Back to Journals » International Medical Case Reports Journal » Volume 17
Successful Outcome of a Patient with Concomitant Pancreatic and Renal Carcinoma Receiving Secoisolariciresinol Diglucoside Therapy Alone: A Case Report
Authors Wu H, Zhang XH, Wang LP, Tian HD, Liu GR, Yang DH, Liu SL
Received 23 October 2023
Accepted for publication 6 March 2024
Published 15 March 2024 Volume 2024:17 Pages 167—175
DOI https://doi.org/10.2147/IMCRJ.S446184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xudong Zhu
Hao Wu,1– 6 Xing-Hua Zhang,1,3– 6 Li-Ping Wang,7,8 Hong-Da Tian,1,3– 6 Gui-Rong Liu,1,3– 6 Dong-Hui Yang,9 Shu-Lin Liu1,3– 8,10
1Genomics Research Center (State-Province Key Laboratory of Biomedicine-Pharmaceutics of China), College of Pharmacy, Harbin Medical University, Harbin, People’s Republic of China; 2Key Laboratory of Tumor Biotherapy of Heilongjiang Province, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 3Key Laboratory of Gut Microbiota and Pharmacogenomics of Heilongjiang Province, Harbin Medical University, Harbin, People’s Republic of China; 4National Key Laboratory of Frigid Zone Cardiovascular Diseases, Harbin, People’s Republic of China; 5Heilongjiang Academy of Medical Sciences, Harbin Medical University, Harbin, People’s Republic of China; 6Translational Medicine Research and Cooperation Center of Northern China, Harbin Medical University, Harbin, People’s Republic of China; 7KangYuan Hospital, Harbin, People’s Republic of China; 8Xun-Qi Medicine Clinic, Harbin, People’s Republic of China; 9State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, People’s Republic of China; 10Department of Microbiology, Immunology and Infectious Diseases, University of Calgary, Calgary, Canada
Correspondence: Shu-Lin Liu, Genomics Research Center, College of Pharmacy, Harbin Medical University, Harbin, 150081, People’s Republic of China, Email [email protected] Dong-Hui Yang, State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, 100191, People’s Republic of China, Email [email protected]
Introduction: Pancreatic cancer (PC) is among the deadliest malignancies. Kidney cancer (KC) is a common malignancy globally. Chemo- or radio-therapies are not very effective to control PC or KC, and overdoses often cause severe site reactions to the patients. As a result, novel treatment strategies with high efficacy but without toxic side effects are urgently desired. Secoisolariciresinol diglucoside (SDG) belongs to plant lignans with potential anticancer activities, but clinical evidence is not available in PC or KC treatment.
Patient Concerns: We report a rare case of an 83-year-old female patient with pancreatic and kidney occupying lesions that lacked the conditions to receive surgery or chemo- or radiotherapy.
Diagnosis: Pancreatic and kidney cancers.
Interventions: We gave dietary SDG to the patient as the only therapeutics.
Outcomes: SDG effectively halted progression of both PC and KC. All clinical manifestations, including bad insomnia, loss of appetite, stomach symptoms, and skin itching over the whole body, all disappeared. The initial massive macroscopic hematuria became microscopic and infrequent, and other laboratory results also gradually returned to normal. Most of the cancer biomarkers, initially high such as CEA, CA199, CA724, CA125, came down rapidly, among which CA199 changed most radically. This patient has had progression-free survival of one year so far.
Conclusion: These results demonstrate the potent inhibitory effects of SDG on PC and KC of this patient and provide promising novel therapeutics for refractory malignant tumors.
Keywords: pancreatic cancer, kidney cancer, lignans, secoisolariciresinol diglucoside, CA199
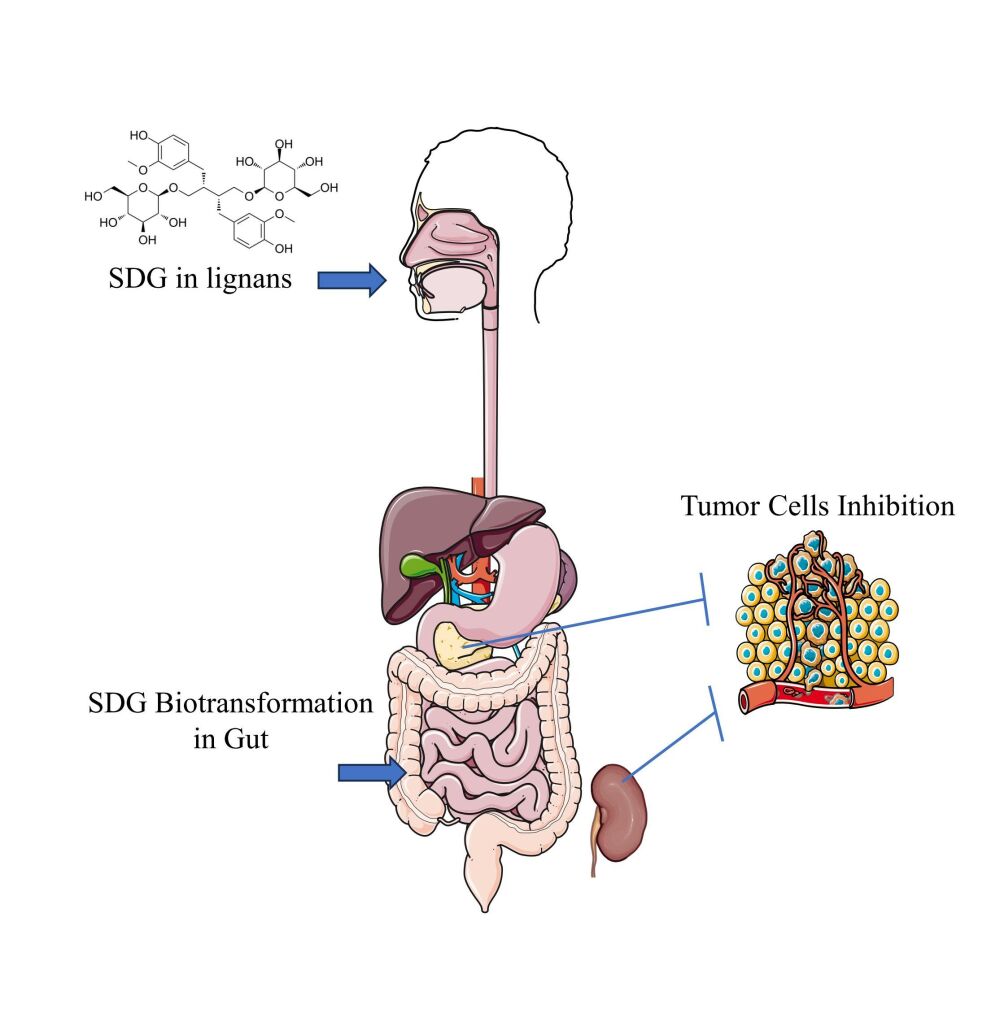
Graphical Abstract:
Introduction
Cancer is a leading cause of death and a major barrier to natural life expectancy worldwide.1,2 Over the past decades, the treatment of cancer has undergone tremendous changes, leading to continuous improvement of clinical outcomes.3–5 Although surgical resection is still among the top choices of treatment for cancer patients, it is suitable only for early stages of very limited kinds of tumors, before metastasis occurs. Even so, usually significant sizes of normal tissues surrounding the tumor or even the whole organ containing the tumor would also have to be removed.6,7 Chemotherapy as the routine method for treatment of most malignancies, on the other hand, could cause severe systemic side effects, including serious damage to tissues and organs such as the immune and hemopoietic systems; similarly, radiotherapy also causes local or systemic damage to the patients, particularly DNA damage.5,8 Immunotherapy as new development of cancer treatment has brought in unprecedented effects and hope for a cure of some malignant diseases, but significant problems remain to be resolved, such as immune function interference and de novo or acquired resistance to immune checkpoint inhibitors (ICIs).9–11 Therefore, innovative and more effective treatment strategies without toxic side effects are urgently desired.
Pancreatic cancer (PC) is a deadly disease with very low (11%) 5-year overall survival (OS).12 In 2020, the global PC death number (ca. 466,000) was close to the number of new cases (ca. 496,000) due to the lack of effective treatment methods and the strong tendency of PC to develop resistance to the current therapeutic strategies, which lead to rapid progression and low rates of pathologic complete response.2,8 Although some novel targeted therapeutic drugs or strategies might contribute to improve treatment outcomes in PC patients, including increasing immune surveillance, regulating cell metabolism and drug combinations,13–15 new possibilities need to be further explored to improve the clinical outcomes of PC patients.
Kidney cancer (KC) ranks 16th among the most frequently diagnosed cancers, with 431,288 new cases reported in 2020 globally.2 Treatment for KC has improved dramatically in recent years, shifting from high-dose cytokine therapy in combination with surgical resection to targeted therapy, immunotherapy or combination therapies.16 However, effective chemotherapies are not available for KC treatment, and ICIs often cause toxic effects due to overtreatment.17,18 Therefore, new therapeutic strategies against KC also need to be developed.
Secoisolariciresinol diglucoside (SDG) belongs to plant lignans, which are phenolic compounds found in a broad range of edible plants, such as the seed of flax.19,20 SDG has strong suppressive effects on cancers, but to date is mostly considered in the prevention rather than treatment of malignancies.21,22 Many studies have demonstrated that SDG functions by affecting the gut microbiome.23,24 However, little is known about the effects of SDG on the highly intractable cancers such as PC or KC.
Here, we report a case of an elderly female patient with both PC and KC diagnosed at the same time. The senior age of the patient and the advanced stages of the two distinct kinds of primary cancers made it impossible for the patient to undergo surgery or chemo- or radiotherapy. We then gave dietary SDG to the patient as the only therapeutics. Consequently, both PC and KC of the patient stopped progression soon after the oral administration of dietary SDG, with clinical manifestations quickly vanishing and laboratory results gradually becoming normal.
Case Report
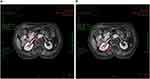
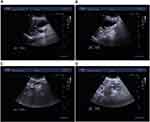
An 83-year-old woman, with one year of weakness, painless macroscopical hematuresis, jaundice, loss of appetite and poor sleep quality, was admitted on November 8th 2021. A gadolinium-enhanced total abdomen magnetic resonance imaging (MRI; detected on November 15th 2021) scan revealed enlarged gallbladder and dilated intrahepatic bile duct, common hepatic duct and bile duct, image of which was truncated by the head of the pancreas. A nodule, 23×16×18 mm, was found in the uncinate process of the pancreas (Figure 1A). The T1-weighted imaging (T1WI) showed slightly low signal, and T2WI showed slightly high signal. The enhanced scan showed progressive enhancement, distal pancreatic parenchyma atrophy, and pancreatic duct dilation. The boundary between the lesion and the descendant duodenum was not clear, the contact with the superior duodenal vein was less than 180°, and the boundary with the superior duodenal artery was clear. Multiple long T1 and T2 signals could be seen around the lesion without enhancement, which seemed to be connected with the pancreatic duct. In addition, a right renal pelvis nodule, 23×9 mm with clear boundary, was found (Figure 1B). T1WI and T2WI showed a little low and high signal, respectively. The enhanced scan showed mild non-uniform enhancement. The MRI findings suggested that the nodule in the uncinate process of the pancreas might be pancreatic cancer that created the low biliary obstruction, and that the nodule in the right renal pelvis might also be malignant. The results of three-dimensional ultrasound images (detected on November 12th 2021) of the whole abdomen also showed that the gallbladder was enlarged (about 79×41 mm) with clear outline, the intrahepatic bile duct was dilated, and the downward part of the common bile duct was widened to about 18 mm (Figure 2A and B). The main pancreatic duct was dilated (about 11 mm, Figure 2C) and coupled with low biliary obstruction (Figure 2D). The patient had no family history of malignant tumors or mental illnesses. These imaging results indicated that the patient had both pancreatic cancer and renal pelvis cancer.
The patient underwent bile duct dilation operation on November 26th 2021, the jaundice was alleviated after the intervention. She did not receive the conventional treatment for pancreatic or kidney cancer, such as surgery, chemo- or radiotherapy, or targeted therapy. She agreed to start oral SDG administration at a dose of 7 g twice per day on January 12th, 2022. After 5 days of ingesting SDG, the patient felt substantial improvement of overall physical state and, particularly, her sleep status became fully recovered after severe insomnia.
After 3 months of oral SDG administration, the frequency of macroscopic hematuria decreased from daily to less than once a week. Since April of 2022, the patient has shown further improvement of general condition.
On July 2nd 2022, the patient experienced a brief period of fever and received oral cephalosporin treatment. During this period, she stopped the oral SDG administration. In the meantime, she complained of loss of appetite, stomach ache and distension after meals, itching all over her body, and severe insomnia again. Her body weight decreased from 52 kg to 46 kg during that period. On August 4th 2022, she restarted the oral SDG administration once again. Two days later, the itching was diminishing, and she began to have normal appetite and bowel movements, but the stomach ache and distension still remained. However, to our delight, her body weight became stable on August 9th 2022, according to the patient, her stomach symptoms were significantly alleviated. Laboratory examinations of urine also showed significant improvement on August 10th 2022 compared with the previous results (see Table 1). Laboratory studies of the blood also demonstrated significant improvement, although serum cystatin C, albumin, ALP, GGT, cholinesterase and prealbumin were still abnormal. Importantly, the recovery of urine and blood glucose levels (see Table 1) suggested that SDG might have potential clinical value in regulating glucose metabolism.
 |
Table 1 Laboratory Results of Routine Blood, Urine, Blood Biochemistry, and Tumor Markers in Patient at Different Time Points |
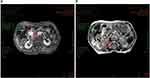
Her body weight increased from the previous 46 kg to 48 kg on August 20th 2022 and this trend continued. On August 26th 2022, her overall condition further improved, including much better complexion and mental state, improved appetite and increased food ingestion, complete disappearance of stomach and intestinal symptoms, and continuously increasing body weight (50 kg on September 16th 2022). MRI scan images (detected on November 7th 2022) showed extremely encouraging results: no further growth of either tumor compared with the MRI results of November 15th 2021, with the current size of the pancreatic tumor being 23×16 mm (Figure 3A) and that of the right renal pelvis tumor being 22×9 mm (Figure 3B), unambiguously demonstrating that the patient’s diseases were under effective control. Whole blood and serum examination results showed a general trend of improvement from April to November, 2022 (Table 1). Most of the biomarkers, initially high, such as CEA, CA199, CA724, CA125, came down rapidly, among which CA199 changed most radically: it was surging from April to August, then remained relatively unchanged from August 10th to November 6th, 2022, and finally dropped dramatically eighteen days afterwards on November 24th, 2022. HE4 kept going down, and the postmenopausal ROMA index first went up slightly and then came down to normal on November 6th, 2022. The hematuria became microscopic upon examination on November 24th, 2022; in the meantime, urine protein and glucose both remained negative (see Table 1). The patient had routine blood and biochemistry tests on August 1th (see Table 1). We have been following-up this patient and she remains well up until the last follow-up on September 3th, 2023.
Discussion
Occurrence of multiple primary cancers in the same individual around the same time is rare, with a frequency of 1–3% among all reported malignancies.25 The frequency of pancreatic cancer concomitant with other cancers is 1–20%,26 predominately in the stomach, colon, thyroid, or genitourinary tract.27 In this report, we presented a female patient with synchronous primary PC and KC.
Despite great advances in the treatment of pancreatic and renal cancers as single diseases over the past decades, there are few reports on the treatment of patients with these two primary cancers diagnosed at the same time. Mahfoud et al reported a 70-year-old female with synchronous primary PC and renal cell carcinoma, who was treated with a combination of gemcitabine, sunitinib and denosumab, and eventually she had a prolonged progression-free survival of 9 months.28 Several laboratories have demonstrated the effects of lignans from flaxseed, which contain SDG, for improving human health as well as for cancer prevention or treatment.20,29 Previously, we used dietary SDG to successfully control malignant diseases.30 Since the elderly patient we reported here was not eligible for surgery or chemo- or radiotherapy, we treated her with dietary SDG as the only therapy and the patient has had progression-free survival of one year. During the treatment, the patient regained body weight, which was significantly decreased during cancer progression, and other symptoms, including bad insomnia, loss of appetite, stomach ache and distension, skin itching over the whole body, all disappeared. Of great significance, the initially macroscopic hematuria became microscopic, and its frequency was also decreased from daily to one or two times a week, all of which demonstrates the potent inhibitory effects of SDG on the malignancies of this patient.
The improvement of laboratory results also demonstrated the efficacy of SDG as treatment, including the recovery of blood cell count and classification as well as the amelioration of levels of several cancer biomarkers, such as CA199, CA724 and HE4. Additionally, the postmenopausal ROMA index was also significantly reduced during SDG treatment, indicating that the risk of ovarian cancer was lowered.
The gut microbiota is now considered as one of the most important factors contributing to host health and influencing disease occurrence.31,32 Changes in the composition of gut microbiota are associated with many human diseases, including cancers.33,34 It is worth noting that after this patient stopped oral SDG ingestion and began receiving antibiotics, her body weight was significantly reduced. We speculate that antibiotics might disrupt the composition and population structure of the gut microbiota, which in turn would greatly weaken the microbial cooperation to support the anticancer activities of SDG, resulting in tumor progression. This speculation is supported by findings that the human intestinal microbiota could convert the plant lignan SDG into mammalian lignans enterodiol and enterolactone, which have potent anticancer activities.35–42 Therefore, the gut microbiota of this patient might act as a mediator in the metabolism and activity of SDG.
Previously, we treated a patient with gastrointestinal stromal tumor through oral administration of SDG.30 The patient had a serum CA724 level of more than 300 U/mL (the upper limit of detection; reference value: 0–6.9 U/mL) before treatment. After one month of oral administration, CA724 was 1.39 U/mL. We have been following-up with him and he was well, working and full of energy up until the last follow-up on August 28th, 2023.
It is worth noting that this patient lacks pathological diagnosis information and has not undergone tumor resection, chemotherapy, radiotherapy or targeted treatment. Judging from clinical experience and laboratory examination results, this patient is in line with the clinical manifestations of pancreatic and renal malignant occupying lesions. The reason why biopsy was not performed was because the patient did not meet the conditions for biopsy at her initial visit in hospital on 2021. At the same time, her old age and poor overall condition meant she was not suitable for surgery or systemic treatment.
The most important point of this study is that we showed evidence to demonstrate the potent suppressive effects of SDG on synchronous primary PC and renal cell carcinoma. Therefore, dietary SDG may become a widely used curative therapy for patients of a variety of cancer types, especially for those who are overly elderly or have other vulnerable conditions.
Conclusion
We reported a case of synchronous primary PC and KC who received oral SDG and had the malignant diseases under control, with significant improvement of clinical manifestations and laboratory examination results. This study demonstrates the potential role of SDG in inhibiting some refractory malignant tumors as a curative therapy.
Abbreviations
PC, Pancreatic cancer; KC, Kidney cancer; SDG, Secoisolariciresinol diglucoside; CEA, Carcinoembryonic antigen; CA199, Carbohydrate antigen 199; CA724, Carbohydrate antigen 724; CA125, Carbohydrate antigen 125; ICIs, Immune checkpoint inhibitors; OS, Overall survival; MRI, Magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging.
Data Sharing Statement
We confirm that all the supporting data mentioned in this manuscript can be provided.
Ethical Approval
Written informed consent for publication was obtained from the patient.
Consent for Publication
We confirm that the work described has not been published before; that it is not under consideration for publication elsewhere; that its publication has been approved by all the authors; that its publication has been approved by the responsible authorities at the institution where the work was carried out. Written informed consent was obtained from the patient for publication of this case report and accompanying materials.
Acknowledgments
We thank the patient for her cooperation. We also acknowledge all the research staff for their contributions to this project. We thank Qiu-Cheng Wang for providing representative enhanced MRI and ultrasound images.
Funding
National Natural Science Foundation of China, Hao Wu, Grant Number: NSFC82202996. National Natural Science Foundation of China, Gui-Rong Liu, Grant Number: NSFC81971910. National Natural Science Foundation of China, Shu-Lin Liu, Grant Numbers: NSFC81871623, NSFC82020108022. Haiyan Fund Project of Harbin Medical University Cancer Hospital, Hao Wu, Grant Number: JJMS 2022-06. The Fundamental Research Funds for the Provincial Universities, Hao Wu, Grant Number: 2022-KYYWF-0288. Heilongjiang Postdoctoral Financial Assistance, Hao Wu, Grant Number: LBH-Z22219. Heilongjiang Provincial Natural Science Foundation Outstanding Youth Project, Hao Wu, Grant Number: YQ2023 H022. China Postdoctoral Science Foundation, Hao Wu, Grant Number: 2023MD734172.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi:10.1002/cncr.33587
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54. doi:10.1186/s13045-019-0738-1
4. Thanarajasingam G, Minasian LM, Baron F, et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol. 2018;5(11):e563–e598. doi:10.1016/S2352-3026(18)30051-6
5. Malyszko J, Tesarova P, Capasso G, Capasso A. The link between kidney disease and cancer: complications and treatment. Lancet. 2020;396(10246):277–287. doi:10.1016/S0140-6736(20)30540-7
6. Barnes H, See K, Barnett S, Manser R. Surgery for limited-stage small-cell lung cancer. Cochrane Database Syst Rev. 2017;4(4):CD011917. doi:10.1002/14651858.CD011917.pub2
7. Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1(3–4):144–158. doi:10.1159/000343828
8. Grossberg AJ, Chu LC, Deig CR, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. Ca a Cancer J Clinicians. 2020;70(5):375–403. doi:10.3322/caac.21626
9. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:6477. doi:10.1126/science.aax0182
10. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi:10.1038/s41575-019-0126-x
11. Dhar R, Seethy A, Singh S, et al. Cancer immunotherapy: recent advances and challenges. J Cancer Res Ther. 2021;17(4):834–844. doi:10.4103/jcrt.JCRT_1241_20
12. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. Ca a Cancer J Clinicians. 2022;72(1):7–33. doi:10.3322/caac.21708
13. Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nature Med. 2016;22(8):851–860. doi:10.1038/nm.4123
14. Pecoraro C, De Franco M, Carbone D, et al. 1,2,4-Amino-triazine derivatives as pyruvate dehydrogenase kinase inhibitors: synthesis and pharmacological evaluation. Eur J Med Chem. 2023;249(115134):115134. doi:10.1016/j.ejmech.2023.115134
15. Valles-Marti A, Mantini G, Manoukian P, et al. Phosphoproteomics guides effective low-dose drug combinations against pancreatic ductal adenocarcinoma. Cell Rep. 2023;42(6):112581.
16. Wang Y, Suarez ER, Kastrunes G, et al. Evolution of cell therapy for renal cell carcinoma. Mol Cancer. 2024;23(1):8. doi:10.1186/s12943-023-01911-x
17. Wang Z, An HW, Hou D, et al. Addressable peptide self-assembly on the cancer cell membrane for sensitizing chemotherapy of renal cell carcinoma. Adv Mater. 2019;31(11):e1807175. doi:10.1002/adma.201807175
18. Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17(3):137–150. doi:10.1038/s41585-020-0282-3
19. Toure A, Xueming X. Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr Rev Food Sci Food Saf. 2010;9(3):261–269. doi:10.1111/j.1541-4337.2009.00105.x
20. Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients. 2019;11(5):1171. doi:10.3390/nu11051171
21. DeLuca JAA, Garcia-Villatoro EL, Allred CD. Flaxseed bioactive compounds and colorectal cancer prevention. Curr Oncol Rep. 2018;20(8):59. doi:10.1007/s11912-018-0704-z
22. Calado A, Neves PM, Santos T, Ravasco P. The effect of flaxseed in breast cancer: a literature review. Frontiers in Nutrition. 2018;5:4. doi:10.3389/fnut.2018.00004
23. Lampe JW, Kim E, Levy L, et al. Colonic mucosal and exfoliome transcriptomic profiling and fecal microbiome response to a flaxseed lignan extract intervention in humans. Am J Clin Nutrit. 2019;110(2):377–390. doi:10.1093/ajcn/nqy325
24. Lagkouvardos I, Klaring K, Heinzmann SS, et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol Nutr Food Res. 2015;59(8):1614–1628. doi:10.1002/mnfr.201500125
25. Miyaguni T, Muto Y, Kusano T, Yamada M, Matsumoto M, Shiraishi M. Synchronous double cancers of the remnant stomach and pancreas: report of a case. Surgery Today. 1995;25(12):1038–1042. doi:10.1007/BF00311689
26. Eriguchi N, Aoyagi S, Hara M, et al. Synchronous or metachronous double cancers of the pancreas and other organs: report on 12 cases. Surgery Today. 2000;30(8):718–721. doi:10.1007/s005950070083
27. Gerdes B, Ziegler A, Ramaswamy A, Wild A, Langer P, Bartsch DK. Multiple primaries in pancreatic cancer patients: indicator of a genetic predisposition? Int J Epidemiol. 2000;29(6):999–1003. doi:10.1093/ije/29.6.999
28. Mahfoud T, Tanz R, Khmamouche MR, et al. Synchronous primary renal cell carcinoma and pancreatic ductal adenocarcinoma: case report and literature review. Case Rep Oncol. 2017;10(3):1050–1056. doi:10.1159/000484552
29. De Silva SF, Alcorn J. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals. 2019;12(2). doi:10.3390/ph12020068
30. Hao Wu YW, Chen J, Wang L, Liu G, Shulin L, Liu S-L. Anticancer effects of dietary administration of secoisolariciresinol diglucoside in a patient of gastrointestinal stromal tumor: a case report. Internat J Surg Oncol. 2021;5:e103. doi:10.1097/IJ9.0000000000000103
31. de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi:10.1136/gutjnl-2021-326789
32. Hou K, Wu ZX, Chen XY, et al. Microbiota in health and diseases. Signal Transd Target Ther. 2022;7(1):135. doi:10.1038/s41392-022-00974-4
33. Park EM, Chelvanambi M, Bhutiani N, Kroemer G, Zitvogel L, Wargo JA. Targeting the gut and tumor microbiota in cancer. Nature Med. 2022;28(4):690–703. doi:10.1038/s41591-022-01779-2
34. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371:6536. doi:10.1126/science.abc4552
35. Wang CZ, Ma XQ, Yang DH, et al. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol. 2010;10(115). doi:10.1186/1471-2180-10-115
36. Possemiers S, Bolca S, Eeckhaut E, Depypere H, Verstraete W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. FEMS Microbiol Ecol. 2007;61(2):372–383. doi:10.1111/j.1574-6941.2007.00330.x
37. Zhuang H, Cheng L, Wang Y, et al. Dysbiosis of the gut microbiome in lung cancer. Front Cell Infect Microbiol. 2019;9:112. doi:10.3389/fcimb.2019.00112
38. Liu H, Liu J, Wang S, et al. Enterolactone has stronger effects than enterodiol on ovarian cancer. Jovarian Res. 2017;10(1):49. doi:10.1186/s13048-017-0346-z
39. Tao YL, Yang DH, Zhang YT, et al. Cloning, expression, and characterization of the beta-glucosidase hydrolyzing secoisolariciresinol diglucoside to secoisolariciresinol from Bacteroides uniformis ZL1. Appl Microbiol Biotechnol. 2014;98(6):2519–2531. doi:10.1007/s00253-013-5111-7
40. Wang YF, Xu ZK, Yang DH, et al. The antidepressant effect of secoisolariciresinol, a lignan-type phytoestrogen constituent of flaxseed, on ovariectomized mice. J Nat Med. 2013;67(1):222–227. doi:10.1007/s11418-012-0655-x
41. Li MX, Zhu HY, Yang DH, et al. Production of secoisolariciresinol from defatted flaxseed by bacterial biotransformation. J Appl Microbiol. 2012;113(6):1352–1361. doi:10.1111/j.1365-2672.2012.05436.x
42. Zhu Hongyun LM, Donghui Y, Yilun T, Ying Z, Shulin L. Biotransformation of the SDG in defatted flaxseed into END co-cultured by three single bacterial colonies. Process Biochem. 2013;49(2014):19–24.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.