Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Successful Treatment of Chronic Actinic Dermatitis with Tofacitinib
Authors Zhong J , Ali K , Yang P, Zhao X, Wu L
Received 4 December 2023
Accepted for publication 6 February 2024
Published 9 February 2024 Volume 2024:17 Pages 399—407
DOI https://doi.org/10.2147/CCID.S451824
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Jianbo Zhong,1,* Kamran Ali,1,2,* Ping Yang,1,* XingYun Zhao,1 LiMing Wu1
1Department of Dermatology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Oncology, the Fourth Affiliated Hospital, International Institutes of Medicine, Zhejiang University School of Medicine, Yiwu, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: LiMing Wu, Department of Dermatology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, No. 261, Huansha Road, Hangzhou, People’s Republic of China, Tel/Fax +86 13750837205, Email [email protected]
Abstract: We present the case of a 58-year-old male patient who presented with pruritic skin plaques and papules on the scalp, face, back, and back of the hands for over a year. The symptoms worsened upon exposure to sunlight and improved on cloudy days. Despite previous attempts at treatment with glucocorticoid ointment and antihistamine drugs, the patient experienced progressive aggravation of symptoms. Physical examination revealed hypertrophic and infiltrating nodules, with significant scratches and local exudation. Skin biopsy confirmed the diagnosis of sun-induced dermatosis. The patient was initiated on tofacitinib, an oral Janus Kinase inhibitor, along with a halometasone ointment, oral ebastine tablets, and strict sun protection. Over the course of four revisits spanning four months, the patient experienced a significant improvement in symptoms, with the rash almost disappearing and pruritus subsiding. The treatment was well tolerated and no adverse effects were observed. Follow-up for six months post-treatment showed no recurrence of symptoms. This case highlights the efficacy of tofacitinib in managing sun-induced pruritic plaques and suggests it as a potential therapeutic option in similar cases.
Keywords: tofacitinib, sun-induced dermatosis, chronic actinic dermatitis, pruritic plaques
Introduction
Photodermatoses, also known as disorders of cutaneous photosensitivity, encompass a diverse array of inflammatory skin conditions that are triggered or exacerbated by exposure to solar radiation.1,2 Among these, chronic actinic dermatitis (CAD) is a rare, persistent immunological photodermatosis that manifests as a pruritic eczematous eruption on sun-exposed skin when subjected to ultraviolet (UV) light,3,4 encompassing both ultraviolet A and ultraviolet B, as well as visible light,5 accompanied by the development of papules and lichenified plaques.6 In severe cases, it can progress to erythroderma with palmoplantar hyperkeratosis,7 and in some instances, it can transform into cutaneous lymphoma.8 However, the exact pathophysiology remains unknown.3 Some hypotheses suggest that the disease mechanism may involve a delayed-type hypersensitivity reaction to an endogenous photoinduced antigen, postulated as UV-altered DNA.9,10 It is believed that the recognition of UV-induced neoantigens by lymphocytes in the skin may underlie this process.7 While CAD affects individuals of any sex and age, it primarily develops in men over 50 years of age,11,12 particularly those with fair skin,13 often engaging in outdoor activities.10 These sex and age patterns appear to be more pronounced in Asian populations.3,14 CAD follows a relapsing and remitting course, which significantly compromises the affected individual’s quality of life.6
The diagnosis of CAD is based on clinical presentation, phototesting, and histological examination. Phototesting is a crucial diagnostic tool for quantifying light-related symptoms, and histological examination of skin biopsy specimens aids in confirming the diagnosis.15 The management of CAD includes strict sun protection, photoprotective clothing, and the use of UVA and UVB sunscreens.10 Currently, no curative treatment for CAD.5 Topical calcineurin inhibitors (TCIs) and topical and systemic corticosteroids are first-line pharmacological management options. In severe cases, indicated systemic immunosuppression usually involves cyclosporine, methotrexate, mycophenolate mofetil, and azathioprine.6,16 However, these treatment options may not always be capable of controlling symptoms and can lead to inadequate outcomes or serious side effects in many instances.10,11,17
Case Presentation
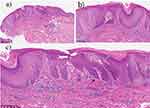
A 58-year-old male patient presented with pruritic skin plaques and papules on the scalp, face, back, and back of the hands that had persisted for over a year. The symptoms worsened upon exposure to sunlight and improved on cloudy and rainy days. The patient had sought medical help previously and received a diagnosis of “eczema.” Treatment with glucocorticoid ointment and oral antihistamines provided only short-lived relief, leading to the progressive aggravation of symptoms. Physical examination revealed hypertrophic and infiltrating nodules of varying sizes with significant scratches and local exudation (Figure 1a and b). A biopsy of the occipital skin confirmed the diagnosis, demonstrating epithelial keratosis, hyperkeratosis, and lymphocytic infiltration around the blood vessels and skin appendages (Figure 2a-c). The patient was initiated on tofacitinib at a dosage of 5 mg twice a day(bid), along with halometasone ointment and oral ebastine (20mg qd) tablets. During the first revisit (4th weeks after the first dose administration), the patient reported improved symptoms, including alleviation of itching and a decline in rash (Figure 1c and d). Blood tests, including routine coagulation and liver and kidney function tests, revealed no abnormalities (8th weeks after the first dose). The patient underwent subsequent revisits after one month (12th weeks after the first dose) (Figure 1e and f). The symptoms continued to improve steadily. The blood test results were within normal ranges. By the fourth revisit (16th weeks after the first dose), the rash was almost imperceptible and pruritus was no longer prominent (Figure 1g and h). The patient discontinued the use of halometasone ointment and oral ebastine tablets, while strictly adhering to sun protection measures. Follow-up for six months showed no recurrence of symptoms, and the patient continued to receive oral tofacitinib once daily.
Discussion
The disease presents as persistent eczematous infiltrated papules and plaques, primarily affecting sun-exposed areas including the face, scalp, neck, dorsal hands, and forearms. Consequently, the management of Chronic Actinic Dermatitis (CAD) involves prevention of sun exposure. However, in some instances, even a brief exposure to sunlight or skin coverage can trigger the development of eczema. As the disease advances, the affected skin undergoes thickening and roughening, characterized by mossy lesions and intensified pruritus, potentially resulting in skin scratching and complications such as localized skin infections and irritant dermatitis.5 CAD is a debilitating disease that significantly restricts the quality of life of those who suffer from it as well as their close associates. These patients must entirely avoid outdoor activities, and some may even lose their jobs.11
Although topical corticosteroids, antihistamines, and other first-line medications are frequently used to manage this disease, some patients may experience treatment resistance or temporary relief of symptoms. Chronic actinic dermatitis is a rare and severe type of photodermatitis that is often be debilitating. Conventional treatments, such as photoprotection, topical steroids, and immunosuppressive agents, might not provide satisfactory results in stubborn cases.18 Moreover, long-term use of these traditional medications to treat CAD carries significant health-related risks and side effects.10 In the scientific literature, research has explored novel therapies and drugs for CAD treatment, including phototherapy, thalidomide, apremilast, and biological therapies such as dupilumab and tofacitinib, which have shown effectiveness in CAD treatment16 with minimal adverse effects.10
Dupilumab, a monoclonal antibody antagonist inhibiting interleukin-4 and interleucin-13, has demonstrated excellent clinical benefits in treating drug-resistant CAD, including good safety and tolerance. Additionally, it has been used in combination with methotrexate for severe refractory CAD, yielding successful outcomes.10,16,17
The JAK-STAT pathway is a common signaling pathway used by many cytokines that are important for skin health. Inhibition of this pathway using JAK inhibitors could be a promising treatment for a variety of skin disorders.19,20 These inhibitors target various cell types, including CD8-positive T cells, and suppress the interferon-gamma and interleukin pathways by blocking the action of four key tyrosine kinases: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). Tofacitinib, a selective oral Janus Kinase inhibitor (JAK1 inhibitor), has proven effective in treating several T cell-mediated dermatoses such as psoriasis, atopic dermatitis, dermatomyositis, alopecia areata, and vitiligo, which do not respond to classical immunosuppressive therapy.7,19,21 Considering the growing evidence that Chronic Actinic Dermatitis (CAD) involves cytotoxic T cell activity inhibition of the JAK pathway holds potential as a therapeutic approach. In a case report by Dev et al, in which the patient exhibited persistent CAD and conventional medications had no effect, the decision to use tofacitinib was made. Subsequently, the symptoms significantly improved, with complete resolution of the rash and relief of the itching. The treatment was well tolerated and no adverse effects were observed. The observed sustained response suggests the potential of this drug for long-term control of CAD.21 Vesely et al reported another case of severe CAD that did not respond to any of the following treatments: potent topical steroids, 0.1% tacrolimus ointment, prednisone, hydroxychloroquine, methotrexate, azathioprine, mycophenolate mofetil, cyclosporine, omalizumab, acitretin, oral bexarotene, extracorporeal photopheresis, or various combinations of these treatments. Tofacitinib citrate was then decided upon, resulting in successful remission of the symptoms of the disease.7 Another reported case informed of a patient with CAD who did not respond to conventional treatment. Subsequently, after the administration of tofacitinib, there was a remission of symptoms without the presence of adverse effects.22 The patient in our study also used first-line drugs for their condition, such as topical glucocorticoid ointments, loratadine, cetirizine, and other antihistamines. However, these results were unexpected. Consequently, we opted to administer tofacitinib at twice a daily dose of 5mg bid, combined with ebastine (20 mg/day) and halometasone ointment, leading to significant symptom improvement. Additionally, although photobiology and photopatch tests are recommended for patients with clinically compatible CAD findings,4,6,12 we did not employ these tests in our patient because of their inability to perform the procedure.
Recent studies have offered exciting prospects for treating Chronic Actinic Dermatitis (CAD) using JAK inhibitors, a class of drugs that target specific inflammatory pathways. These studies demonstrated significant improvements in the disease burden and encouraging safety profiles. Pappa et al explored the potential of upadacitinib, a selective and reversible JAK inhibitor, in the treatment of CAD. While its efficacy in managing other skin conditions, such as atopic dermatitis, has been established, its impact on CAD remains unknown. This study showed promising results, suggesting that upadacitinib is a potential therapeutic option for patients with CAD.23 Maguire et al reported a case of a 69-year-old man with severe CAD who achieved complete clinical and phototest remission after receiving baricitinib, another JAK inhibitor. This case adds to the growing body of evidence supporting the potential of JAK inhibitors for the treatment of CAD.24 Jin et al reported the effectiveness of brobrobrocitinib in a patient with CAD, highlighting the growing range of JAK inhibitors that show promise for this challenging condition.25 These preliminary studies offer a glimmer of hope for CAD patients. Summary of off-label use of drugs for the treatment of CAD is given in Table 1.
 |
Table 1 Off-Label Use of Drugs for Chronic Actinic Dermatitis |
Although ultraviolet light is the causative factor of CAD, some studies have indicated that ultraviolet light can also be utilized for diagnosing and managing photodermatosis. On the one hand, phototesting can be useful in confirming photosensitivity disorders, particularly immunologically originated photodermatoses. Once the diagnosis is confirmed, phototherapy can be employed to treat many photodermatoses.15,18 Phototherapy is effective for CAD and seems to enhance patient tolerance to sunlight, consequently reducing disease extent, as demonstrated by Wang et al who employed the UV-A rush hardening technique at various intervals.11
Apremilast is an orally administered small-molecule compound that specifically inhibits the enzyme phosphodiesterase-4 (PDE-4) and modulates the immune system by increasing intracellular cyclic adenosine monophosphate (cAMP) levels and inhibiting the production of IL-2 and 8, interferon-gamma, and tumor necrosis factor (TNF).28 Apremilast acts as a broad-spectrum anti-inflammatory.26 The use of this drug has shown significant improvement in skin lesions after four weeks of treatment, resulting in the complete elimination of characteristic CAD lesions. Furthermore, no significant adverse effects were reported during the 12-week follow-up period.28 Apremilast has been suggested as a potential substitute for steroid medications.26 On the other hand, severe chronic actinic dermatitis symptoms have also been successfully controlled by prescribing thalidomide. However, its limitations include numerous adverse effects such as drowsiness, dizziness, digestive issues, constipation, teratogenicity, irreversible peripheral neuropathy, and venous thromboembolism.18
Nutrients and natural compounds can also exert therapeutic effects one numerous diseases.29,30 Colostrum-derived macrophage-activating factor (colostrum-MAF) is a health-promoting substance containing degalactosylated/desialylated bovine colostrum, which is frequently used in patients with atopic dermatitis and autism. Ichihashi et al suggested that this compound could also be effective in the treatment of CAD. The potential mechanism may be associated with the modulation of M1 and M2 macrophages and regulation of inflammation.27
Several studies have reported that CAD development is linked to dysregulated inflammation and oxidative stress.5,20 Additionally, it’s also observed that CAD patients are more susceptible to infections following skin biopsy.3 Photo allergy triggers the alteration of endogenous skin antigens, followed by inflammation mediated by CD8+ T cells, a process similar to the delayed-type hypersensitivity in allergic contact dermatitis. Studies have shown increased CD8+ T-cell aggregation within the epidermis and a low CD4:CD8 ratio in patients with CAD. Repeated exposure to ultraviolet light triggers an inflammatory response, leading to the formation of characteristic lesions. Long-standing lesions can display lichenification and pigmentary changes.17,26 In contrast, mast cells may be involved in the early process of CAD, whereas dendritic cells and tissue-resident memory T cells may be related to the chronic process of the disease.3
Expression of the SPAG5/FOXM1 axis is associated with UV-mediated inflammation, keratinocyte proliferation, oxidative stress, and increased apoptosis. In a study by Chen et al, utilizing in vitro models, it was observed that UV rays could enhance the expression of the SPAG5/FOXM1 axis in cells. Conversely, the use of curcumin, a natural compound from turmeric, delayed and restricted the expression of SPAG5/FOXM1, successfully reversing the effects caused by UV rays. The results demonstrated decreased keratinocyte proliferation, and lower levels of inflammation, oxidative stress, and apoptosis.5
CAD has also been associated with other pathologies. For instance, individuals with contact allergy to plants, compounds such as sesquiterpene lactones, fragrances, sunscreens components, or those presenting with other types of photodermatoses have a higher risk of developing CAD.4 In a case report by Fujii et al, in which the patient had an allergy to sesquiterpene lactones present in chrysanthemums, CAD also developed. However, CAD symptoms and photosensitivity also improve upon being informed to avoid contact with the plant.31 CAD develops more frequently during periods of high sun exposure, such as the summer, and can persist throughout the year in severe cases. Recent studies indicate that while solar exposure is indeed the primary risk factor, climate change, air pollutants, and air quality are exacerbating factors for symptomatology.1,3,8
It has been reported that CAD patients have decreased albumin and increased uric acid levels. Additionally, they present a higher risk of skin infections following biopsy. These effects can be attributed to the destruction of the skin barrier, exudation, and desquamation of the skin.3 At the genetic level, the expression of the WAKMAR2 gene might be associated with the dermatological barrier in CAD,32 whereas UV-induced hsa-miR-31-3p has been correlated with the severity of CAD, and plays a significant role in regulating the keratinocyte permeability barrier by targeting CLDN1, a gene involved in regulating epithelial barrier permeability and barrier function.8
Tofacitinib appears to be a promising therapeutic option for patients with sun-induced pruritic plaques that do not respond adequately to conventional treatment. This case report underscores the importance of considering alternative therapeutic approaches in refractory cases and suggests that tofacitinib could be an effective and well-tolerated treatment in such scenarios.
Limitations
Effective long-term treatments for CAD are lacking and the disease course tends to be persistent and chronic. Limited studies exist regarding the use of tofacitinib for the treatment of CAD. Further studies and broader trials are warranted to assess the long-term efficacy and safety of tofacitinib in this patient population.
Data Sharing Statement
The case study data are described in the manuscript.
Consent to Participate
The patients in this manuscript provided written informed consent for the publication of their case details and images to be published.
Acknowledgment
Jianbo Zhong, Kamran Ali and Ping Yang are co-first authors for this study. The patients in this manuscript provided informed written consent for the publication of their case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that the research was conducted without any funding.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
References
1. Kim HJ, Kim KH. Increased incidence of chronic actinic dermatitis in relation to climate changes and air pollution during the past 15 years in Korea. Photodermatol Photoimmunol Photomed. 2018;34(6):387–392. doi:10.1111/phpp.12402
2. Burfield L, Rutter KJ, Thompson B, Marjanovic EJ, Neale RE, Rhodes LE. Systematic review of the prevalence and incidence of the photodermatoses with meta-analysis of the prevalence of polymorphic light eruption. J Eur Acad Dermatol Venereol. 2023;37(3):511–520. doi:10.1111/jdv.18772
3. Lin N, Huang X, Ma C, Han J. Clinical and pathological findings of chronic actinic dermatitis. Photodermatol Photoimmunol Photomed. 2021;37(4):313–320. doi:10.1111/phpp.12654
4. Wang CX, Belsito DV. Chronic Actinic Dermatitis Revisited. Dermatitis. 2020;31(1):68–74. doi:10.1097/DER.0000000000000531
5. Chen Q, Tang Y, Deng H, et al. Curcumin Improves Keratinocyte Proliferation, Inflammation, and Oxidative Stress through Mediating the SPAG5/FOXM1 Axis in an In Vitro Model of Actinic Dermatitis by Ultraviolet. Dis. Markers. 2022;2022:5085183. doi:10.1155/2022/5085183
6. Paracha MM, Zahoor H, Khan AQ, Noor SM, Sagheer F. Efficacy Of Methotrexate Versus Azathioprine In The Treatment Of Chronic Actinic Dermatitis: a Randomized Control Trial. J Ayub Med College Abbottabad. 2022;34(Suppl 1):S644–s8. doi:10.55519/JAMC-03-S1-9643
7. Vesely MD, Imaeda S, King BA. Tofacitinib citrate for the treatment of refractory, severe chronic actinic dermatitis. JAAD case reports. 2017;3(1):4–6. doi:10.1016/j.jdcr.2016.09.008
8. Tu Y, Wu W, Guo Y, et al. Upregulation of hsa-miR-31-3p induced by ultraviolet affects keratinocytes permeability barrier by targeting CLDN1. Biochem. Biophys. Res. Commun. 2020;532(4):626–632. doi:10.1016/j.bbrc.2020.06.113
9. Bourgaux M, Marcaillou M, Matei I, et al. Man of stone: a case of a chronic actinic dermatitis mimicking a cutis verticis gyrata. J Eur Acad Dermatol Venereol. 2020;34(3):e129–e30. doi:10.1111/jdv.16068
10. Ali K, Wu L, Lou H, et al. Clearance of Chronic Actinic Dermatitis With Dupilumab Therapy in Chinese Patients: a Case Series. Front Med. 2022;9:803692. doi:10.3389/fmed.2022.803692
11. Wang T, Gong Y, Rong W, Li L, Zhang J, Li HZ. Ultraviolet A rush hardening for chronic actinic dermatitis: pilot treatment outcomes. J Dermatol. 2021;48(3):385–388. doi:10.1111/1346-8138.15667
12. Gu Q, Zhang Z, Yang J, et al. Chronic actinic dermatitis: a 5-year clinical analysis of 488 patients in China. Photodermatol Photoimmunol Photomed. 2023;39(3):263–268. doi:10.1111/phpp.12835
13. Maghfour J, Mohney L, Lim HW, Mohammad TF. Demographics and clinical presentations of 844 patients with light and dark skin types with polymorphous light eruption and chronic actinic dermatitis evaluated over 23 years. Photodermatol Photoimmunol Photomed. 2023;39(2):93–99. doi:10.1111/phpp.12863
14. Lee CN, Chen TY, Wong TW. The Immunogenetics of Photodermatoses. Adv Exp Med Biol. 2022;1367:369–381.
15. Jiang AJ, Lim HW. Phototherapy in the Evaluation and Management of Photodermatoses. Dermatologic Clinics. 2020;38(1):71–77. doi:10.1016/j.det.2019.08.007
16. Chen JC, Lian CH. Chronic actinic dermatitis in an old adult significantly improved by dupilumab. Photodermatol Photoimmunol Photomed. 2022;38(2):176–177. doi:10.1111/phpp.12731
17. Patel N, Konda S, Lim HW. Dupilumab for the treatment of chronic actinic dermatitis. Photodermatol Photoimmunol Photomed. 2020;36(5):398–400. doi:10.1111/phpp.12566
18. Lahouel M, Ben Kahla M, Aounallah A, et al. Severe chronic actinic dermatitis treated successfully with Thalidomide. Photodermatol Photoimmunol Photomed. 2020;36(6):493–495. doi:10.1111/phpp.12588
19. Duraisamy P, Jagadeesan S, Thomas J. Tofacitinib in the treatment of refractory eczemas - a case series. J Dermatological Treatment. 2022;33(6):2873–2875. doi:10.1080/09546634.2022.2082355
20. Kim JH, Kim HJ, Yoo DW, et al. A postulated model for photo immunopathogenesis of chronic actinic dermatitis around adaptive immunity, including Th17 cells, Tregs, TRMs, cytotoxic T cells, and/or common-γ chain receptor+ cells. Photodermatol Photoimmunol Photomed. 2023;39(2):147–154. doi:10.1111/phpp.12848
21. Dev A, Bishnoi A, Narang T, Vinay K. Recalcitrant chronic actinic dermatitis responding to tofacitinib: a case report. Indian J Dermatol Venereol Leprol. 2023;89(4):600–602. doi:10.25259/IJDVL_744_2022
22. Wang Y-J, Hui H-Z, Cheng J-R, Mao H, Diao Q-C, Shi B-J. Improvement of Refractory Chronic Actinic Dermatitis During Tofacitinib Treatment. Am j Therapeutics. 2023;30(6):e547–e548. doi:10.1097/MJT.0000000000001616
23. Pappa G, Sgouros D, Kanelleas A, Koumaki D, Bozi E, Katoulis A. JAK-ing up chronic actinic dermatitis with upadacitinib. Clin Exp Dermatol. 2023.
24. Maguire J, Gleeson D, Corso R, Pink A, Smith C, Ferguson J. Remission of chronic actinic dermatitis on baricitinib: a case report. Skin Health Dis. 2023;3(6):e243. doi:10.1002/ski2.243
25. Jin X, Qiao J. Effectiveness of Abrocitinib in a Patient With Chronic Actinic Dermatitis. Am j Therapeutics. 2023. doi:10.1097/MJT.0000000000001671
26. Kaushik A, Narang T, Handa S. Successful use of apremilast as a steroid-sparing agent in chronic actinic dermatitis. Dermatologic Therapy. 2020;33(6):e13809. doi:10.1111/dth.13809
27. Ichihashi M, Nakamura Y, Muto M, Nishikata T, Inui T, Uto Y. A case of chronic actinic dermatitis that responded completely to treatment with oral colostrum-macrophage-activating factor (colostrum-MAF). Photodermatol Photoimmunol Photomed. 2019;35(4):290–292. doi:10.1111/phpp.12469
28. Nassim D, Alajmi A, Jfri A, Pehr K. Apremilast in dermatology: a review of literature. Dermatologic Therapy. 2020;33(6):e14261. doi:10.1111/dth.14261
29. Xu Lou I, Ali K, Chen Q. Effect of nutrition in Alzheimer’s disease: a systematic review. Front Neurosci. 2023;17:1147177. doi:10.3389/fnins.2023.1147177
30. Xu Lou I, Gil-García E, Cáceres-Matos R, Ali K, Molina E. Nutritional aspects in chronic non-cancer pain: a systematic review. Frontiers in Nutrition. 2022;9:931090. doi:10.3389/fnut.2022.931090
31. Fujii S, Washio K, Masaki T. Case report of chronic actinic dermatitis accompanied by ultraviolet A photosensitivity in a Chrysanthemum farmer. J Dermatol. 2019;46(7):e229–e30. doi:10.1111/1346-8138.14818
32. Tu Y, Wang L, Wang X, et al. LncRNA-WAKMAR2 regulates expression of CLDN1 to affect skin barrier through recruiting c-Fos. Contact Dermatitis. 2023;88(3):188–200. doi:10.1111/cod.14256
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.