the dysregulation of keratinization in porokeratosis. Br J Dermatol. 2002;147(3):498–502. doi:10.1046/j.1365-2133.2002.04853.x
13. Shimamoto Y, Shimamoto H. Differentiation of disseminated superficial actinic porokeratosis from the superficial disseminated eruptive form of porokeratosis of Mibelli. Cutis. 1988;42(4):345–348.
14. Reed RJ, Leone P. Porokeratosis--A mutant clonal keratosis of the epidermis. I. Histogenesis. Arch Dermatol. 1970;101(3):340–347. doi:10.1001/archderm.1970.04000030084014
15. Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–328.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.